In Vivo Efficacy Testing of Peptide Receptor Radionuclide Therapy Radiosensitization Using Olaparib
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture Conditions
2.2. Animal Experimental Conditions and Tissue Collection
2.3. Olaparib Measurements
2.4. Tissue Processing and Immunofluorescent Stainings
2.5. Statistical Analyses
3. Results
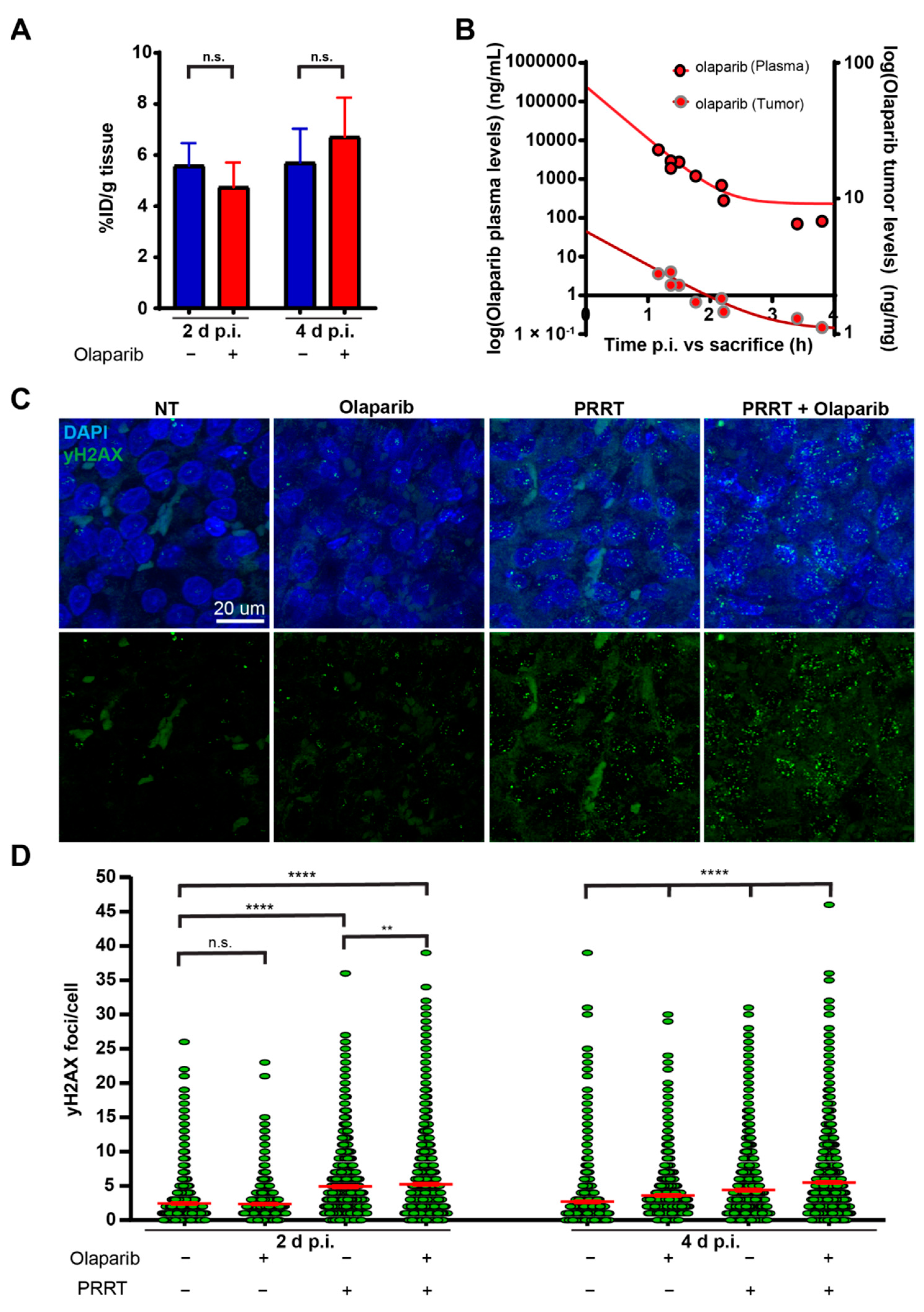
3.1. Olaparib Enhanced the Induction of DNA Damage during PRRT in Mice Bearing CA20948 Tumors
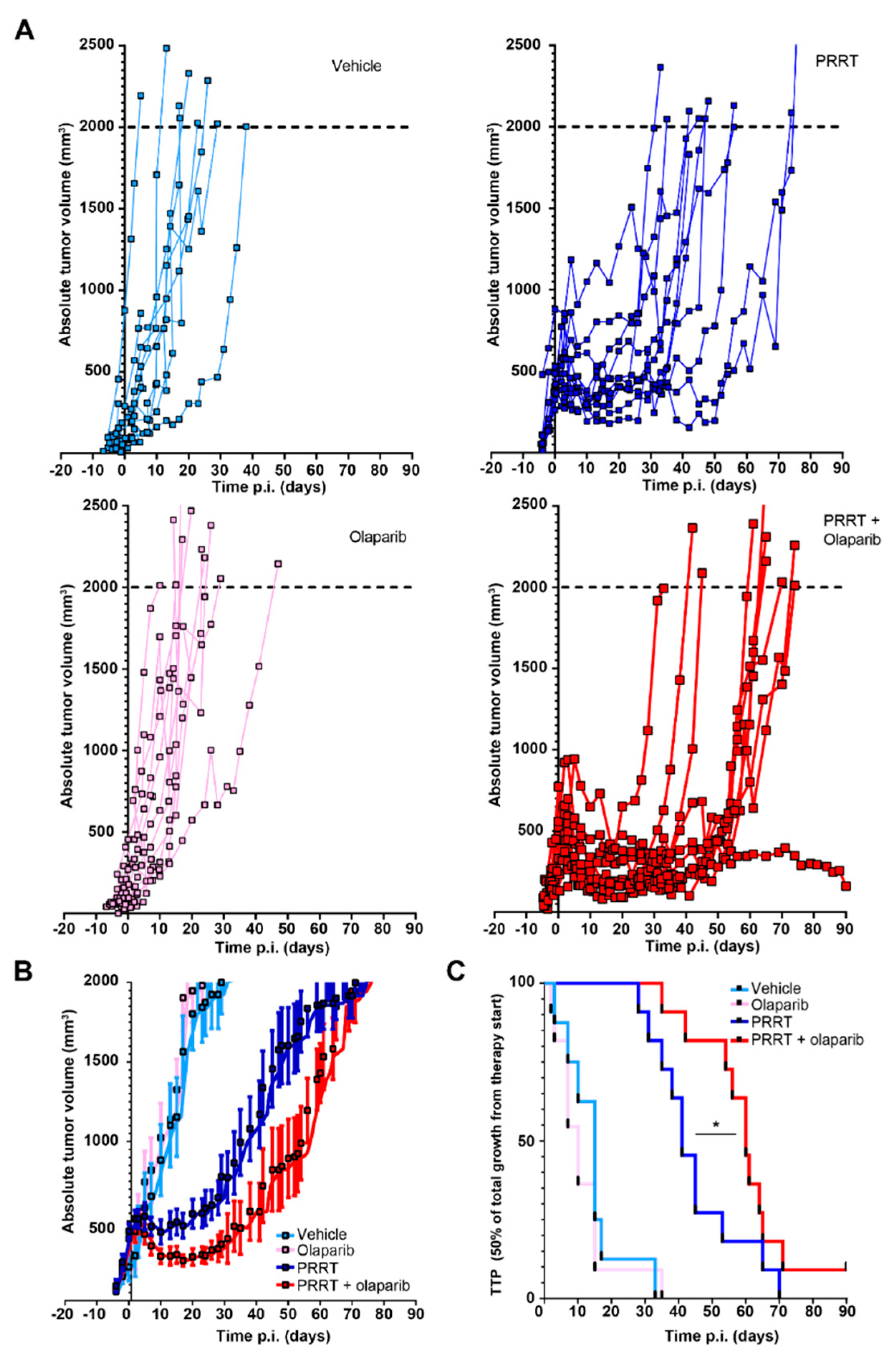
3.2. Combination of PRRT and Olaparib Synergistically Improves CA20948 Tumor Control
3.3. Combining PRRT with Olaparib Does Not Improve Tumor Control in NCI-H69 Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 177Lu-DOTA-TATE | [177Lu]Lu[DOTA0-Tyr3]octreotate |
| BSA | Bovine serum albumin |
| γH2AX | Phosphorylated histone 2A |
| DAPI | 4’,6-diamidino-2-phenylindole |
| DSB | Double strand break |
| DMSO | Dimethyl sulfoxide |
| GEP-NETs | Gastroenteropancreatic-neuroendocrine tumors |
| HBSS | Hanks balanced salt solution |
| IF | Immunofluorescent |
| NETs | Neuroendocrine tumors |
| PARP | Poly(ADP-ribose)-polymerase |
| PARPi | Poly(ADP-ribose)-polymerase inhibitor |
| PBS | Phosphate buffered saline |
| PBST | Phosphate buffered saline containing Triton X-100 |
| PEG300 | Polyethylene glycol 300 |
| P-gp | P-glycoprotein efflux pump |
| P.i. | Post-injection |
| PRRT | Peptide receptor radionuclide therapy |
| SSB | Single strand break |
| SSTR2 | Somatostatin receptor subtype 2 |
| TTP | Time to progression |
| UPLC-MS/MS | Ultra performance liquid chromatography-tandem mass spectrometer |
References
- Kwekkeboom, D.J.; Bakker, W.H.; Kooij, P.P.; Konijnenberg, M.W.; Srinivasan, A.; Erion, J.L.; Schmidt, M.A.; Bugaj, J.L.; de Jong, M. [177Lu-DOTAOTyr3]octreotate: Comparison with [111In-DTPAo]octreotide in patients. Eur. J. Nucl. Med. 2001, 28, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Sundlöv, A.; Sjögreen-Gleisner, K.; Svensson, J.; Ljungberg, M.; Olsson, T.; Bernhardt, P.; Tennvall, J. Individualised (177)Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1480–1489. [Google Scholar] [CrossRef] [PubMed]
