Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Immune-Related Adverse Events
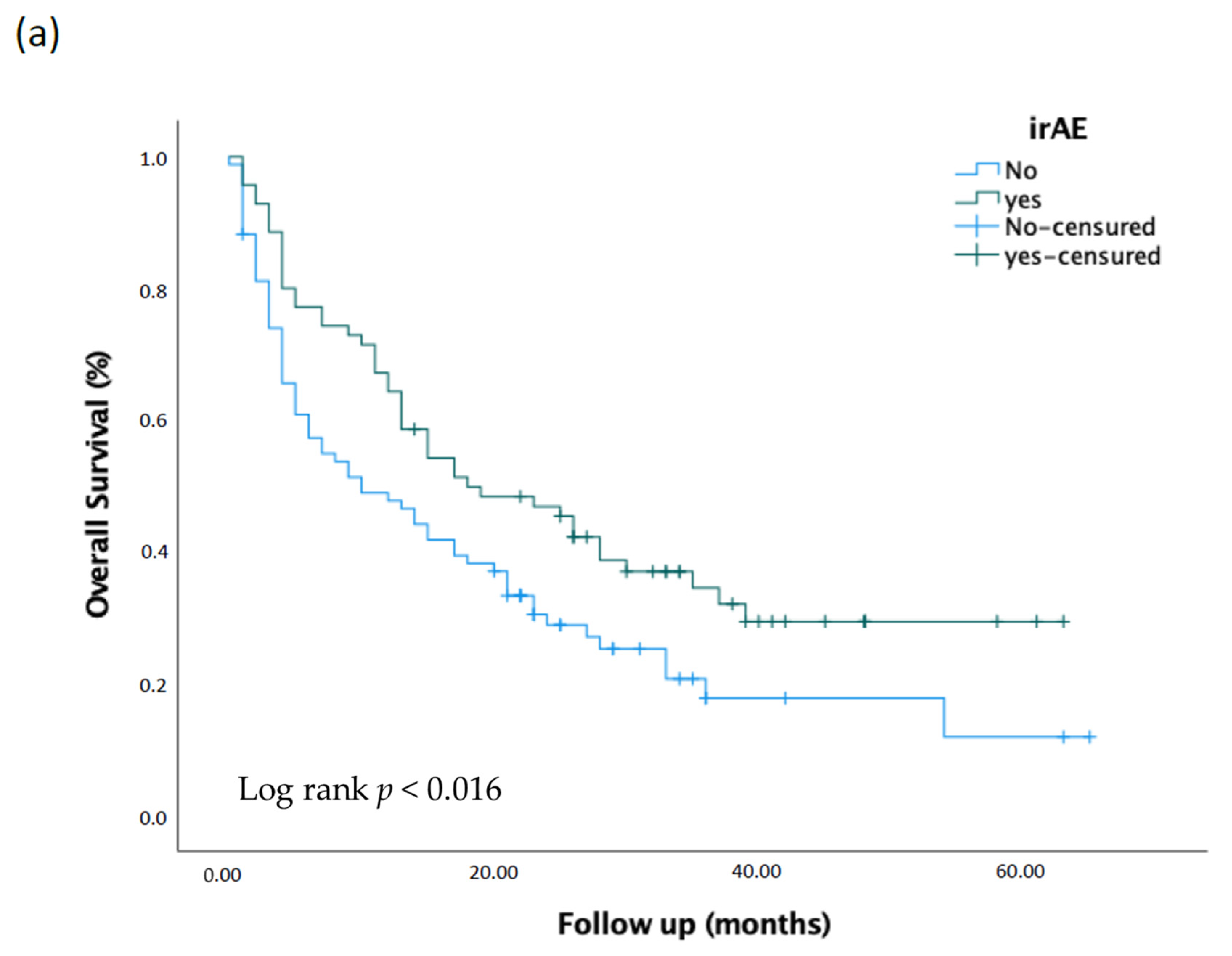
3.3. Response Rate and Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.M.; Lenardo, M.J. Development of immune checkpoint therapy for cancer. J. Exp. Med. 2019, 216, 1244–1254. [Google Scholar] [CrossRef]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Williams. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K.; Hospital, W.; Immunology, E. functions in immune regulation. Immunity 2017, 44, 989–1004. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Hellmann. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Gangadhar, T.C.; Vonderheide, R.H. Mitigating the toxic effects of anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2014, 11, 91–99. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American society of clinical oncology clinical practice guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv264–iv266. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-J.; Kuen, D.-S.; Chung, Y. Future prospects of immune checkpoint blockade in cancer: From response prediction to overcoming resistance. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Horvat, T.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef]
- Petrelli, F.; Grizzi, G.; Ghidini, M.; Ghidini, A.; Ratti, M.; Panni, S.; Cabiddu, M.; Ghilardi, M.; Borgonovo, K.; Parati, M.C.; et al. Immune-related Adverse Events and Survival in Solid Tumors Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. J. Immunother. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Suresh, K.; Naidoo, J. Lower Survival in Patients Who Develop Pneumonitis Following Immunotherapy for Lung Cancer. Clin. Lung Cancer 2020, 21, e169–e170. [Google Scholar] [CrossRef]
- Weber, J.S.; Yang, J.C.; Atkins, M.B.; Disis, M.L. Toxicities of immunotherapy for the practitioner. J. Clin. Oncol. 2015, 33, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Hodi, F.S.; Wolchok, J.D.; Topalian, S.L.; Schadendorf, D.; Larkin, J.; Sznol, M.; Long, G.; Li, H.; Waxman, I.M.; et al. Safety profile of nivolumab monotherapy: A pooled analysis of patients with advanced melanoma. J. Clin. Oncol. 2017, 35, 785–792. [Google Scholar] [CrossRef]
