Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
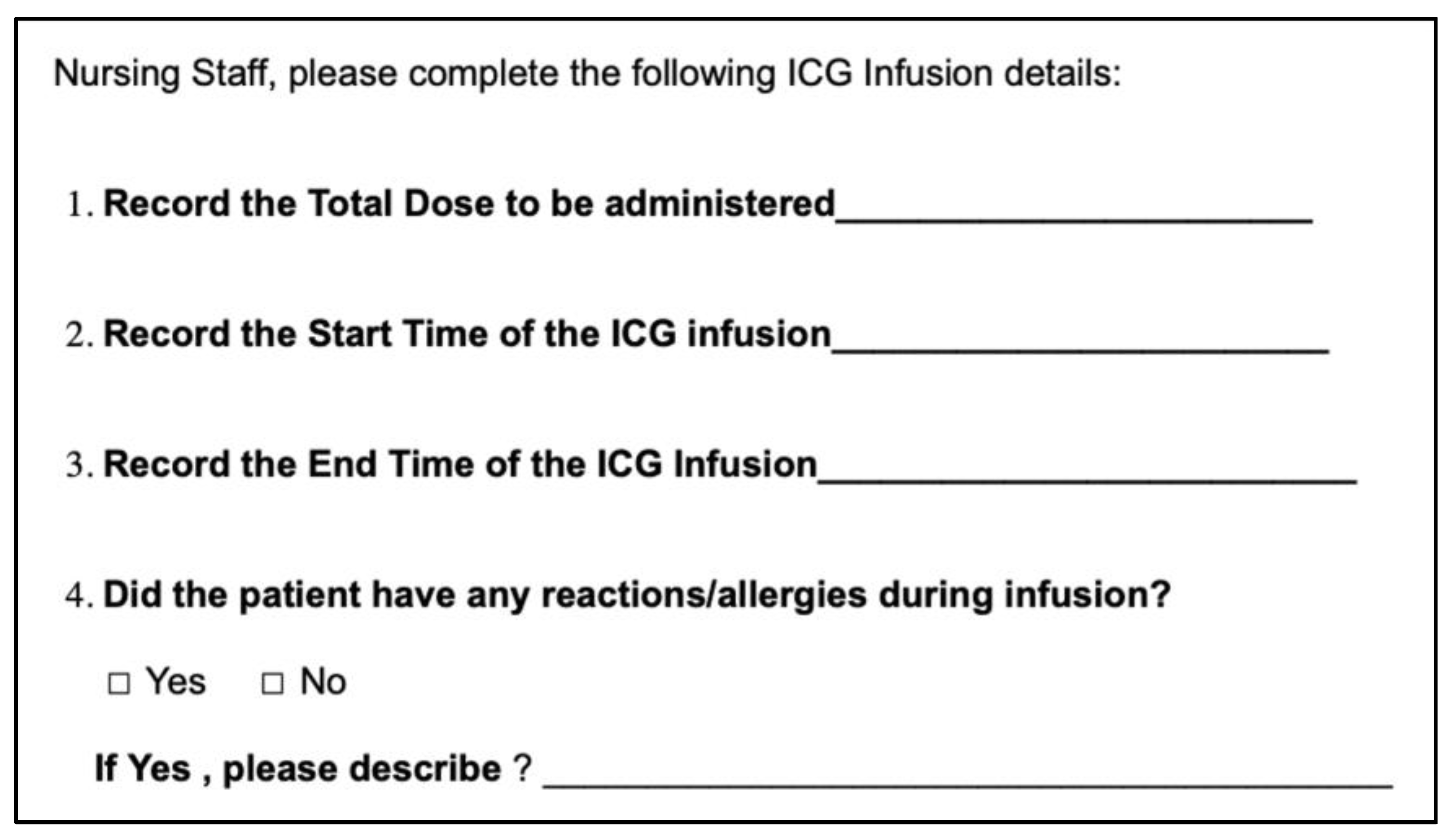
2.2. Study Protocol
2.3. Statistics
3. Results
3.1. Demographics
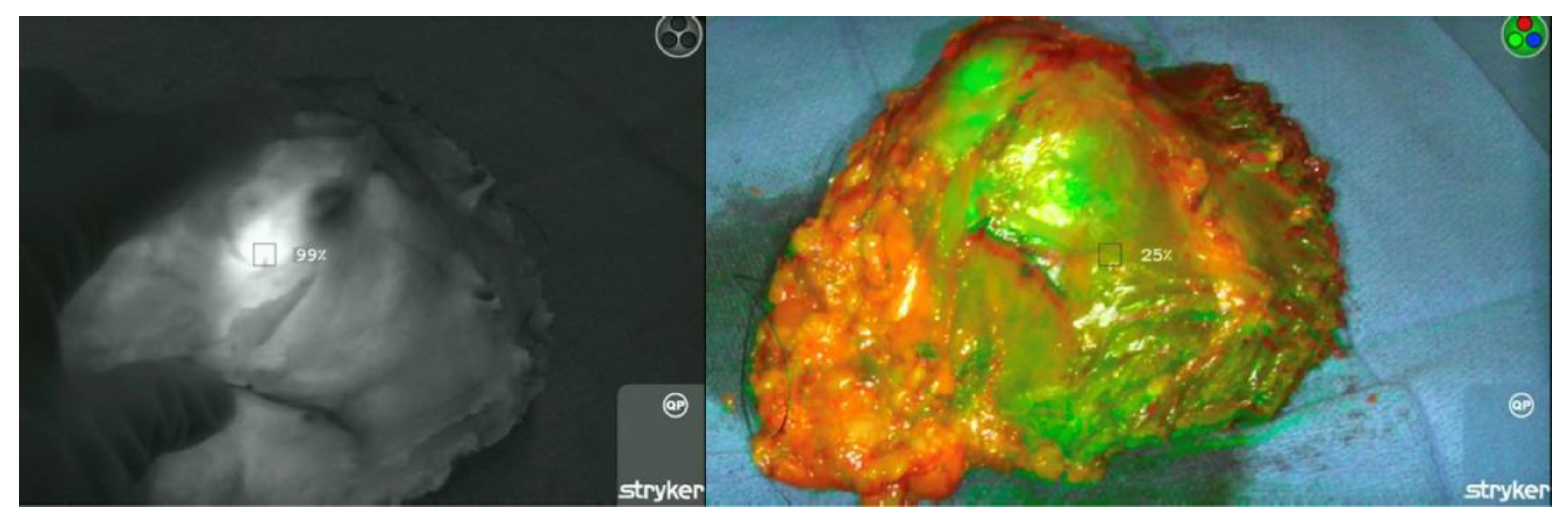
3.2. ICG Dosage, Duration, and Detection
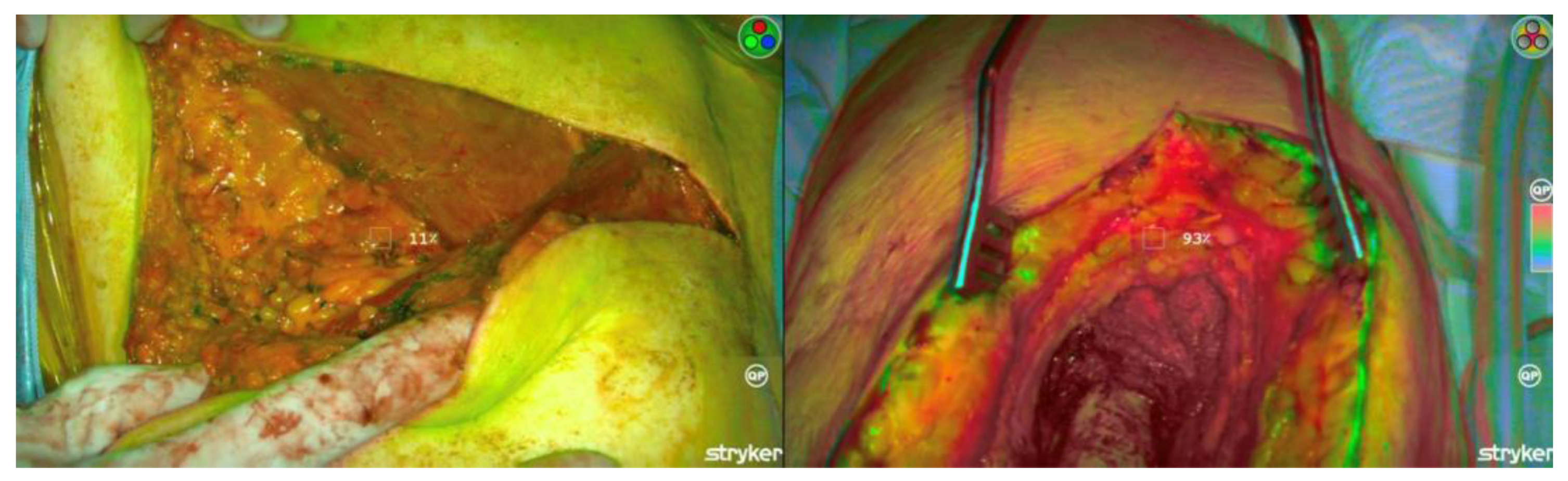
3.3. ICG Margin Comparison
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Von Mehren, M.; Kane, J.M.; Agulnik, M.; Bui, M.M.; Carr-Ascher, J.; Choy, E.; Connelly, M.; Dry, S.; Ganjoo, K.N.; Gonzalez, R.J.; et al. Soft tissue sarcoma, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 815–833. [Google Scholar] [CrossRef]
- Morrison, B.A. Soft tissue sarcomas of the extremities. Bayl. Univ. Med. Cent. Proc. 2003, 16, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, R.T.; Hill-Harmon, M.B.; Murray, T.; Thun, M. Cancer statistics, 2001. CA Cancer J. Clin. 2001, 51, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Jeys, L.M.; Thorne, C.J.; Parry, M.; Gaston, C.L.L.; Sumathi, V.P.; Grimer, J.R. A novel system for the surgical staging of primary high-grade osteosarcoma: The Birmingham classification. Clin. Orthop. Relat. Res. 2017, 475, 842–850. [Google Scholar] [CrossRef]
- O’Donnell, P.W.; Griffin, A.M.; Eward, W.C.; Sternheim, A.; Catton, C.N.; Chung, P.W.; O’Sullivan, B.; Ferguson, P.C.; Wunder, J.S. The effect of the setting of a positive surgical margin in soft tissue sarcoma. Cancer 2014, 120, 2866–2875. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Jacobson, A.; Hornicek, F.; Haynes, A.B.; Choy, E.; Cote, G.; Nielsen, G.P.; Chen, Y.-L.; DeLaney, T.F.; Mullen, J.T. The width of the surgical margin does not influence outcomes in extremity and truncal soft tissue sarcoma treated with radiotherapy. Oncologist 2016, 21, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Biau, D.J.; Weiss, K.R.; Bhumbra, R.S.; Davidson, D.; Brown, C.; Griffin, A.; Wunder, J.S.; Ferguson, P.C. Monitoring the adequacy of surgical margins after resection of bone and soft-tissue sarcoma. Ann. Surg. Oncol. 2013, 20, 1858–1864. [Google Scholar] [CrossRef]