- Scheinberg, D.A.; McDevitt, M.R. Actinium-225 in targeted alpha-particle therapeutic applications. Curr. Radiopharm. 2011, 4, 306–320. [Google Scholar] [CrossRef]
- Feijtel, D.; de Jong, M.; Nonnekens, J. Peptide Receptor Radionuclide Therapy: Looking Back, Looking Forward. Curr. Top. Med. Chem. 2020, 20, 2959–2969. [Google Scholar] [CrossRef]
- Nonnekens, J.; van Kranenburg, M.; Beerens, C.E.; Suker, M.; Doukas, M.; van Eijck, C.H.; De Jong, M.; Van Gent, D.C. Potentiation of Peptide Receptor Radionuclide Therapy by the PARP Inhibitor Olaparib. Theranostics 2016, 6, 1821–1832. [Google Scholar] [CrossRef]
- Purohit, N.K.; Shah, R.G.; Adant, S.; Hoepfner, M.; Shah, G.M.; Beauregard, J.M. Potentiation of (177)Lu-octreotate peptide receptor radionuclide therapy of human neuroendocrine tumor cells by PARP inhibitor. Oncotarget 2018, 9, 24693–24706. [Google Scholar] [CrossRef]
- Cullinane, C.; Waldeck, K.; Kirby, L.; Rogers, B.E.; Eu, P.; Tothill, R.W.; Hicks, R.J. Enhancing the anti-tumour activity of (177)Lu-DOTA-octreotate radionuclide therapy in somatostatin receptor-2 expressing tumour models by targeting PARP. Sci. Rep. 2020, 10, 10196. [Google Scholar] [CrossRef]
- Ruigrok, E.A.M.; Verkaik, N.S.; de Blois, E.; de Ridder, C.; Stuurman, D.; Roobol, S.J.; Van Gent, D.C.; de Jong, M.; Van Weerden, W.M.; Nonnekens, J. Preclinical Assessment of the Combination of PSMA-Targeting Radionuclide Therapy with PARP Inhibitors for Prostate Cancer Treatment. Int. J. Mol. Sci. 2022, 23, 8037. [Google Scholar] [CrossRef]
- Prasad, V.; Zengerling, F.; Steinacker, J.P.; Bolenz, C.; Beer, M.; Wiegel, T.; Eiber, M.; Fleshner, N.; Beer, A.J. First Experiences with (177)Lu-PSMA Therapy in Combination with Pembrolizumab or After Pretreatment with Olaparib in Single Patients. J. Nucl. Med. 2021, 62, 975–978. [Google Scholar] [CrossRef]