- Conroy, M.; Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat. Commun. 2022, 13, 392. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, W.; Huang, H.; Wang, Y.; Li, G.; Geng, Y.; Hao, Y.; Zhang, Z. Association of Immune Related Adverse Events With Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Cancers: A Systemic Review and Meta-analysis. Front. Oncol. 2021, 11, 633032. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Jin, B.; Chen, J.; Wang, H.; Lin, S.; Dang, J.; Li, G. Comparative risk of serious and fatal treatment-related adverse events caused by 19 immune checkpoint inhibitors used in cancer treatment: A network meta-analysis. Ther. Adv. Med Oncol. 2020, 12, 1758835920940927. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Doberstein, T.; Amberker, R.R.; Garje, R.; Field, E.H.; Singh, N. Immune-related adverse events in cancer patients treated with immune checkpoint inhibitors: A single-center expe-rience. Medicine 2019, 98, e17348. [Google Scholar] [CrossRef]
- Möhn, N.; Beutel, G.; Gutzmer, R.; Ivanyi, P.; Satzger, I.; Skripuletz, T. Neurological Immune Related Adverse Events Associated with Nivolumab, Ipilimumab, and Pembrolizumab Therapy—Review of the Literature and Future Outlook. J. Clin. Med. 2019, 8, 1777. [Google Scholar] [CrossRef]
- Xu, C.; Chen, Y.-P.; Du, X.-J.; Liu, J.-Q.; Huang, C.-L.; Chen, L.; Zhou, G.-Q.; Li, W.-F.; Mao, Y.-P.; Hsu, C.; et al. Comparative safety of immune checkpoint inhibitors in cancer: Systematic review and network meta-analysis. BMJ 2018, 363, k4226. [Google Scholar] [CrossRef]
- Di Giacomo, A.M.; Grimaldi, A.M.; Ascierto, P.A.; Queirolo, P.; Del Vecchio, M.; Ridolfi, R.; De Rosa, F.; De Galitiis, F.; Testori, A.; Cognetti, F.; et al. Correlation between efficacy and toxicity in pts with pretreated advanced melanoma treated within the Italian cohort of the ipilimumab expanded access programme (EAP). J. Clin. Oncol. 2013, 31, 9065. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Kicinski, M.; Blank, C.U.; Mandala, M.; Long, G.; Atkinson, V.; Dalle, S.; Haydon, A.; Khattak, A.; Carlino, M.S.; et al. Association Between Immune-Related Adverse Events and Recurrence-Free Survival Among Patients With Stage III Melanoma Randomized to Receive Pembrolizumab or Placebo A Secondary Analysis of a Randomized Clinical Trial Supplemental content. JAMA Oncol. 2020, 6, 519–527. [Google Scholar] [CrossRef]
- Maher, V.E.; Fernandes, L.L.; Weinstock, C.; Tang, S.; Agarwal, S.; Brave, M.; Ning, Y.-M.; Singh, H.; Suzman, D.; Xu, J.; et al. Analysis of the Association Between Adverse Events and Outcome in Patients Receiving a Programmed Death Protein 1 or Programmed Death Ligand 1 Antibody. J. Clin. Oncol. 2019, 37, 2730–2737. [Google Scholar] [CrossRef]
