Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Tumor Treating Fields Overview
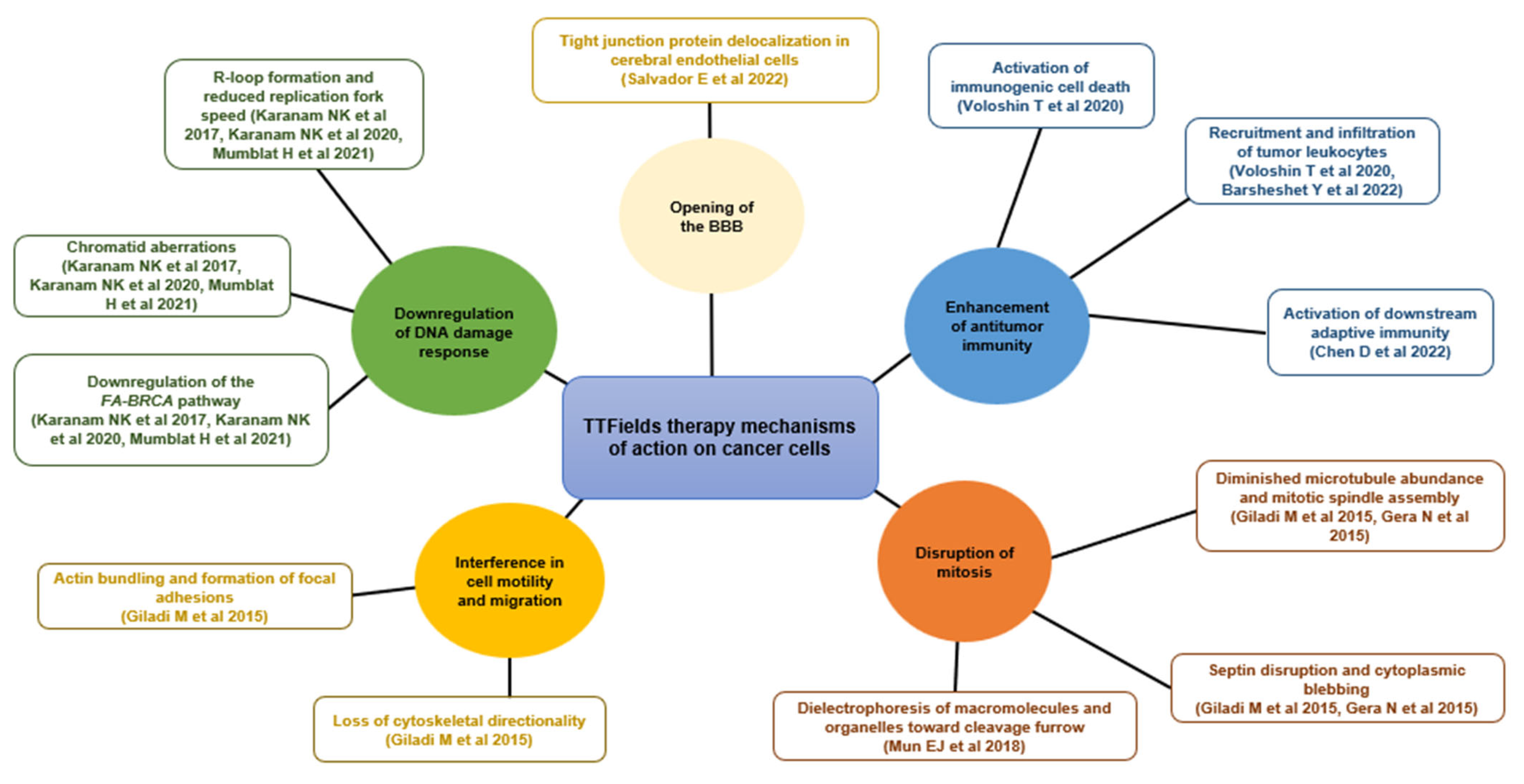
3. The TTFields Mechanism of Action
4. TTFields Therapy Concomitant with Systemic and Localized Anticancer Treatments
4.1. TTFields Therapy Concomitant with Radiation
4.2. TTFields Therapy Concomitant with Immunotherapy
4.3. TTFields Therapy Concomitant with Targeted Therapy
4.4. TTFields Therapy Concomitant with Chemotherapy
4.4.1. TTFields Therapy Concomitant with Chemotherapy: Overview
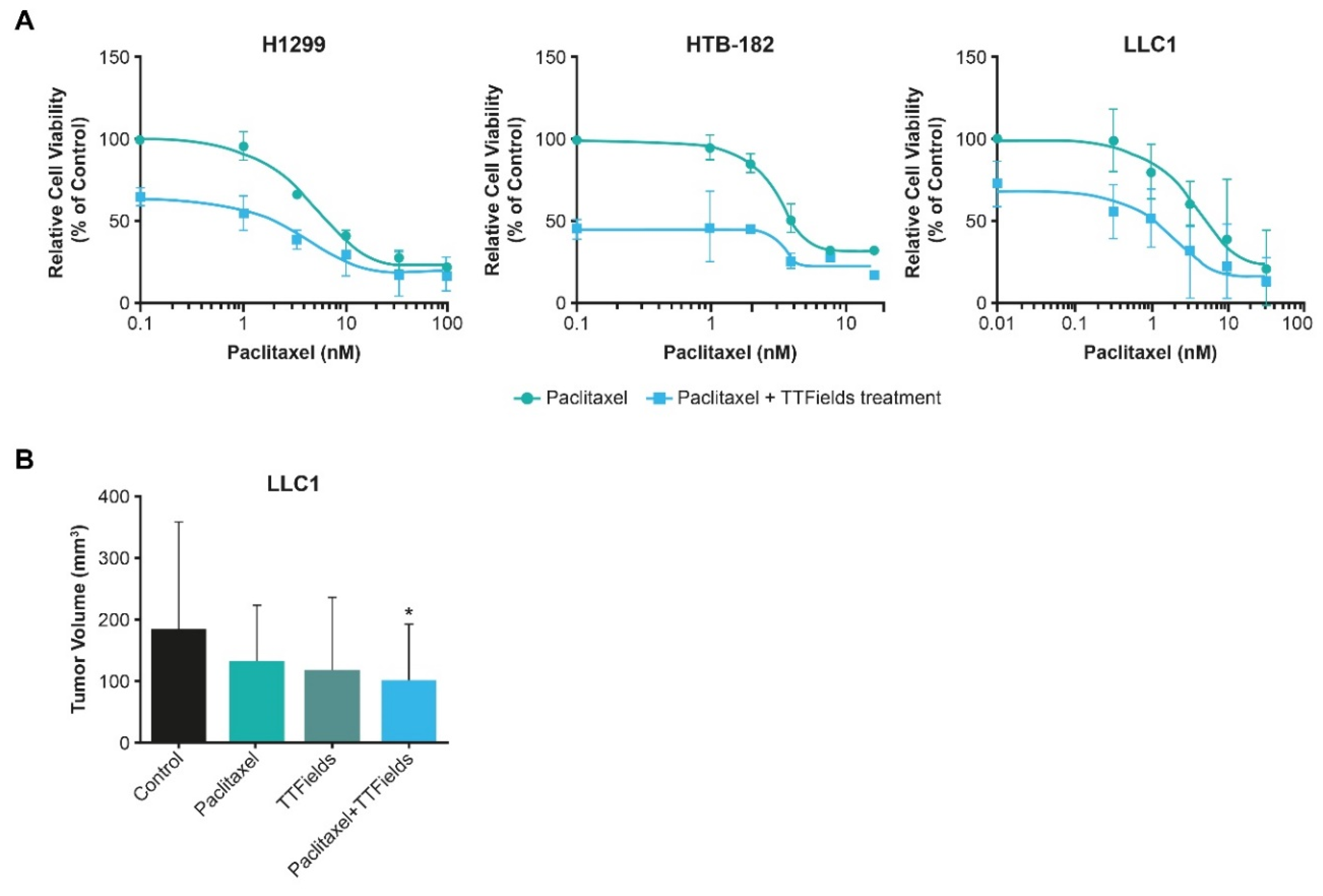
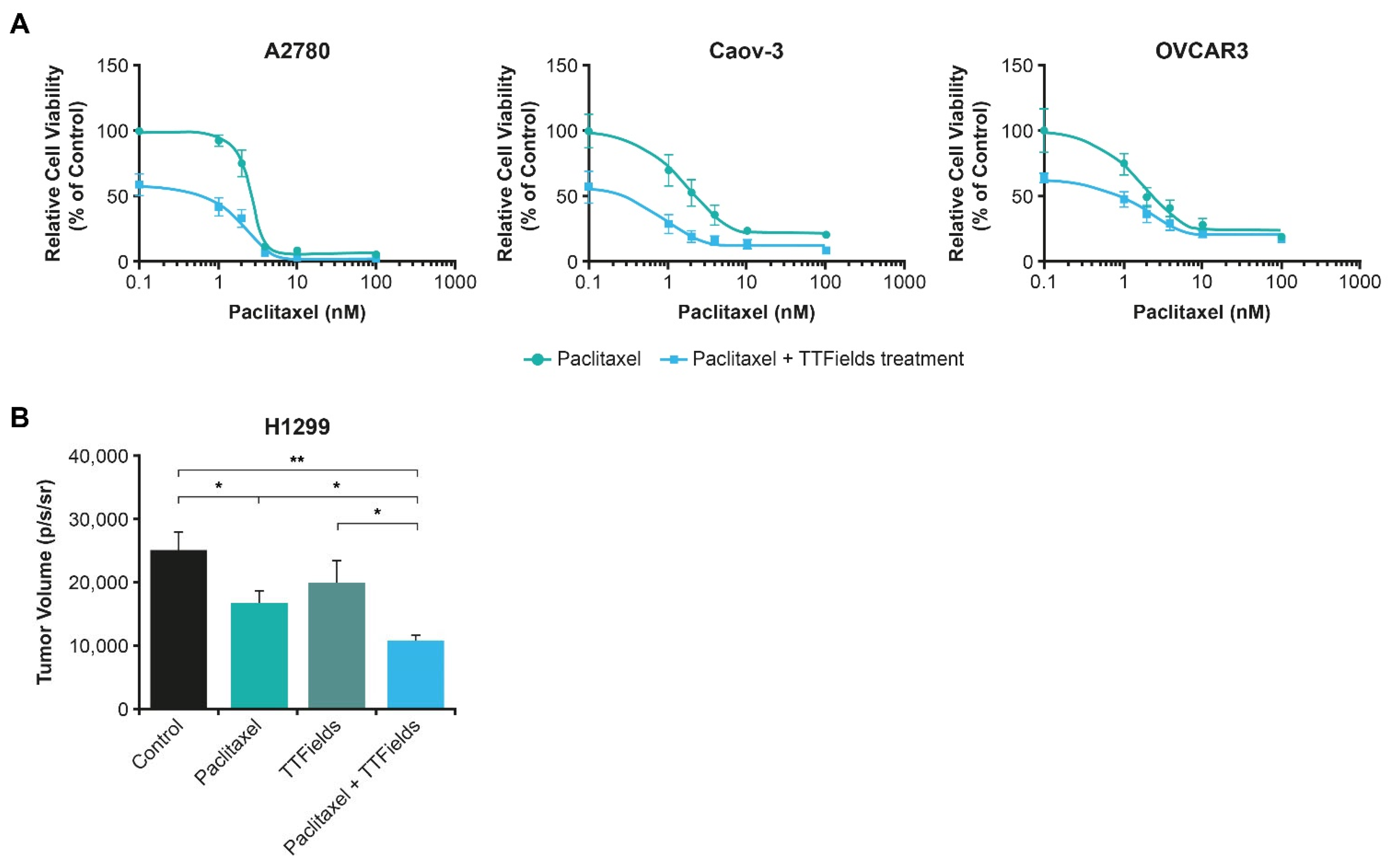
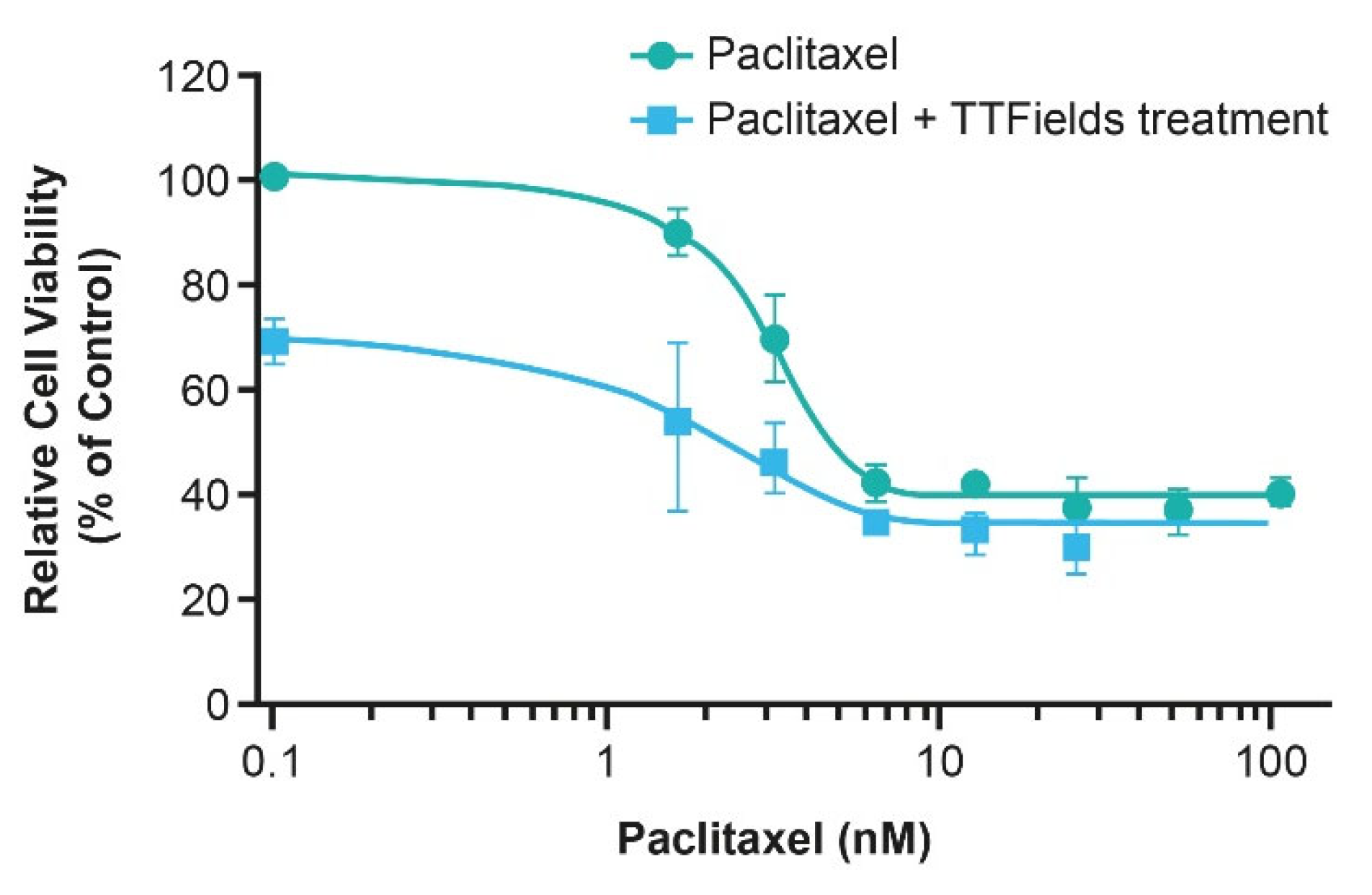
4.4.2. TTFields Therapy Concomitant with Taxanes
5. TTFields Therapy Concomitant with Taxanes in NSCLC, Ovarian Cancer, and Pancreatic Cancer
5.1. TTFields Therapy Concomitant with Taxanes: NSCLC
5.2. TTFields Therapy Concomitant with Taxanes: Ovarian Cancer
5.3. TTFields Therapy Concomitant with Taxanes: Pancreatic Cancer
6. TTFields Therapy Concomitant with Taxanes: Other Cancers
7. TTFields Therapy Concomitant with Taxanes: Summary of Clinical Efficacy and Safety
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 16 August 2022).
