SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Considerations
3. Results
Correlative Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wagner, M.J.; Ismaila, L.; Duh, M.; Korves, C.; Solleza, F.; Manson, S.; Diaz, J.; Neary, M.; Demetri, G. A retrospective chart review of drug treatment patterns and clinical outcomes among patients with metastatic or recurrent soft tissue sarcoma refractory to one or more prior chemotherapy treatments. BMC Cancer 2015, 15, 175. [Google Scholar] [CrossRef] [PubMed]
- Mocellin, S.; Rossi, C.; Brandes, A.; Nitti, D. Adult soft tissue sarcomas: Conventional therapies and molecularly targeted approaches. Cancer Treat. Rev. 2006, 32, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Takyar, S.; Manson, S.; Powell, S.; Penel, N. Efficacy and safety of pharmacological interventions in second- or later-line treatment of patients with advanced soft tissue sarcoma: A systematic review. BMC Cancer 2013, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Petek, B.J.; Loggers, E.T.; Pollack, S.M.; Jones, R.L. Trabectedin in Soft Tissue Sarcomas. Mar. Drugs 2015, 13, 974–983. [Google Scholar] [CrossRef]
- Judson, I.; Verweij, J.; Gelderblom, H.; Hartmann, J.T.; Schöffski, P.; Blay, J.Y.; Kerst, J.M.; Sufliarsky, J.; Whelan, J.; Hohenberger, P.; et al. Doxorubicin alone versus intensified doxorubicin plus ifosfamide for firstline treatment of advanced or metastatic soft-tissue sarcoma: A randomised controlled phase 3 trial. Lancet Oncol. 2014, 15, 415–423. [Google Scholar] [CrossRef]
- Nagar, S.P.; Mytelka, D.S.; Candrilli, S.D.; D’yachkova, Y.; Lorenzo, M.; Kasper, B.; Lopez-Martin, J.A.; Kaye, J.A. Treatment Patterns and Survival among Adult Patients with Advanced Soft Tissue Sarcoma: A Retrospective Medical Record Review in the United Kingdom, Spain, Germany, and France. Sarcoma 2018, 2018, 5467057. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef]
- Coens, C.; van der Graaf, W.T.A.; Blay, J.-Y.; Chawla, S.P.; Judson, I.; Sanfilippo, R.; Manson, S.C.; Hodge, R.A.; Marreaud, S.; Prins, J.B.; et al. Health-related quality-of-life results from PALETTE: A randomized, double-blind, phase 3 trial of pazopanib versus placebo in patients with soft tissue sarcoma whose disease has progressed during or after prior chemotherapy-a European Organization for research and treatment of cancer soft tissue and bone sarcoma group global network study (EORTC 62072). Cancer 2015, 121, 2933–2934. [Google Scholar] [CrossRef]
- Schoffski, P.; Chawla, S.; Maki, R.G.; Italiano, A.; Gelderblom, H.; Choy, E.; Grignani, G.; Camargo, V.; Bauer, S.; Rha, S.Y.; et al. Eribulin versus dacarbazine in previously treated patients with advanced liposarcoma or leiomyosarcoma: A randomised, open-label, multicentre, phase III trial. Lancet 2016, 387, 1629–1637. [Google Scholar] [CrossRef]
- In, G.K.; Hu, J.S.; Tseng, W.W. Treatment of advanced, metastatic soft tissue sarcoma: Latest evidence and clinical considerations. Ther. Adv. Med. Oncol. 2017, 9, 533–550. [Google Scholar] [CrossRef]
- Ferre, A.; Álvarez, R.Á.; Herráez, A.C.; Jurado, J.C.; González, A.E.; Martin-Broto, J.; Marín, V.M.; Vega, A.M.; García, A.S.; Morales, C.V.; et al. SEOM Clinical Guideline of management of soft-tissue sarcoma. Clin. Transl. Oncol. 2021, 23, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.-Y.; Le Cesne, A.; Demetri, G.D. The current reality of soft tissue sarcomas: Advances, controversies, areas for improvement, and promising new treatments. Expert. Rev. Anticancer Ther. 2020, 20, 29–39. [Google Scholar] [CrossRef]
- Lee, A.; Huang, P.; DeMatteo, R.P.; Pollack, S.M. Immunotherapy for Soft Tissue Sarcoma: Tomorrow Is Only a Day Away. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, 281–290. [Google Scholar] [CrossRef]
- Clemente, O.; Ottaiano, A.; Di Lorenzo, G.; Bracigliano, A.; Lamia, S.; Cannella, L.; Pizzolorusso, A.; Di Marzo, M.; Santorsola, M.; Chiara, D.; et al. Is immunotherapy in the future of therapeutic management of sarcomas? J. Transl. Med. 2021, 19, 173. [Google Scholar] [CrossRef]
- Yervoy pi. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s115lbl.pdf (accessed on 6 June 2017).