- Bertrand, M.M.; Carrère, S.; Delmond, L.; Mehta, S.; Rouanet, P.; Canaud, L.; Alric, P.; Quénet, F. Oncovascular compartmental resection for retroperitoneal soft tissue sarcoma with vascular involvement. J. Vasc. Surg. 2016, 64, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.-Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Keller, J.; Safwat, A.; Baerentzen, S.; Pedersen, A.B. Relative mortality in soft tissue sarcoma patients: A Danish population-based cohort study. BMC Cancer 2014, 14, 682. [Google Scholar] [CrossRef]
- Salipas, A.; Dowsey, M.M.; May, D.; Choong, P.F.M. “Beware the lump in the foot!”: Predictors of recurrence and survival in bone and soft-tissue sarcomas of the foot and ankle. ANZ J. Surg. 2014, 84, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, J.J.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.; Pollock, R.; Aston, W.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; Wunder, J.S.; et al. Individualised risk assessment for local recurrence and distant metastases in a retrospective transatlantic cohort of 687 patients with high-grade soft tissue sarcomas of the extremities: A multistate model. BMJ Open 2017, 7, e012930. [Google Scholar] [CrossRef]
- Stojadinovic, A.; Leung, D.H.Y.; Hoos, A.; Jaques, D.P.; Lewis, J.J.; Brennan, M.F. Analysis of the prognostic significance of microscopic margins in 2084 localized primary adult soft tissue sarcomas. Ann. Surg. 2002, 235, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Trovik, C.S.; Bauer, H.C.; Alvegård, T.A.; Anderson, H.; Blomqvist, C.; Berlin, O.; Gustafson, P.; Saeter, G.; Wallöe, A. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the Scandinavian Sarcoma Group Register. Eur. J. Cancer 2000, 36, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, M.; Klein, A.; Lindner, L.H.; Nachbichler, S.; Knösel, T.; Birkenmaier, C.; Baur-Melnyk, A.; Dürr, H.R. Amputation for extremity sarcoma: Indications and outcomes. Cancers 2021, 13, 5125. [Google Scholar] [CrossRef]
- Stevenson, M.G.; Musters, A.H.; Geertzen, J.H.B.; van Leeuwen, B.L.; Hoekstra, H.J.; Been, L.B. Amputations for extremity soft tissue sarcoma in an era of limb salvage treatment: Local control and survival. J. Surg. Oncol. 2018, 117, 434–442. [Google Scholar] [CrossRef]