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Malhotra, J.; Jabbour, S.K.; Aisner, J. Current state of immunotherapy for non-small cell lung cancer. Transl. Lung. Cancer Res. 2017, 6, 196–211. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.; Zhu, L.; Feng, J.; Huang, X.; Baak, J.P.A. “How Long Have I Got?” in Stage IV NSCLC Patients With at Least 3 Months Up to 10 Years Survival, Accuracy of Long-, Intermediate-, and Short-Term Survival Prediction Is Not Good Enough to Answer This Question. Front. Oncol. 2021, 11, 761042. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.E.; Singh, N.; Ismaila, N.; Antonoff, M.B.; Arenberg, D.A.; Bradley, J.; David, E.; Detterbeck, F.; Früh, M.; Gubens, M.A.; et al. Management of Stage III Non–Small-Cell Lung Cancer: ASCO Guideline. J. Clin. Oncol. 2022, 40, 1356–1384. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv192–iv237, Updated 2020 Version. Available online: https://www.esmo.org/content/download/347819/6934778/6934771/ESMO-CPG-mNSCLC-6934715SEPT6932020.pdf (accessed on 2 August 2022). [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- National Cancer Institute. For Early-Stage Lung Cancer, Nivolumab and Chemo before Surgery Proves Effective. Available online: https://www.cancer.gov/news-events/cancer-currents-blog/2022/nivolumab-chemotherapy-neoadjuvant-lung-cancer (accessed on 16 November 2022).