- Longnecker, D.S.; Lilja, H.S.; French, J.; Kuhlmann, E.; Noll, W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979, 7, 197–202. [Google Scholar] [CrossRef] [PubMed]
- De Blois, E.; Chan, H.S.; de Zanger, R.; Konijnenberg, M.; Breeman, W.A. Application of single-vial ready-for-use formulation of 111In- or 177Lu-labelled somatostatin analogs. Appl. Radiat. Isot. 2014, 85, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Feijtel, D.; Doeswijk, G.N.; Verkaik, N.S.; Haeck, J.C.; Chicco, D.; Angotti, C.; Konijnenberg, M.W.; de Jong, M.; Nonnekens, J. Inter and intra-tumor somatostatin receptor 2 heterogeneity influences peptide receptor radionuclide therapy response. Theranostics 2021, 11, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Bogdanova, N.; Ohlendorf, F.; Ramachandran, D.; Werner, R.A.; Ross, T.L.; Christiansen, H.; Bengel, F.; Henkenberens, C. Assessment of γ-H2AX and 53BP1 Foci in Peripheral Blood Lymphocytes to Predict Subclinical Hematotoxicity and Response in Somatostatin Receptor-Targeted Radionuclide Therapy for Advanced Gastroenteropancreatic Neuroendocrine Tumors. Cancers 2021, 13, 1516. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.; Modlin, I.M.; Severi, S.; Drozdov, I.; Nicolini, S.; Kwekkeboom, D.J.; Krenning, E.P.; Baum, R.P.; Paganelli, G. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Ezziddin, S.; Opitz, M.; Attassi, M.; Biermann, K.; Sabet, A.; Guhlke, S.; Brockmann, H.; Willinek, W.; Wardelmann, E.; Biersack, H.-J.; et al. Impact of the Ki-67 proliferation index on response to peptide receptor radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 459–466. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, X.A.; Zhang, N.; Wang, J. Evolving insights: How DNA repair pathways impact cancer evolution. Cancer Biol. Med. 2020, 17, 805–827. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Gioia, U.; Francia, S.; Cabrini, M.; Brambillasca, S.; Michelini, F.; Jones-Weinert, C.W.; di Fagagna, F.D. Pharmacological boost of DNA damage response and repair by enhanced biogenesis of DNA damage response RNAs. Sci. Rep. 2019, 9, 6460. [Google Scholar] [CrossRef]