- Opdivo pi. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s070lbl.pdf (accessed on 6 June 2017).
- Yervoy. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/207953s005lbl.pdf (accessed on 6 June 2017).
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Storer, B.E. Design and analysis of phase I clinical trials. Biometrics 1989, 45, 925–937. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, G.; Kim, K.; Lee, C.; Yoon, S.; Chae, Y.; Tirumani, S.; Ramaiya, N. Comparison of RECIST 1.1 and iRECIST in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 120. [Google Scholar] [CrossRef]
- NCI Common Terminology Criteria for Adverse Events Version 4.03. 2010; pp. 1–78. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf (accessed on 6 June 2017).
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Selby, M.J.; Engelhardt, J.J.; Johnston, R.J.; Lu, L.S.; Han, M.; Thudium, K.; Yao, D.; Quigley, M.; Valle, J.; Wang, C.; et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, In Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS ONE 2016, 11, e0161779. [Google Scholar]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501, Erratum in: Lancet Oncol. 2018, 19, e8. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Mahoney, M.R.; Van Tine, B.A.; Atkins, J.; Milhem, M.M.; Jahagirdar, B.N.; Antonescu, C.R.; Horvath, E.; Tap, W.D.; Schwartz, G.K.; et al. A non-comparative multi-center randomized phase II study of nivolumab +/− ipilimumab for patients with metastatic sarcoma (Alliance A091401). Lancet Oncol. 2018, 19, 416–426. [Google Scholar] [CrossRef]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.P.; Sankhala, K.; Ravicz, J.; Kang, G.; Liu, S.; Stumpf, N.; Leong, B.; Kim, S.; Arasheben, S.; Tseng, W.; et al. Clinical Experience with Combination Chemo-/Immunotherapy using Trabectedin and Nivolumab for Advanced Soft Tissue Sarcoma. J. Sarcoma Res. 2018, 2, 1009. [Google Scholar] [CrossRef]