- Pils, D.; Hager, G.; Tong, D.; Aust, S.; Heinze, G.; Kohl, M.; Schuster, E.; Wolf, A.; Sehouli, J.; Braicu, I.; et al. Validating the impact of a molecular subtype in ovarian cancer on outcomes: A study of the OVCAD Consortium. Cancer Sci. 2012, 103, 1334–1341. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Ovarian Cancer Research Alliance. Stages of Ovarian Cancer. Available online: https://ocrahope.org/patients/about-ovarian-cancer/staging/ (accessed on 16 August 2022).
- European Society for Medical Oncology. ESMO Ovarian Cancer Guidelines. Available online: https://www.esmo.org/content/download/10097/201883/1/EN-Ovarian-Cancer-Guide-for-Patients.pdf (accessed on 16 August 2022).
- Vergote, I.; Gonzalez-Martin, A.; Lorusso, D.; Gourley, C.; Mirza, M.R.; Kurtz, J.-E.; Okamoto, A.; Moore, K.; Kridelka, F.; McNeish, I.; et al. Clinical research in ovarian cancer: Consensus recommendations from the Gynecologic Cancer InterGroup. Lancet Oncol. 2022, 23, e374–e384. [Google Scholar] [CrossRef] [PubMed]
- European Society for Medical Oncology. eUpdate–Cancer of the Pancreas Treatment Recommendations. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/gastrointestinal-cancers/pancreatic-cancer/eupdate-cancer-of-the-pancreas-treatment-recommendations (accessed on 6 September 2022).