- Steinkamp, P.J.; Pranger, B.K.; Li, M.-F.; Linssen, M.D.; Voskuil, F.J.; Been, L.B.; van Leeuwen, B.L.; Suurmeijer, A.J.H.; Nagengast, W.B.; Kruijff, S.; et al. Fluorescence-guided visualization of soft-tissue sarcomas by targeting vascular endothelial growth factor A: A phase 1 single-center clinical trial. J. Nucl. Med. 2021, 62, 342–347. [Google Scholar] [CrossRef]
- Samkoe, K.S.; Sardar, H.S.; Bates, B.D.; Tselepidakis, N.N.; Gunn, J.R.; Hoffer-Hawlik, K.A.; Feldwisch, J.; Pogue, B.W.; Paulsen, K.D.; Henderson, E.R. Preclinical imaging of epidermal growth factor receptor with ABY-029 in soft-tissue sarcoma for fluorescence-guided surgery and tumor detection. J. Surg. Oncol. 2019, 119, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Tummers, Q.R.J.G.; Hoogstins, C.E.S.; Peters, A.A.W.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van de Velde, C.J.H.; Frangioni, J.V.; Vahrmeijer, A.L.; Gaarenstroom, K.N. The value of intraoperative near-infrared fluorescence imaging based on enhanced permeability and retention of indocyanine green: Feasibility and false-positives in ovarian cancer. PLoS ONE 2015, 10, e0129766. [Google Scholar] [CrossRef] [PubMed]
- Okusanya, O.T.; Holt, D.; Heitjan, D.; Deshpande, C.; Venegas, O.; Jiang, J.; Judy, R.; DeJesus, E.; Madajewski, B.; Oh, K.; et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann. Thorac. Surg. 2014, 98, 1223–1230. [Google Scholar] [CrossRef]
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A review of indocyanine green fluorescent imaging in surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, W.; Tong, M. Intraoperative indocyanine green fluorescence guidance for excision of nonpalpable breast cancer. World J. Surg. Oncol. 2016, 14, 266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lin, H.; Fu, R.; Zhang, T.; Nie, Q.; Dong, S.; Yang, X.-N.; Wu, Y.-L.; Zhong, W.-Z. Application of indocyanine green fluorescence for precision sublobar resection. Thorac. Cancer 2019, 10, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Alford, R.; Simpson, H.M.; Duberman, J.; Hill, G.C.; Ogawa, M.; Regino, C.; Kobayashi, H.; Choyke, P.L. Toxicity of organic fluorophores used in molecular imaging: Literature review. Mol. Imaging 2009, 8, 341–354. [Google Scholar] [CrossRef]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef]