- Birdi, H.K.; Jirovec, A.; Cortés-Kaplan, S.; Werier, J.; Nessim, C.; Diallo, J.S.; Ardolino, M. Immunotherapy for sarcomas: New frontiers and unveiled opportunities. J. Immunother. Cancer 2021, 9, e001580. [Google Scholar] [CrossRef]
- Pautier, P.; Italiano, A.; Piperno-Neumann, S.; Chevreau, C.; Penel, N.; Firmin, N.; Boudou-Rouquette, P.; Bertucci, F.; Balleyguier, C.; Lebrun-Ly, V.; et al. Doxorubicin alone versus doxorubicin with trabectedin followed by trabectedin alone as first-line therapy for metastatic or unresectable leiomyosarcoma (LMS-04): A randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2022, 23, 1044–1054. [Google Scholar] [CrossRef]
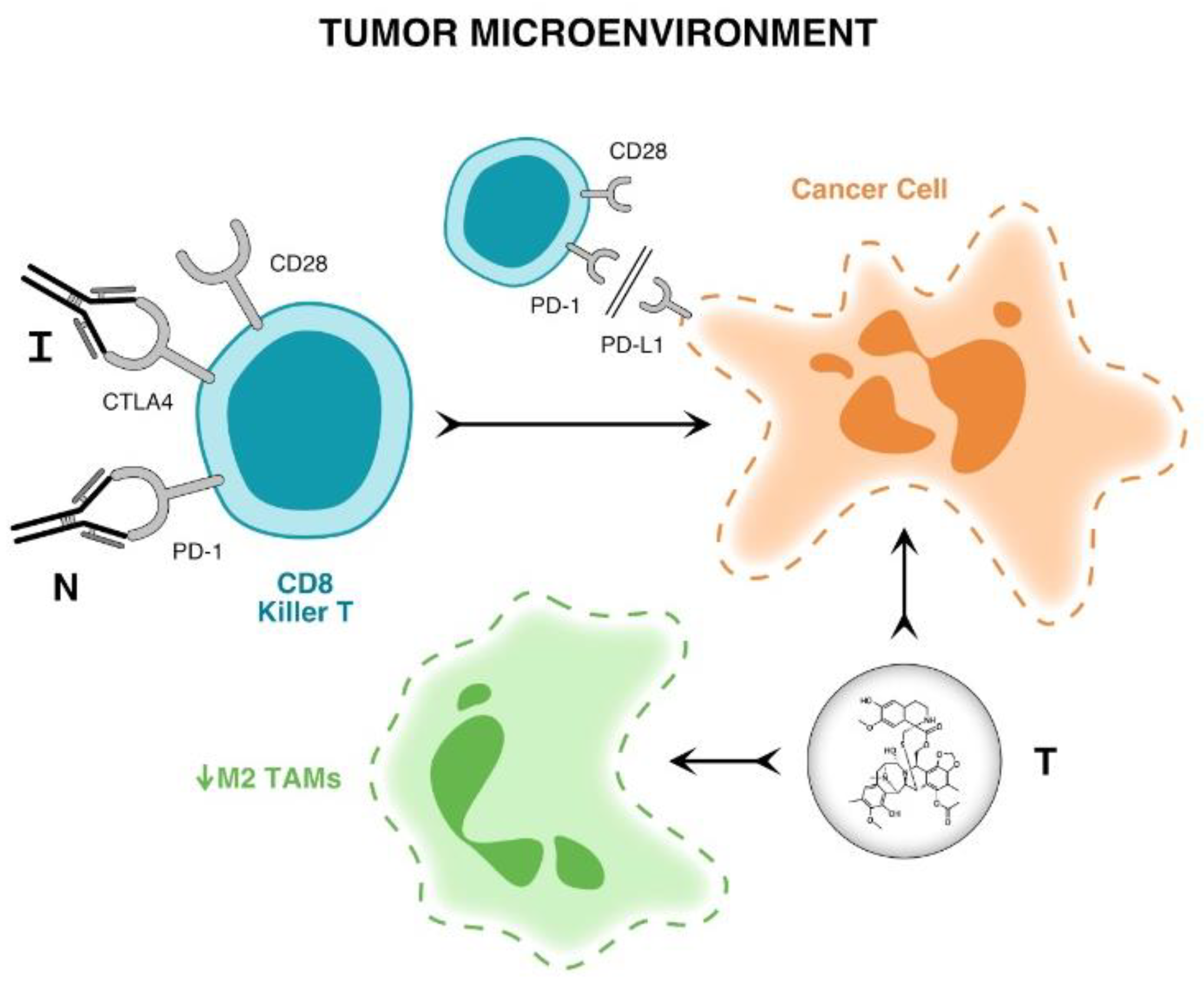
| Patients | n = 101 |
|---|---|
| Age | |
| 18–28 | 8 (7.9%) |
| 29–39 | 12 (11.9%) |
| 40–50 | 13 (12.9%) |
| 51–61 | 30 (29.7%) |
| 62–72 | 26 (25.7% |
| 73–83 | 12 (11.9%) |
| Sex | |
| Men | 44 (43.6%) |
| Women | 57 (56.4% |
| ECOG Score | |
| ≤1 | 101 (100%) |
| Histological type | |
| Liposarcoma | 14 (13.7%) |
| Leiomyosarcoma | 26 (25.5%) |
| Undifferentiated pleomorphic sarcoma | 9 (8.8%) |
| Rhabdomyosarcoma | 7 (6.9%) |
| Synovial sarcoma | 5 (4.9%) |
| Clear cell sarcoma | 4 (3.9%) |
| Pleomorphic sarcoma | 4 (3.9%) |
| Myxofibrosarcoma | 4 (3.9%) |
| Peripheral nerve sheath tumor | 3 (2.9%) |
| Myxoid liposarcoma | 3 (2.9%) |
| Carcinosarcoma | 2 (2.0%) |
| Desmoplastic small round cell tumor | 2 (2.0%) |
| NOS sarcoma | 2 (2.0%) |
| Phase I—Dose Level 1 (n = 3) | ||||||
| Adverse Event | Trabectedin | Nivolumab | Ipilimumab | |||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Investigations | ||||||
| TSH Increased | 1 (33.3%) | 1 (33.3%) | ||||
| Phase I—Dose Level 2 (n = 6) | ||||||
| Adverse Event | Trabectedin | Nivolumab | Ipilimumab | |||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Blood and lymphatic system disorders | ||||||