- Moore, N.; Houghton, J.; Lyle, S. Slow-cycling therapy-resistant cancer cells. Stem Cells Dev. 2012, 21, 1822–1830. [Google Scholar] [CrossRef] [PubMed]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; Freshney, R.I.; Murray, A.M.; Merry, S.; Plumb, J.A.; McNicol, A.M. Identification and characterisation in vitro of cells with a non-SCLC cell-like phenotype derived from a continuous SCLC cell line. Anticancer Res. 1991, 11, 1687–1695. [Google Scholar] [PubMed]
- Verwijnen, S.; Capello, A.; Bernard, B.; van den Aardweg, G.; Konijnenberg, M.; Breeman, W.; Krenning, E.; de Jong, M. Low-dose-rate irradiation by 131I versus high-dose-rate external-beam irradiation in the rat pancreatic tumor cell line CA20948. Cancer Biother. Radiopharm. 2004, 19, 285–292. [Google Scholar] [CrossRef]
- Krohn, A.; Ahrens, T.; Yalcin, A.; Plönes, T.; Wehrle, J.; Taromi, S.; Wollner, S.; Follo, M.; Brabletz, T.; Mani, S.A.; et al. Tumor cell heterogeneity in Small Cell Lung Cancer (SCLC): Phenotypical and functional differences associated with Epithelial-Mesenchymal Transition (EMT) and DNA methylation changes. PLoS ONE 2014, 9, e100249. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, A.; Sawers, L.; Gannon, A.L.; Chakravarty, P.; Scott, A.L.; Bray, S.E.; Ferguson, M.J.; Smith, G. ABCB1 (MDR1) induction defines a common resistance mechanism in paclitaxel- and olaparib-resistant ovarian cancer cells. Br. J. Cancer 2016, 115, 431–441. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; van der Burg, E.; Nygren, A.O.; Zander, S.A.; Derksen, P.W.B.; De Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; Sol, W.; van Deemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; et al. Loss of 53BP1 Causes PARP Inhibitor Resistance in Brca1-Mutated Mouse Mammary Tumors. Cancer Discov. 2013, 3, 68–81. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Shi, Y.; Rodriguez, L.E.; Solomon, L.R.; Donawho, C.K.; DiGiammarino, E.L.; Panchal, S.C.; Wilsbacher, J.L.; Gao, W.; Olson, A.M.; et al. Mechanistic Dissection of PARP1 Trapping and the Impact on In vivo Tolerability and Efficacy of PARP Inhibitors. Mol. Cancer Res. 2015, 13, 1465–1477. [Google Scholar] [CrossRef]