- Sohal, D.P.S.; Kennedy, E.B.; Cinar, P.; Conroy, T.; Copur, M.S.; Crane, C.H.; Garrido-Laguna, I.; Lau, M.W.; Johnson, T.; Krishnamurthi, S.; et al. Metastatic Pancreatic Cancer: ASCO Guideline Update. J. Clin. Oncol. 2020, 38, 3217–3230. [Google Scholar] [CrossRef]
- Adel, N. Current treatment landscape and emerging therapies for pancreatic cancer. Am. J. Manag. Care 2019, 25 (Suppl. 1), S3–S10. [Google Scholar] [PubMed]
- Sheikh, R.; Walsh, N.; Clynes, M.; O’Connor, R.; McDermott, R. Challenges of drug resistance in the management of pancreatic cancer. Expert Rev. Anticancer Ther. 2010, 10, 1647–1661. [Google Scholar] [CrossRef]
- Long, J.; Zhang, Y.; Yu, X.; Yang, J.; LeBrun, D.G.; Chen, C.; Yao, Q.; Li, M. Overcoming drug resistance in pancreatic cancer. Expert Opin. Ther. Targets 2011, 15, 817–828. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Mun, E.J.; Babiker, H.M.; Weinberg, U.; Kirson, E.D.; Von Hoff, D.D. Tumor-Treating Fields: A Fourth Modality in Cancer Treatment. Clin. Cancer Res. 2018, 24, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Schneiderman, R.S.; Volodin, A.; Shamir, R.R.; Kaynan, N.; Zeevi, E.; Koren, L.; Klein-Goldberg, A.; Paz, R.; Giladi, M.; et al. Tumor Treating Fields (TTFields) hinder cancer cell motility through regulation of microtubule and actin dynamics. Cancers 2020, 12, 3016. [Google Scholar] [CrossRef] [PubMed]
- Karanam, N.K.; Story, M.D. An overview of potential novel mechanisms of action underlying Tumor Treating Fields-induced cancer cell death and their clinical implications. Int. J. Radiat. Biol. 2021, 97, 1044–1054. [Google Scholar] [CrossRef]
- Wenger, C.; Giladi, M.; Bomzon, Z.; Salvador, R.; Basser, P.J.; Miranda, P.C. Modeling Tumor Treating Fields (TTFields) application in single cells during metaphase and telophase. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2015, 2015, 6892–6895. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbalý, V.; Tovaryš, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef] [PubMed]
- Voloshin, T.; Munster, M.; Blatt, R.; Shteingauz, A.; Roberts, P.C.; Schmelz, E.M.; Giladi, M.; Schneiderman, R.S.; Zeevi, E.; Porat, Y.; et al. Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int. J. Cancer 2016, 139, 2850–2858. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Itzhaki, A.; Mordechovich, D.; Cahal, S.; Kirson, E.D.; Weinberg, U.; Palti, Y. Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 2014, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Weinberg, U.; Schneiderman, R.S.; Porat, Y.; Munster, M.; Voloshin, T.; Blatt, R.; Cahal, S.; Itzhaki, A.; Onn, A.; et al. Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 2014, 41 (Suppl. S6), S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Trainito, C.I.; Sweeney, D.C.; Cemazar, J.; Schmelz, E.M.; Francais, O.; Le Pioufle, B.; Davalos, R.V. Characterization of sequentially-staged cancer cells using electrorotation. PLoS ONE 2019, 14, e0222289. [Google Scholar] [CrossRef] [PubMed]
- Oberheim-Bush, N.A.; Shi, W.; McDermott, M.W.; Grote, A.; Stindl, J.; Lustgarten, L. The safety profile of Tumor Treating Fields (TTFields) therapy in glioblastoma patients with ventriculoperitoneal shunts. J. Neurooncol. 2022, 158, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Natour, Z.A.; Mustafa, F.; Rizvi, T.A. Electrical characterization of normal and cancer cells. IEEE Access 2018, 6, 25979–25986. [Google Scholar] [CrossRef]
- Novocure. Optune®: Instructions for Use. Available online: https://www.optune.com/Content/pdfs/Optune_IFU_8.5x11.pdf (accessed on 9 November 2022).
- Novocure. NovoTTF™-100L System: Instructions for Use for Unresectable Pleural Malignant Mesothelioma. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf18/H180002D.pdf (accessed on 20 June 2022).
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef] [PubMed]
- Taphoorn, M.J.B.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of treatment with Tumor-Treating Fields on health-related quality of life of patients with newly diagnosed glioblastoma: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Kinzel, A.; Ambrogi, M.; Varshaver, M.; Kirson, E.D. Tumor Treating Fields for Glioblastoma Treatment: Patient Satisfaction and Compliance With the Second-Generation Optune® System. Clin. Med. Insights Oncol. 2019, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbalý, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Blumenthal, D.T.; Oberheim Bush, N.A.; Kebir, S.; Lukas, R.V.; Muragaki, Y.; Zhu, J.J.; Glas, M. Global post-marketing safety surveillance of Tumor Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J. Neurooncol. 2020, 148, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: A randomized clinical trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Ceresoli, G.L.; Aerts, J.; Madrzak, J.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; Hiddinga, B.; VanMeerbeeck, J.; Mencoboni, M.; Planchard, D.; et al. Final results of Phase II STELLAR trial: TTFields with chemotherapy in unresectable malignant pleural mesothelioma. Cancer Res. 2019, 79, CT201. [Google Scholar] [CrossRef]
- Grosso, F.; Ceresoli, G.L. Radiological response patterns in the phase 2 STELLAR trial of TTFields with chemotherapy for first-line treatment of malignant pleural mesothelioma (MPM). J. Clin. Oncol. 2019, 37, 8551. [Google Scholar] [CrossRef]
- Rivera, F.; Benavides, M.; Gallego, J.; Guillen-Ponce, C.; Lopez-Martin, J.; Küng, M. Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: Results of the PANOVA phase 2 study. Pancreatology 2019, 19, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Benavides, M.; Guillen, C.; Rivera, F.; Gallego, J.; Lopez-Martin, J.A.; Küng, M. PANOVA: A phase II study of TTFields (150 kHz) concomitant with standard chemotherapy for front-line therapy of advanced pancreatic adenocarcinoma—Updated efficacy results. J. Clin. Oncol. 2017, 35 (Suppl. 15), e15790. [Google Scholar] [CrossRef]