- Chan, C.D.; Brookes, M.J.; Tanwani, R.; Hope, C.; Pringle, T.A.; Knight, J.C.; Rankin, K.S. Investigating the mechanisms of indocyanine green (ICG) cellular uptake in sarcoma. BioRxiv 2021. [Google Scholar] [CrossRef]
- Brookes, M.J.; Chan, C.D.; Nicoli, F.; Crowley, T.P.; Ghosh, K.M.; Beckingsale, T.; Saleh, D.; Dildey, P.; Gupta, S.; Ragbir, M.; et al. Intraoperative near-infrared fluorescence guided surgery using indocyanine green (ICG) for the resection of sarcomas may reduce the positive margin rate: An extended case series. Cancers 2021, 13, 6284. [Google Scholar] [CrossRef]
- Nicoli, F.; Saleh, D.B.; Baljer, B.; Chan, C.D.; Beckingsale, T.; Ghosh, K.M.; Ragbir, M.; Rankin, K.S. Intraoperative near-infrared fluorescence (NIR) imaging with indocyanine green (ICG) can identify bone and soft tissue sarcomas which may provide guidance for oncological resection. Ann. Surg. 2021, 273, e63–e68. [Google Scholar] [CrossRef]
- Veys, I.; Pop, C.-F.; Barbieux, R.; Moreau, M.; Noterman, D.; De Neubourg, F.; Nogaret, J.-M.; Liberale, G.; Larsimont, D.; Bourgeois, P. ICG fluorescence imaging as a new tool for optimization of pathological evaluation in breast cancer tumors after neoadjuvant chemotherapy. PLoS ONE 2018, 13, e0197857. [Google Scholar] [CrossRef] [PubMed]
- Pop, F.-C.; Veys, I.; Vankerckhove, S.; Barbieux, R.; Chintinne, M.; Moreau, M.; Donckier, V.; Larsimont, D.; Bourgeois, P.; Liberale, G. Absence of residual fluorescence in the surgical bed at near-infrared fluorescence imaging predicts negative margins at final pathology in patients treated with breast-conserving surgery for breast cancer. Eur. J. Surg. Oncol. 2021, 47, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Fourman, M.S.; Mahjoub, A.; Mandell, J.B.; Yu, S.; Tebbets, J.C.; Crasto, J.A.; Alexander, P.E.; Weiss, K.R. Quantitative primary tumor indocyanine green measurements predict osteosarcoma metastatic lung burden in a mouse model. Clin. Orthop. Relat. Res. 2018, 476, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, A.; Morales-Restrepo, A.; Fourman, M.S.; Mandell, J.B.; Feiqi, L.; Hankins, M.L.; Watters, R.J.; Weiss, K.R. Tumor resection guided by intraoperative indocyanine green dye fluorescence angiography results in negative surgical margins and decreased local recurrence in an orthotopic mouse model of osteosarcoma. Ann. Surg. Oncol. 2019, 26, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Wilke, B.K.; Schultz, D.S.; Huayllani, M.T.; Boczar, D.; Spaulding, A.C.; Sherman, C.; Murray, P.; Forte, A.J. A prospective evaluation of intraoperative indocyanine green fluorescence angiography for soft tissue sarcomas. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 2021, 5, e21.00187-6. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Zaidi, N.; Berber, E. An initial report on the intraoperative use of indocyanine green fluorescence imaging in the surgical management of liver tumorss. J. Surg. Oncol. 2016, 114, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Kaplan-Marans, E.; Fulla, J.; Tomer, N.; Bilal, K.; Palese, M. Indocyanine green (ICG) in urologic surgery. Urology 2019, 132, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Predina, J.D.; Newton, A.D.; Corbett, C.; Shin, M.; Sulfyok, L.F.; Okusanya, O.T.; Delikatny, E.J.; Nie, S.; Gaughan, C.; Jarrar, D.; et al. Near-infrared intraoperative imaging for minimally invasive pulmonary metastasectomy for sarcomas. J. Thorac. Cardiovasc. Surg. 2019, 157, 2061–2069. [Google Scholar] [CrossRef]
- Holt, D.; Parthasarathy, A.B.; Okusanya, O.; Keating, J.; Venegas, O.; Deshpande, C.; Karakousis, G.; Madajewski, B.; Durham, A.; Nie, S.; et al. Intraoperative near-infrared fluorescence imaging and spectroscopy identifies residual tumor cells in wounds. J. Biomed. Opt. 2015, 20, 76002. [Google Scholar] [CrossRef]
- Jiang, J.X.; Keating, J.J.; Jesus, E.M.D.; Judy, R.P.; Madajewski, B.; Venegas, O.; Okusanya, O.T.; Singhal, S. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 390–400. [Google Scholar]