- Juhász, S.; Smith, R.; Schauer, T.; Spekhardt, D.; Mamar, H.; Zentout, S.; Chapuis, C.; Huet, S.; Timinszky, G. The chromatin remodeler ALC1 underlies resistance to PARP inhibitor treatment. Sci. Adv. 2020, 6, eabb8626. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijtel, D.; Reuvers, T.G.A.; van Tuyll-van Serooskerken, C.; de Ridder, C.M.A.; Stuurman, D.C.; de Blois, E.; Verkaik, N.S.; de Bruijn, P.; Koolen, S.L.W.; de Jong, M.; et al. In Vivo Efficacy Testing of Peptide Receptor Radionuclide Therapy Radiosensitization Using Olaparib. Cancers 2023, 15, 915. https://doi.org/10.3390/cancers15030915
Feijtel D, Reuvers TGA, van Tuyll-van Serooskerken C, de Ridder CMA, Stuurman DC, de Blois E, Verkaik NS, de Bruijn P, Koolen SLW, de Jong M, et al. In Vivo Efficacy Testing of Peptide Receptor Radionuclide Therapy Radiosensitization Using Olaparib. Cancers. 2023; 15(3):915. https://doi.org/10.3390/cancers15030915
Chicago/Turabian StyleFeijtel, Danny, Thom G. A. Reuvers, Christine van Tuyll-van Serooskerken, Corrina M. A. de Ridder, Debra C. Stuurman, Erik de Blois, Nicole S. Verkaik, Peter de Bruijn, Stijn L. W. Koolen, Marion de Jong, and et al. 2023. "In Vivo Efficacy Testing of Peptide Receptor Radionuclide Therapy Radiosensitization Using Olaparib" Cancers 15, no. 3: 915. https://doi.org/10.3390/cancers15030915
APA StyleFeijtel, D., Reuvers, T. G. A., van Tuyll-van Serooskerken, C., de Ridder, C. M. A., Stuurman, D. C., de Blois, E., Verkaik, N. S., de Bruijn, P., Koolen, S. L. W., de Jong, M., & Nonnekens, J. (2023). In Vivo Efficacy Testing of Peptide Receptor Radionuclide Therapy Radiosensitization Using Olaparib. Cancers, 15(3), 915. https://doi.org/10.3390/cancers15030915