- Pless, M.; Droege, C.; von Moos, R.; Salzberg, M.; Betticher, D. A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 2013, 81, 445–450. [Google Scholar] [CrossRef]
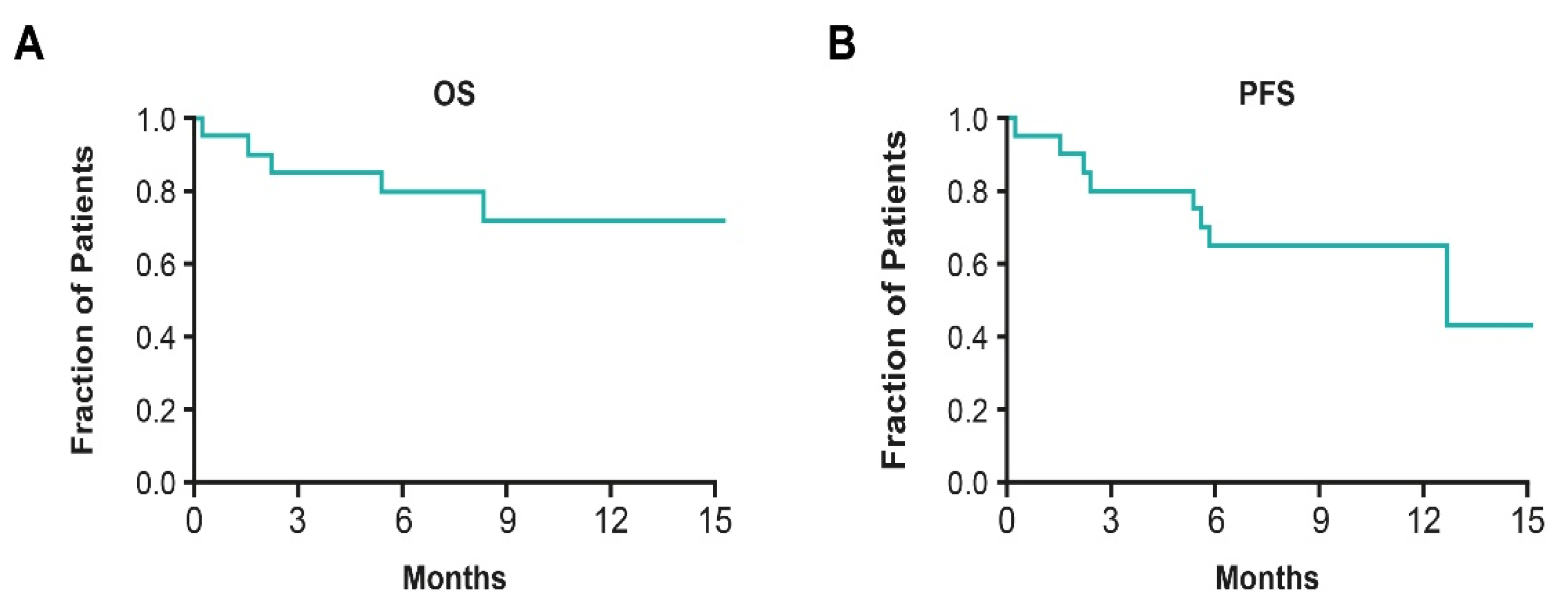
- Vergote, I.; von Moos, R.; Manso, L.; Van Nieuwenhuysen, E.; Concin, N.; Sessa, C. Tumor Treating Fields in combination with paclitaxel in recurrent ovarian carcinoma: Results of the INNOVATE pilot study. Gynecol. Oncol. 2018, 150, 471–477. [Google Scholar] [CrossRef]
- Giladi, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Munster, M.; Blat, R.; Sherbo, S.; Bomzon, Z.; Urman, N.; Itzhaki, A.; et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 2015, 5, 18046. [Google Scholar] [CrossRef] [PubMed]
- Gera, N.; Yang, A.; Holtzman, T.S.; Lee, S.X.; Wong, E.T.; Swanson, K.D. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS ONE 2015, 10, e0125269. [Google Scholar] [CrossRef]
- Li, X.; Yang, F.; Rubinsky, B. A Theoretical Study on the Biophysical Mechanisms by Which Tumor Treating Fields Affect Tumor Cells During Mitosis. IEEE Trans. Bio-Med. Eng. 2020, 67, 2594–2602. [Google Scholar] [CrossRef]
- Salvador, E.; Kessler, A.F.; Domröse, D.; Hörmann, J.; Schaeffer, C.; Giniunaite, A.; Burek, M.; Tempel-Brami, C.; Voloshin, T.; Volodin, A.; et al. Tumor Treating Fields (TTFields) Reversibly Permeabilize the Blood–Brain Barrier In Vitro and In Vivo. Biomolecules 2022, 12, 1348. [Google Scholar] [CrossRef]
- Voloshin, T.; Kaynan, N.; Davidi, S.; Porat, Y.; Shteingauz, A.; Schneiderman, R.S.; Zeevi, E.; Munster, M.; Blat, R.; Tempel Brami, C.; et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol. Immunother. 2020, 69, 1191–1204. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Le, S.B.; Hutchinson, T.E.; Calinescu, A.A.; Sebastian, M.; Jin, D.; Liu, T.; Ghiaseddin, A.; Rahman, M.; Tran, D.D. Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Invest. 2022, 132, e149258. [Google Scholar] [CrossRef] [PubMed]
- Gutin, P.H.; Wong, E.T. Noninvasive Application of Alternating Electric Fields in Glioblastoma: A Fourth Cancer Treatment Modality. Am. Soc. Clin. Oncol. Educ. Book 2012, 32, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Karanam, N.K.; Ding, L.; Aroumougame, A.; Story, M.D. Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: Implications for cancer therapy. Transl. Res. 2020, 217, 33–46. [Google Scholar] [CrossRef]
- Karanam, N.K.; Srinivasan, K.; Ding, L.; Sishc, B.; Saha, D.; Story, M.D. Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 2017, 8, e2711. [Google Scholar] [CrossRef] [PubMed]
- Mumblat, H.; Martinez-Conde, A.; Braten, O.; Munster, M.; Dor-On, E.; Schneiderman, R.S.; Porat, Y.; Voloshin, T.; Davidi, S.; Blatt, R.; et al. Tumor Treating Fields (TTFields) downregulate the Fanconi Anemia-BRCA pathway and increase the efficacy of chemotherapy in malignant pleural mesothelioma preclinical models. Lung Cancer 2021, 160, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Barsheshet, Y.; Voloshin, T.; Brant, B.; Cohen, G.; Koren, L.; Blatt, R.; Cahal, S.; Haj Khalil, T.; Zemer Tov, E.; Paz, R.; et al. Tumor Treating Fields (TTFields) Concomitant with Immune Checkpoint Inhibitors Are Therapeutically Effective in Non-Small Cell Lung Cancer (NSCLC) In Vivo Model. Int. J. Mol. Sci. 2022, 23, 14073. [Google Scholar] [CrossRef]
- Shteingauz, A.; Porat, Y.; Voloshin, T.; Schneiderman, R.S.; Munster, M.; Zeevi, E.; Kaynan, N.; Gotlib, K.; Giladi, M.; Kirson, E.D.; et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Silginer, M.; Weller, M.; Stupp, R.; Roth, P. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017, 8, e2753. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Jo, Y.; Sai, S.; Park, M.-J.; Kim, J.-Y.; Kim, J.S.; Lee, Y.-J.; Cho, J.-M.; Kwak, S.-Y.; Baek, J.-H.; et al. Tumor-treating fields induce autophagy by blocking the Akt2/miR29b axis in glioblastoma cells. Oncogene 2019, 38, 6630–6646. [Google Scholar] [CrossRef] [PubMed]
- Davidi, S.; Jacobovitch, S.; Shteingauz, A.; Martinez-Conde, A.; Braten, O.; Tempel-Brami, C.; Zeevi, E.; Frechtel-Gerzi, R.; Ene, H.; Dor-On, E.; et al. Tumor Treating Fields (TTFields) Concomitant with Sorafenib Inhibit Hepatocellular Carcinoma In Vitro and In Vivo. Cancers 2022, 14, 2959. [Google Scholar] [CrossRef] [PubMed]
- Diamant, G.; Simchony Goldman, H.; Gasri Plotnitsky, L.; Roitman, M.; Shiloach, T.; Globerson-Levin, A.; Eshhar, Z.; Haim, O.; Pencovich, N.; Grossman, R.; et al. T Cells Retain Pivotal Antitumoral Functions under Tumor-Treating Electric Fields. J. Immunol. 2021, 207, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.C.; Salvador, E.; Deniz, K.; Swanson, K.; Tusynski, J.; Carlson, K.W.; Karanam, N.K.; Patel, C.B.; Story, M.; Lou, E.; et al. The mechanisms of action of Tumor Treating Fields. Cancer Res. 2022, 82, 3650–3658. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.-M.; Arami, H.; Natarajan, A.; et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Oh, G.; Gi, Y.; Sung, H.; Joo, E.B.; Lee, S.; Yoon, M. Tumor treating fields (TTF) treatment enhances radiation-induced apoptosis in pancreatic cancer cells. Int. J. Radiat. Biol. 2020, 96, 1528–1533. [Google Scholar] [CrossRef]
- Kim, E.H.; Kim, Y.H.; Song, H.S.; Jeong, Y.K.; Lee, J.Y.; Sung, J.; Yoo, S.H.; Yoon, M. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget 2016, 7, 62267–62279. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Munster, M.; Schneiderman, R.S.; Voloshin, T.; Porat, Y.; Blat, R.; Zielinska-Chomej, K.; Haag, P.; Bomzon, Z.; Kirson, E.D.; et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 2017, 12, 206. [Google Scholar] [CrossRef]