| Anemia | 2 (33.3%) | |||||
| General disorders and administration site conditions | ||||||
| Fatigue | 1 (16.7%) | 1 (16.7%) | ||||
| Investigations | ||||||
| Alanine aminotransferase increased | 2 (33.3%) | 2 (33.3%) | ||||
| Platelet count decreased | 1 (16.7%) | |||||
| TSH decreased | 1 (16.7%) | 1 (16.7%) | ||||
| T4 increased | 1 (16.7%) | |||||
| Aspartate aminotransferase increased | 1 (16.7%) | 1 (16.7%) | ||||
| TSH increased | 3 (50%) | 2 (33.3%) | ||||
| CPK increased | 2 (33.3%) | |||||
| Alkaline phosphatase increased | 1 (16.7%) | 1 (16.7%) | ||||
| Musculoskeletal and connective tissue disorders | ||||||
| Asthenia | 1 (16.7%) | |||||
| Expanded Phase II (n = 92) | ||||||
| Adverse Event | Trabectedin | Nivolumab | Ipilimumab | |||
| 3 | 4 | 3 | 4 | 3 | 4 | |
| Blood and lymphatic system disorders | ||||||
| Anemia | 7 (7.6%) | 1 (1.1%) | ||||
| Gastrointestinal disorders | ||||||
| Nausea | 1 (1.1%) | |||||
| Vomiting | 1 (1.1%) | |||||
| General disorders and administration site conditions | ||||||
| Fatigue | 8 (8.7%) | |||||
| Fever | 2 (2.2%) | |||||
| Exhaustion | 1 (1.1%) | 1 (1.1%) | ||||
| Infections and infestations | ||||||
| Cellulitis, port-a-catheter | 2 (2.2%) | |||||
| Investigations | ||||||
| Aspartate aminotransferase increased | 8 (8.7%) | 2 (2.2%) | 2 (2.2%) | 1 (1.1%) | ||
| Alanine aminotransferase increased | 23 (25%) | 3 (3.3%) | 5 (5.4%) | 3 (3.3%) | ||
| TSH increased | 1 (1.1%) | 1 (1.1%) | ||||
| Neutrophil count decreased | 5 (5.4%) | 1 (1.1%) | ||||
| Platelet count decreased | 2 (2.2%) | 2 (2.2%) | ||||
| Alkaline phosphatase increased | 1 (1.1%) | 1 (1.1%) | ||||
| CPK increased | 2 (2.2%) | 2 (2.2%) | 1 (1.1%) | |||
| White blood cell decreased | 1 (1.1%) | |||||
| Metabolism and nutrition disorders | ||||||
| Hyponatremia | 4 (4.3%) | 2 (2.2%) | ||||
| Dehydration | 1 (1.1%) | 1 (1.1%) | 1 (1.1%) | |||