- Xu, S.; Lai, R.; Zhao, Q.; Zhao, P.; Zhao, R.; Guo, Z. Correlation Between Immune-Related Adverse Events and Prognosis in Hepatocellular Carcinoma Patients Treated With Immune Checkpoint Inhibitors. Front. Immunol. 2021, 12, 5204. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non–Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Soneji, S.; Tanner, N.T.; Silvestri, G.A.; Lathan, C.S.; Black, W. Racial and Ethnic Disparities in Early-Stage Lung Cancer Survival. Chest 2017, 152, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gerber, D.E. Autoimmunity, checkpoint inhibitor therapy and immune-related adverse events: A review. Semin. Cancer Biol. 2020, 64, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Santos, E. Most common autoimmune diseases in relatives of portuguese patients with systemic lupus erythematosus. RFML 2007, 12, 181–186. [Google Scholar]
- Kuusisalo, S.; Koivunen, J.P.; Iivanainen, S. Association of Rare Immune-Related Adverse Events to Survival in Advanced Cancer Patients Treated with Immune Checkpoint Inhibitors: A Real-World Single-Center Cohort Study. Cancers 2022, 14, 2276. [Google Scholar] [CrossRef] [PubMed]
- Chin, I.S.; Khan, A.; Olsson-Brown, A.; Papa, S.; Middleton, G.; Palles, C. Germline genetic variation and predicting immune checkpoint inhibitor induced toxicity. NPJ Genom. Med. 2022, 7, 73. [Google Scholar] [CrossRef] [PubMed]

| Variables | Whole Cohort n (%) | irAE n (%) | non-irAE n (%) |
|---|---|---|---|
| Age (years) | |||
| Median (range) | 64 (21–86) | 64 (36–86) | 65 (21–85) |
| Sex | |||
| Male | 118 (76.1) | 52 (74.3) | 66 (77.6) |
| Female | 37 (23.9) | 18 (25.7) | 19 (22.4) |
| Tumor type | |||
| Lung, non-small cell | 76 (49.0) | 42(60) | 34 (40.0) |
| Melanoma | 28 (18.1) | 16 (22.8) | 12 (14.2) |
| Renal | 18 (11.6) | 7(10.0) | 11 (12.9) |
| Head and neck | 17 (11.0) | 2 (2.9) | 15 (17.6) |
| Bladder | 11 (7.1) | 3 (4.3) | 8 (9.4) |
| Others | 5 (3.2) | - | 5 (6.0) |
| ECOG PS | |||
| 0 | 38 (24.5) | 17 (24.3) | 21 (24.7) |
| 1 | 101 (65.2) | 46 (65.7) | 55 (64.7) |
| 2 | 16 (10.3) | 7 (10.0) | 9 (10.6) |
| Treatment Line | |||
| First | 50 (32.3) | 28 (40.0) | 22 (25.9) |
| Second | 91 (58.7) | 37 (52.9) | 54 (63.5) |
| Third | 11 (7.1) | 5 (7.1) | 6 (7.1) |
| Fifth or later | 3 (1.9) | - | 3 (3.6) |
| Type of Immune Checkpoint inhibitor | |||
| Anti-PD-1/PD-L1 | 146 (94.2) | 66 (94.3) | 80 (94.1) |
| Anti-CTLA-4 | 9 (5.8) | 4 (5.7) | 5 (5.9) |
| Variables | n (%) |
|---|---|
| Treatment-related irAEs | |
| yes | 70 (45.2) |
| no | 85 (54.8) |
| Grade of irAE | |
| <3 | 91 (92.9) |
| ≥3 | 9 (8.1) |
| Frequency of irAEs | |
| 1 | 33(47.1) |
| 2 | 26 (37.1) |
| 3 | 11(15.71) |
| Type of irAE | |
| Dermatologic | 34 (35.4) |
| Pruritus | 18(18.7) |
| Rash | 14(14.7) |