- Karanam, N.K.; Shang, Z.; Story, M.D.; Saha, D. Abstract 3316: Tumor Treating Fields in combination with radiation cause significant delay in tumor growth in in-vivo mice modelsignificant delay in tumor growth in in-vivo mice model. Cancer Res. 2022, 82, 3316. [Google Scholar] [CrossRef]
- Kim, J.S.; Cho, J.M.; Kim, H.; Jeong, Y.K.; Kim, J.K.; Kim, E.H. Tumor treating fields can effectively overcome trastuzumab resistant breast cancer multiplication. Am. J. Cancer Res. 2021, 11, 3935–3945. [Google Scholar] [PubMed]
- Jo, Y.; Kim, E.H.; Sai, S.; Kim, J.S.; Cho, J.M.; Kim, H.; Baek, J.H.; Kim, J.Y.; Hwang, S.G.; Yoon, M. Functional Biological Activity of Sorafenib as a Tumor-Treating Field Sensitizer for Glioblastoma Therapy. Int. J. Mol. Sci. 2018, 19, 3684. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jo, Y.; Oh, H.K.; Kim, E.H. Sorafenib increases tumor treating fields-induced cell death in glioblastoma by inhibiting STAT3. Am. J. Cancer Res. 2020, 10, 3475–3486. [Google Scholar] [PubMed]
- Ceresoli, G.L.; Aerts, J.G.; Dziadziuszko, R.; Ramlau, R.; Cedres, S.; van Meerbeeck, J.P.; Mencoboni, M.; Planchard, D.; Chella, A.; Crino, L.; et al. Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): A multicentre, single-arm phase 2 trial. Lancet Oncol. 2019, 20, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Rao, M.; Zhu, P.; Liang, B.; El-Nazer, R.T.; Fonkem, E.; Bhattacharjee, M.B.; Zhu, J.J. Triple-drug Therapy With Bevacizumab, Irinotecan, and Temozolomide Plus Tumor Treating Fields for Recurrent Glioblastoma: A Retrospective Study. Front. Neurol. 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Song, A.; Ali, A.; Niazi, M.; Bar-Ad, V.; Martinez, N.; Glass, J.; Alnahhas, I.; Andrews, D.; Judy, K.; et al. Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma. Front. Oncol. 2022, 12, 896246. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.R.; Slone, S.A.; Morgan, R.M.; Gruber, L.; Kumar, S.S.; Lightner, D.D.; Villano, J.L. Dose-dense temozolomide for recurrent high-grade gliomas: A single-center retrospective study. Med. Oncol. 2018, 35, 136. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, L.; Schäfer, N.; Schmidt, T.; Stoppek, A.; Weller, J.; Tzaridis, T.; Scheffler, B.; Pierscianek, D.; Kleinschnitz, C.; Stuschke, M.; et al. P14.61 Tumor Treating Fields (TTFields) combined with lomustine (CCNU) and temozolomide (TMZ) in newly diagnosed glioblastoma (GBM) patients–A bi-centric analysis. Neuro Oncol. 2019, 21, iii81. [Google Scholar] [CrossRef]
- Lei, L.; Wang, X.J.; Tang, S.C. Novel taxanes in development: Hopes or hypes? Crit. Rev. Oncol. Hematol. 2022, 176, 103727. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: EMT as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef]
- Pronk, L.C.; Stoter, G.; Verweij, J. Docetaxel (Taxotere): Single agent activity, development of combination treatment and reducing side-effects. Cancer Treat. Rev. 1995, 21, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Figgitt, D.P.; Wiseman, L.R. Docetaxel: An update of its use in advanced breast cancer. Drugs 2000, 59, 621–651. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Rivera, E.; Cianfrocca, M. Overview of neuropathy associated with taxanes for the treatment of metastatic breast cancer. Cancer Chemother. Pharmacol. 2015, 75, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, T.Y.; Salama, J.K.; Vokes, E.E. The concurrent chemoradiation paradigm--general principles. Nat. Clin. Pract. Oncol. 2007, 4, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Khing, T.M.; Choi, W.S.; Kim, D.M.; Po, W.W.; Thein, W.; Shin, C.Y.; Sohn, U.D. The effect of paclitaxel on apoptosis, autophagy and mitotic catastrophe in AGS cells. Sci. Rep. 2021, 11, 23490. [Google Scholar] [CrossRef]
- Sunters, A.; Madureira, P.A.; Pomeranz, K.M.; Aubert, M.; Brosens, J.J.; Cook, S.J.; Burgering, B.M.; Coombes, R.C.; Lam, E.W. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006, 66, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, F.; Amadori, D.; Carloni, S.; Brigliadori, G.; Tesei, A.; Ulivi, P.; Rosetti, M.; Vannini, I.; Arienti, C.; Zoli, W.; et al. Mitotic catastrophe and apoptosis induced by docetaxel in hormone-refractory prostate cancer cells. J. Cell Physiol. 2008, 217, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mang, J.; Merkle, K.; Heller, M.; Schüler, J.; Tolstov, Y.; Li, J.; Hohenfellner, M.; Duensing, S. Molecular complexity of taxane-induced cytotoxicity in prostate cancer cells. Urol. Oncol. 2017, 35, 32.e9–32.e16. [Google Scholar] [CrossRef] [PubMed]
- Carlson, K.W.; Tuszynski, J.A.; Dokos, S.; Paudel, N.; Dreeben, T.; Bomzon, Z. How Do Tumor-Treating Fields Work? In Brain and Human Body Modeling 2020: Computational Human Models Presented at EMBC 2019 and the BRAIN Initiative® 2019 Meeting; Makarov, S.N., Noetscher, G.M., Nummenmaa, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 19–35. [Google Scholar]
- Kirson, E.D.; Schneiderman, R.S.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Massafra, M.; Passalacqua, M.I.; Gebbia, V.; Macrì, P.; Lazzari, C.; Gregorc, V.; Buda, C.; Altavilla, G.; Santarpia, M. Immunotherapeutic Advances for NSCLC. Biologics 2021, 15, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. The Immune Revolution: A Case for Priming, Not Checkpoint. Cancer Cell 2018, 33, 563–569. [Google Scholar] [CrossRef] [PubMed]
- European Society for Medical Oncology. Metastatic Non-Small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours/clinical-practice-living-guidelines-metastatic-non-small-cell-lung-cancer (accessed on 1 August 2022).