- Newton, A.D.; Predina, J.D.; Corbett, C.J.; Frenzel-Sulyok, L.G.; Xia, L.; Petersson, E.J.; Tsourkas, A.; Nie, S.; Delikatny, E.J.; Singhal, S. Optimization of second window indocyanine green for intraoperative near-infrared imaging of thoracic malignancy. J. Am. Coll. Surg. 2019, 228, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Abdelhafeez, A.; Talbot, L.; Murphy, A.J.; Davidoff, A.M. Indocyanine green-guided pediatric tumor resection: Approach, utility, and challenges. Front. Pediatr. 2021, 9, 689612. [Google Scholar] [CrossRef]

| Demographics (n = 18) | |
|---|---|
| Age (years) | 64.1 ± 14.1 |
| Gender | 8 female (44%), 10 male (56%) |
| Local Recurrence | 4/18 (22%) |
| Prior Chemotherapy | 2/18 (11%) |
| Prior Radiation | 6/18 (33%) |
| ICG Dosing and Duration | |
|---|---|
| ICG Dose (mg) | 201.8 ± 52.7 |
| Infusion Duration (min) | 37.6 ± 17.3 |
| Time Between Infusion to Surgical Incision (min) | 115.6 ± 80.4 |
| Tumor Background Ratio | 6.53 ± 2.61 |
| ICG Margin Case-by-Case Comparison | ||||
|---|---|---|---|---|
| Subject | Diagnosis | Margins (Surgeon) | Margins (ICG) | Margins (Pathology) |
| 1 | Pleomorphic liposarcoma | Negative | Positive | Positive |
| 2 | High grade round cell sarcoma | Negative | Positive | Negative |
| 3 | Dedifferentiated liposarcoma | Positive | Negative | Positive |
| 4 | Pleomorphic dermal sarcoma | Negative | Negative | Negative |
| 5 | Epithelioid inflammatory myofibroblastic sarcoma | Negative | Negative | Negative |
| 6 | Leiomyosarcoma | Negative | Negative | Negative |
| 7 | Sclerosing rhabdomyosarcoma | Negative | Negative | Negative |
| 8 | Spindle cell sarcoma | Negative | Negative | Positive |
| 9 | Pleomorphic sarcoma | Negative | Negative | Negative |
| 10 | Myxofibrosarcoma | Negative | Positive | Positive |
| 11 | Dedifferentiated liposarcoma | Positive | Negative | Positive |
| 12 | Myxofibrosarcoma | Negative | Negative | Positive |
| 13 | Spindle cell sarcoma | Negative | Negative | Positive |
| 14 | Synovial sarcoma | Negative | Negative | Negative |
| 15 | Dedifferentiated liposarcoma | Positive | Negative | Negative |
| 16 | Myxofibrosarcoma | Negative | Negative | Positive |
| 17 | Pleomorphic liposarcoma | Positive | Negative | Negative |
| 18 | Pleomorphic sarcoma | Negative | Negative | Positive |