| Vitiligo | 1(1.0) |
| Bullous pemphigoid | 1(1.0) |
| Neurologic/Musculoskeletal | 13 (13.5) |
| Myalgias | 12 (12.5) |
| Immune-mediated necrotizing myopathy | 1(1.0) |
| Endocrin | 14(14.7) |
| Hypothyroidism | 11 (11.5) |
| Hypertiroidism | 3 (3.2) |
| Rheumatologic | 17 (17.7) |
| Artralgias | 16 (16.7) |
| Vasculitis | 1(1.0) |
| Pulmonary | 8(8.3) |
| Pneumonitis | 8 (8.3) |
| Gastrointestinal and Hepatic and biliary | 9 (9.4) |
| Diarrhea | 2 (2.1) |
| Colitis | 2 (2.1) |
| Hepatitis | 3 (3.2) |
| Colangitis | 1 (1.0) |
| Colestases | 1 (1.0) |
| Renal | 1 (1.0) |
| Nephritis | 1 (1.0) |
| Treatment of irAE | |
| Supportive care | 66 (94.3) |
| Oral Corticosteroid | 27 (38.6) |
| Intravenous Corticosteroid | 5 (7.1) |
| Other Immunosuppressor (Methotrexate) | 1(1.4) |
| Predictable Variables | HR Crude (CI 95%) | p Value | HR Adjusted (CI 95%) | p Value |
|---|---|---|---|---|
| irAE Yes vs. No | 0.67 (0.46–0.99) | 0.043 | 0.65 (0.44–0.96) | 0.03 |
| Sex Male vs. female | 0.73 (0.48–1.12) | 0.152 | ||
| ECOG PS 1 vs. 0 | 1.84 (1.12–3.03) | 0.017 | 1.81 (1.10–2.88) | 0.020 |
| 2 vs. 0 | 3.50 (1.72–7.11) | 0.001 | 3.73 (1.83–7.62) | 0.000 |
| Age <65 vs. ≥65 | 0.95 (0.65–1.30) | 0.771 | ||
| Treatment Line 1 vs. ≥2 | 1.29 (0.85–1.98) | 0.230 | ||
| Type of tumor NSCLC vs. other | 0.75 (0.52–1.11) | 0.151 | ||
| Grade of toxicity <3 vs. ≥3 | 0.46 (0.19–1.14) | 0.094 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romão, R.; Mendes, A.S.; Ranchor, R.; Ramos, M.J.; Coelho, J.; Pichel, R.C.; Azevedo, S.X.; Fidalgo, P.; Araújo, A. Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review. Cancers 2023, 15, 888. https://doi.org/10.3390/cancers15030888
Romão R, Mendes AS, Ranchor R, Ramos MJ, Coelho J, Pichel RC, Azevedo SX, Fidalgo P, Araújo A. Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review. Cancers. 2023; 15(3):888. https://doi.org/10.3390/cancers15030888
Chicago/Turabian StyleRomão, Raquel, Ana S. Mendes, Ridhi Ranchor, Maria João Ramos, João Coelho, Rita Carrilho Pichel, Sérgio Xavier Azevedo, Paula Fidalgo, and António Araújo. 2023. "Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review" Cancers 15, no. 3: 888. https://doi.org/10.3390/cancers15030888
APA StyleRomão, R., Mendes, A. S., Ranchor, R., Ramos, M. J., Coelho, J., Pichel, R. C., Azevedo, S. X., Fidalgo, P., & Araújo, A. (2023). Impact of Immune-Related Adverse Events on Immune Checkpoint Inhibitors Treated Cancer Patients’ Survival: Single Center Experience and Literature Review. Cancers, 15(3), 888. https://doi.org/10.3390/cancers15030888