- Weinberg, U.; Farber, O.; Giladi, M.; Bozman, Z.; Kirson, E. 187TiP–Tumor Treating Fields concurrent with standard of care therapy for stage 4 NSCLC following platinum failure: Phase 3 LUNAR study. Ann. Oncol. 2019, 30, ii38–ii68. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Effect of Tumor Treating Fields (TTFields) (150 kHz) Concurrent With Standard of Care Therapies for Treatment of Stage 4 Non-small Cell Lung Cancer (NSCLC) Following Platinum Failure (LUNAR). Available online: https://clinicaltrials.gov/ct2/show/NCT02973789 (accessed on 1 August 2022).
- Hanker, L.C.; Loibl, S.; Burchardi, N.; Pfisterer, J.; Meier, W.; Pujade-Lauraine, E.; Ray-Coquard, I.; Sehouli, J.; Harter, P.; du Bois, A. The impact of second to sixth line therapy on survival of relapsed ovarian cancer after primary taxane/platinum-based therapy. Ann. Oncol. 2012, 23, 2605–2612. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I. Abstract CT174: Phase III INNOVATE study of tumor treating fields (200 kHz) concomitant with weekly paclitaxel for platinum-resistant ovarian cancer (ENGOT-ov50/BGOG study groups). Cancer Res. 2019, 79 (Suppl. S13), CT174. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Effect of Tumor Treating Fields (TTFields, 200 kHz) Concomitant With Weekly Paclitaxel for the Treatment of Recurrent Ovarian Cancer (ENGOT-ov50/GOG-3029/INNOVATE-3). Available online: https://clinicaltrials.gov/ct2/show/NCT03940196 (accessed on 1 August 2022).
- Mohammad, A.A. Advanced pancreatic cancer: The standard of care and new opportunities. Oncol. Rev. 2018, 12, 370. [Google Scholar] [CrossRef] [PubMed]
- Servetto, A.; Santaniello, A.; Napolitano, F.; Foschini, F.; Marciano, R.; Mozzillo, E.; Cascetta, P.; Amato, A.R.; Augurio, M.R.; Maresca, L.; et al. Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study. Cancers 2021, 13, 4939. [Google Scholar] [CrossRef]
- Philip, P.A.; Lacy, J.; Portales, F.; Sobrero, A.; Pazo-Cid, R.; Manzano Mozo, J.L.; Kim, E.J.; Dowden, S.; Zakari, A.; Borg, C.; et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol. Hepatol. 2020, 5, 285–294. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Effect of Tumor Treating Fields (TTFields, 150 kHz) as Front-Line Treatment of Locally-advanced Pancreatic Adenocarcinoma Concomitant With Gemcitabine and Nab-paclitaxel (PANOVA-3). Available online: https://clinicaltrials.gov/ct2/show/NCT03377491 (accessed on 1 August 2022).
- Picozzi, V.J.; Macarulla, T.; Philip, P.A.; Becerra, C.R.; Dragovich, T. PANOVA-3: A phase 3 study of Tumor Treating Fields (TTFields) with gemcitabine and nab-paclitaxel (GnP) for front-line treatment of locally advanced pancreatic adenocarcinoma. J. Clin. Oncol. 2022, 40 (Suppl. S4), TPS629. [Google Scholar] [CrossRef]
- Michelhaugh, S.; Mittal, S. EXTH-31. Combination of Tumor Treating Fields (TTFields) and paclitaxel produces additive reductions in proliferation and clonogenicity in patient-derived metastatic non-small cell lung cancer (NSCLC) cells. Neuro-Oncol. 2019, 21 (Suppl. 6), vi88. [Google Scholar] [CrossRef]
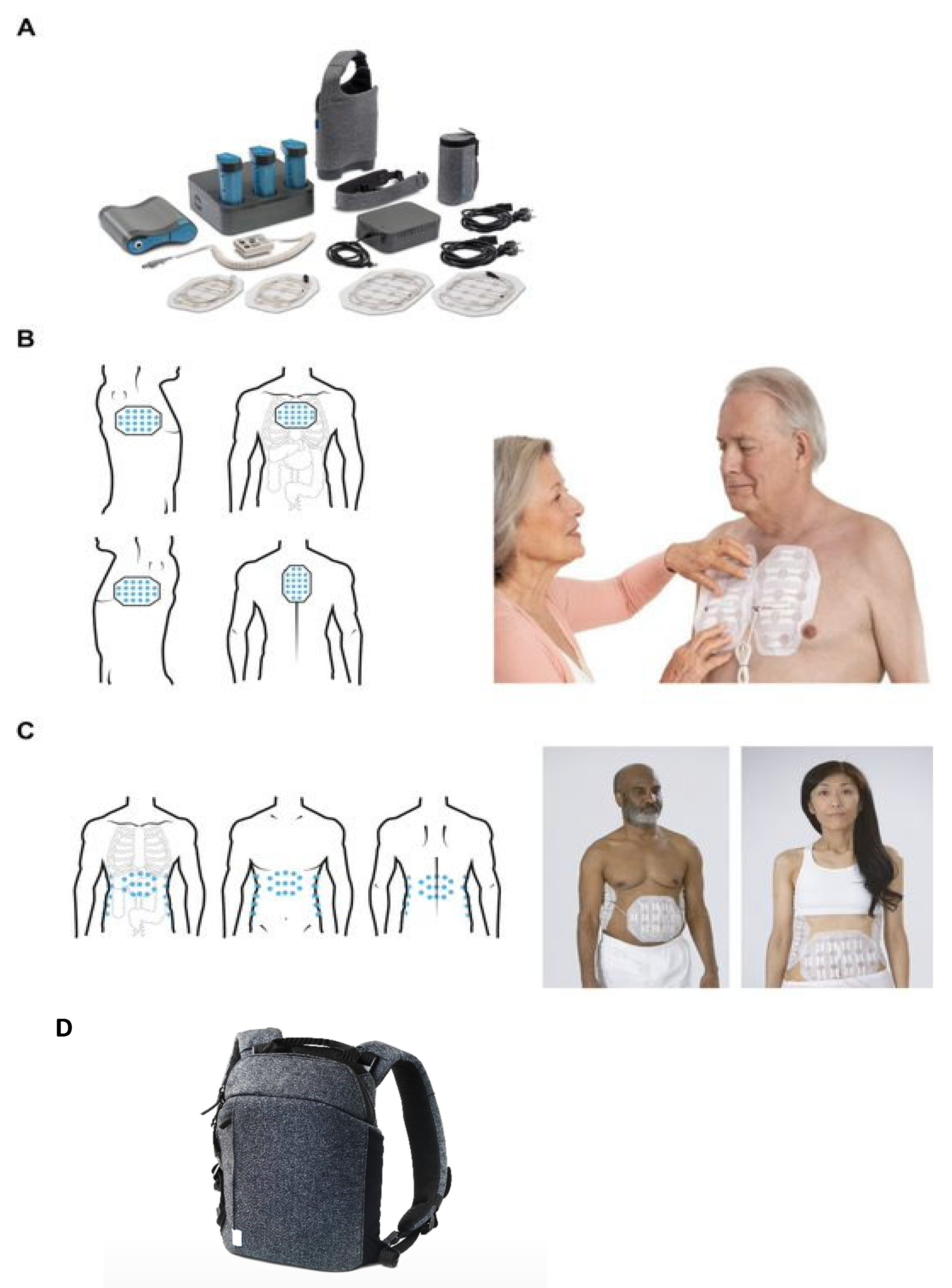


| Disease | Country/Countries Where TTFields Therapy Is Approved |
|---|---|
| GBM | USA |
| Canada | |
| China | |
| Hong Kong | |
| Japan | |
| Europe *,† | |
| Israel | |
| Australia | |
| Pleural mesothelioma | USA |
| Hong Kong | |
| Europe * |
| Study | Disease | Regimen | Key Findings |
|---|---|---|---|
| Immunotherapies | |||
| Kim et al., 2021 [70] | Breast cancer | TTFields therapy concomitant with TRZ | TTFields treatment concomitant with TRZ enhanced penetration of TRZ after inducing apoptosis; TTFields overcame TRZ resistance in vivo and in vitro |
| Voloshin et al., 2020 [52] | NSCLC, colorectal cancer | TTFields therapy concomitant with anti–PD-1 | Immunostimulatory effects from TTFields-induced cell death were observed; TTFields treatment utilized concomitantly with anti–PD-1 enhanced antitumor immunity and decreased tumor volume |