| Sensitivity, Specificity, and Predictive Values Compared to Permanent Pathology. PPV, Positive Predictive Value; NPV, Negative Predictive Value | ||||
|---|---|---|---|---|
| Margin Evaluation | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| ICG | 22.2 | 88.9 | 66.7 | 53.3 |
| Surgeon Impression | 22.2 | 77.8 | 50.0 | 50.0 |
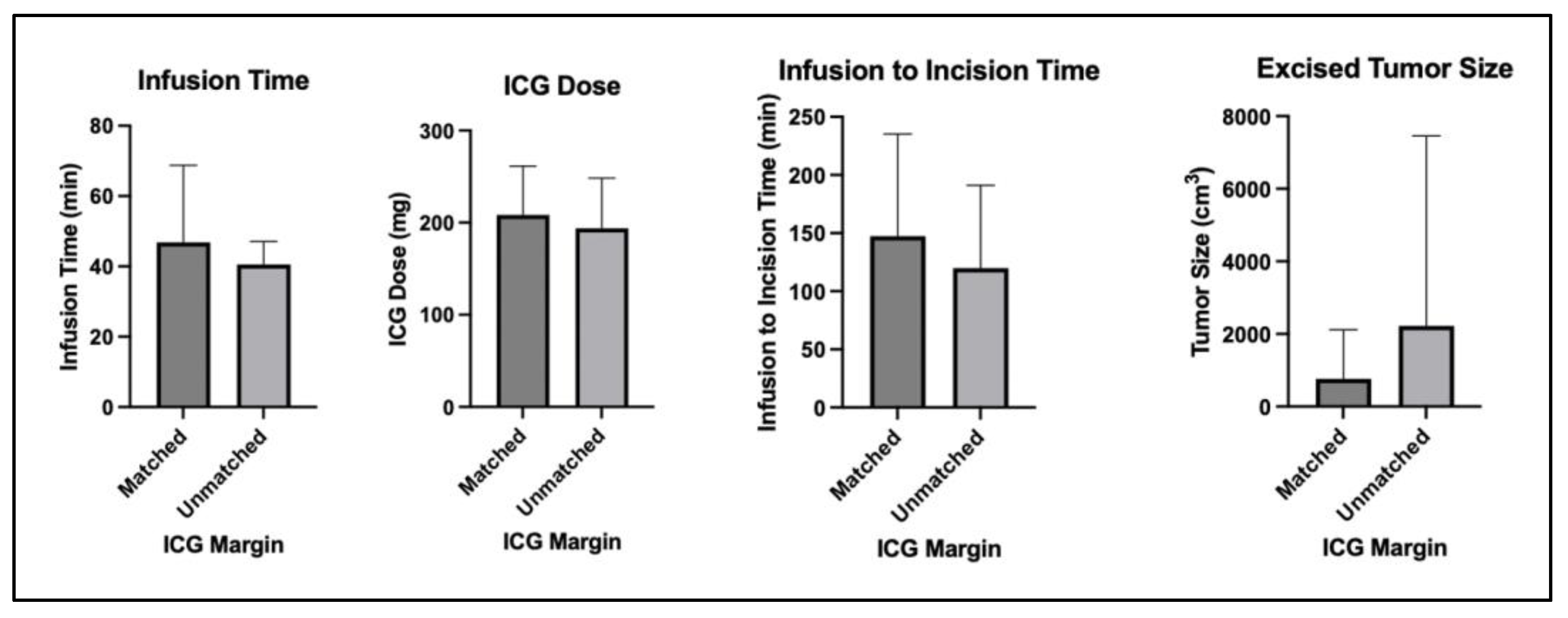
| Comparison between Cases Where ICG Margins Matched or Did Not Match Margin Descriptions on the Case’s Final Pathology Report | |||
|---|---|---|---|
| Matched (n = 10) | Unmatched (n = 8) | Significance (a = 0.05) | |
| Infusion dose (mg) | 208 ± 53 | 194 ± 55 | p = 0.58 |
| Infusion time (min) | 46 ± 22 | 40 ± 6 | p = 0.48 |
| Infusion to incision time (min) | 147 ± 88 | 120 ± 71 | p = 0.50 |
| Excised tumor size (cm3) | 762 ± 1356 | 2220 ± 5237 | p = 0.41 |
| Prior chemotherapy | 1/10 | 1/8 | p = 0.93 |
| Prior radiation | 3/10 | 3/8 | p = 0.89 |
| Local recurrence | 2/10 | 2/8 | p = 0.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.F.; Li, W.T.; Bhogal, S.; Royes, B.; Heim, T.; Silvaggio, M.; Malek, M.; Dhupar, R.; Lee, S.J.; McGough, R.L.; et al. Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging. Cancers 2023, 15, 582. https://doi.org/10.3390/cancers15030582
Gong MF, Li WT, Bhogal S, Royes B, Heim T, Silvaggio M, Malek M, Dhupar R, Lee SJ, McGough RL, et al. Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging. Cancers. 2023; 15(3):582. https://doi.org/10.3390/cancers15030582
Chicago/Turabian StyleGong, Matthew F., William T. Li, Sumail Bhogal, Brittany Royes, Tanya Heim, Maria Silvaggio, Marcus Malek, Rajeev Dhupar, Stella J. Lee, Richard L. McGough, and et al. 2023. "Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging" Cancers 15, no. 3: 582. https://doi.org/10.3390/cancers15030582
APA StyleGong, M. F., Li, W. T., Bhogal, S., Royes, B., Heim, T., Silvaggio, M., Malek, M., Dhupar, R., Lee, S. J., McGough, R. L., & Weiss, K. R. (2023). Intraoperative Evaluation of Soft Tissue Sarcoma Surgical Margins with Indocyanine Green Fluorescence Imaging. Cancers, 15(3), 582. https://doi.org/10.3390/cancers15030582