| Musculoskeletal and connective tissue disorders | ||||||
| Asthenia | 1 (1.1%) | |||||
| Skin and subcutaneous tissue disorders | ||||||
| Pruritus | 1 (1.1%) | |||||
| Psoriasis | 1 (1.1%) | 1 (1.1%) | ||||
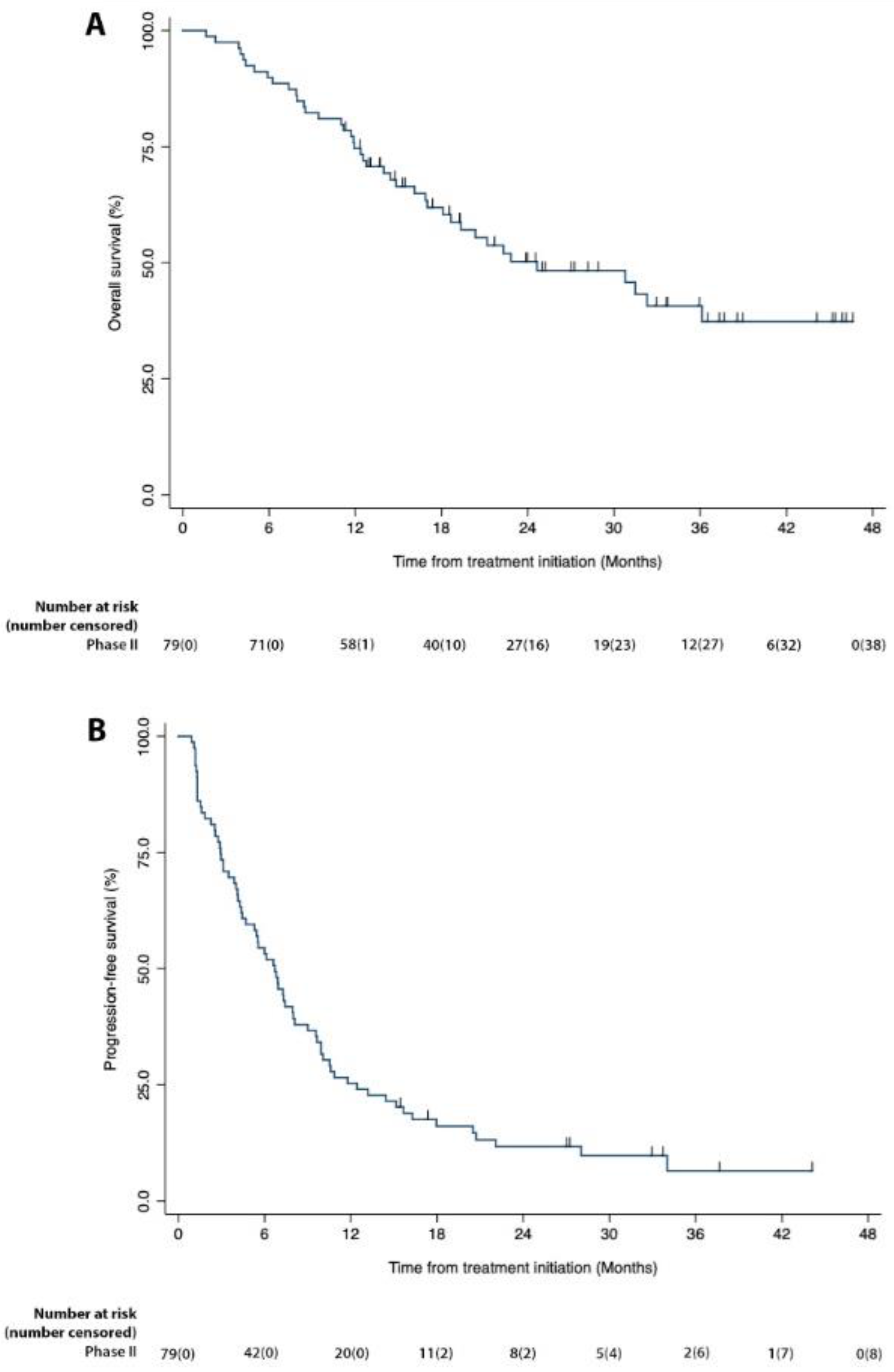
| Best Response | Disease Control Rate | Median OS Months (Range) [CI] | Median PFS Months (Range) [CI] | 6-Month OS Rate | 6-Month PFS Rate |
|---|---|---|---|---|---|
| 6 CR, 14 PR, 49 SD, 10 PD (25.3% ORR) | 87.3% | 24.6 (1.6–46.5) (CI 95%: 17.0–.) | 6.7 (0.9–44.0) (CI 95%: 4.4–7.9) | 89.9% | 53.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, E.M.; Chawla, S.P.; Tellez, W.A.; Younesi, E.; Thomas, S.; Chua-Alcala, V.S.; Chomoyan, H.; Valencia, C.; Brigham, D.A.; Moradkhani, A.; et al. SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma. Cancers 2023, 15, 906. https://doi.org/10.3390/cancers15030906
Gordon EM, Chawla SP, Tellez WA, Younesi E, Thomas S, Chua-Alcala VS, Chomoyan H, Valencia C, Brigham DA, Moradkhani A, et al. SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma. Cancers. 2023; 15(3):906. https://doi.org/10.3390/cancers15030906
Chicago/Turabian StyleGordon, Erlinda Maria, Sant P. Chawla, Walter Andree Tellez, Elan Younesi, Sonu Thomas, Victoria S. Chua-Alcala, Hripsime Chomoyan, Chrysler Valencia, Don Arlen Brigham, Ania Moradkhani, and et al. 2023. "SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma" Cancers 15, no. 3: 906. https://doi.org/10.3390/cancers15030906
APA StyleGordon, E. M., Chawla, S. P., Tellez, W. A., Younesi, E., Thomas, S., Chua-Alcala, V. S., Chomoyan, H., Valencia, C., Brigham, D. A., Moradkhani, A., Quon, D., Srikureja, A., Wong, S. G., Tseng, W., & Federman, N. (2023). SAINT: A Phase I/Expanded Phase II Study Using Safe Amounts of Ipilimumab, Nivolumab and Trabectedin as First-Line Treatment of Advanced Soft Tissue Sarcoma. Cancers, 15(3), 906. https://doi.org/10.3390/cancers15030906