| Barsheshet et al., 2022 [58] | NSCLC | TTFields therapy concomitant with anti–PD-1 and anti-CTLA-4 | TTFields treatment enhanced the immunostimulatory effect of anti–PD-1/anti-CTLA-4, causing tumor leukocyte infiltration and reduced tumor volume |
| Targeted therapies | |||
| Davidi et al., 2022 [62] | HCC | TTFields therapy concomitant with sorafenib | Concomitant use of TTFields treatment and sorafenib led to augmented efficacy through increased cellular stress and apoptosis versus either agent alone |
| Jo et al., 2018 [71] | GBM | TTFields therapy concomitant with sorafenib | Sorafenib and TTFields treatment accelerated apoptosis via ROS generation; TTFields treatment and sorafenib significantly inhibited tumor cell motility, cell invasiveness, and angiogenesis |
| Kim et al., 2020 [72] | GBM | TTFields therapy concomitant with sorafenib | Sorafenib plus TTFields treatment significantly inhibited xenograft tumor growth; STAT3 expression, linked to tumor progression, was also reduced |
| Study | Disease | Phase | Regimen | Patients | Key Findings |
|---|---|---|---|---|---|
| Pivotal studies | |||||
| Stupp et al., 2017 EF-11; NCT00916409 [41] | ndGBM | III | TTFields therapy concomitant with TMZ | N = 695 | Median PFS, (95% CI) months TTFields therapy with TMZ: 6.7 (6.1–8.1) TMZ: 4.0 (3.8–4.4) p < 0.001 |
| Ceresoli et al., 2019 STELLAR; NCT02397928 [73] | Pleural mesothelioma | II | TTFields therapy concomitant with pemetrexed and cisplatin/carboplatin | N = 80 | Median OS, (95% CI) months 18.2 months (12.1–25.8) p value NA |
| Rivera et al., 2019 PANOVA; NCT01971281 [44] | PDAC | II | TTFields therapy concomitant with gemcitabine TTFields therapy concomitant with gemcitabine and nab-paclitaxel | N = 40 | Safety In each cohort, 85% reported grade ≥ 3 AEs No increase in SAEs vs. systemic chemotherapy alone |
| Vergote et al., 2018 INNOVATE; NCT02244502 [47] | PROC | II | TTFields therapy concomitant with paclitaxel | N = 31 | Safety Overall, 55% reported grade ≥ 3% AEs No increase in SAEs vs. systemic chemotherapy alone |
| Other studies | |||||
| Lu et al., 2019 [74] | rGBM | RW | TTFields therapy concomitant with bevacizumab and irinotecan and TMZ TTFields therapy concomitant with bevacizumab-based chemotherapies * | N = 48 | Median OS, (95% CI) months TTFields therapy concomitant with bevacizumab and irinotecan and TMZ: 32.5 (17.0–49.0) TTFields therapy concomitant with bevacizumab-based chemotherapies: * 17.8 (13.3–19.9) p < 0.05 |
| Miller et al., 2022 NCT03477110 [75] | ndGBM | I | TTFields therapy concomitant with TMZ | N = 30 | Safety No grade ≥ 3 AEs TTFields therapy-related AE reported Grade 1 and 2 skin toxicity reported in 73.3% and 10%, respectively |
| Garcia et al., 2018 [76] | rGBM | RW | TTFields therapy concomitant with TMZ | N = 21 | Safety TMZ and TTFields therapy were well-tolerated, few AEs reported |
| Lazaridis et al., 2019 [77] | ndGBM | RW | TTFields therapy concomitant with lomustine and TMZ | N = 16 | Safety Grade ≥ 3 hematologic and grade ≥ 3 hepatotoxic AEs were observed in 44% and 25% of patients, respectively |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vergote, I.; Macarulla, T.; Hirsch, F.R.; Hagemann, C.; Miller, D.S. Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment. Cancers 2023, 15, 636. https://doi.org/10.3390/cancers15030636
Vergote I, Macarulla T, Hirsch FR, Hagemann C, Miller DS. Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment. Cancers. 2023; 15(3):636. https://doi.org/10.3390/cancers15030636
Chicago/Turabian StyleVergote, Ignace, Teresa Macarulla, Fred R. Hirsch, Carsten Hagemann, and David Scott Miller. 2023. "Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment" Cancers 15, no. 3: 636. https://doi.org/10.3390/cancers15030636
APA StyleVergote, I., Macarulla, T., Hirsch, F. R., Hagemann, C., & Miller, D. S. (2023). Tumor Treating Fields (TTFields) Therapy Concomitant with Taxanes for Cancer Treatment. Cancers, 15(3), 636. https://doi.org/10.3390/cancers15030636






