Elevation of Cytoplasmic Calcium Suppresses Microtentacle Formation and Function in Breast Tumor Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Reagents
2.3. Kinetic Calcium Assay
2.4. Cell Viability
2.5. McTNs Scoring and Analysis
2.6. Cellular Clustering Assay
2.7. Cell-Electrode Impedance Reattachment Assay
2.8. Immunoblotting
2.9. Confocal Microscopy
2.10. Statistical Analysis
3. Results
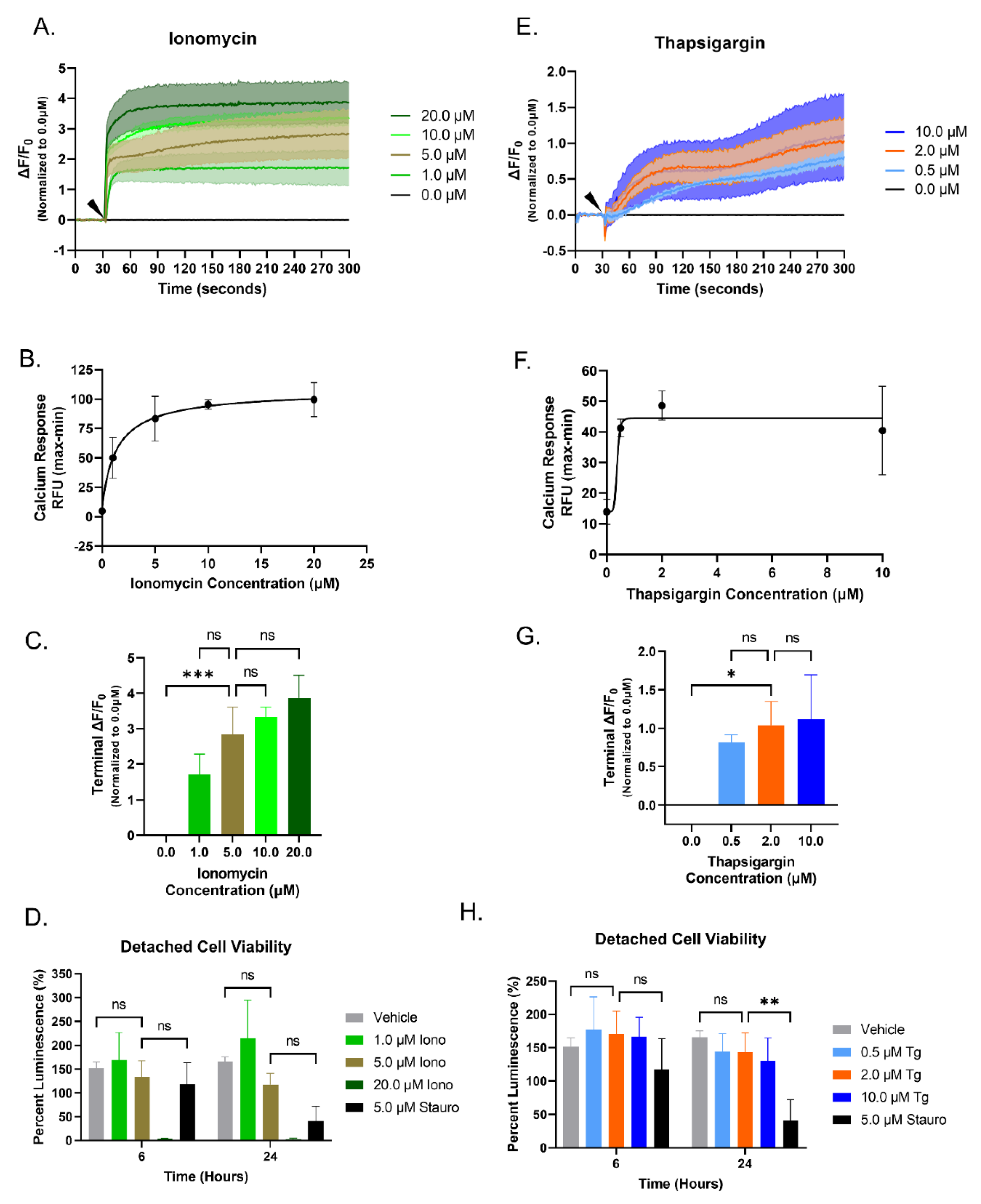
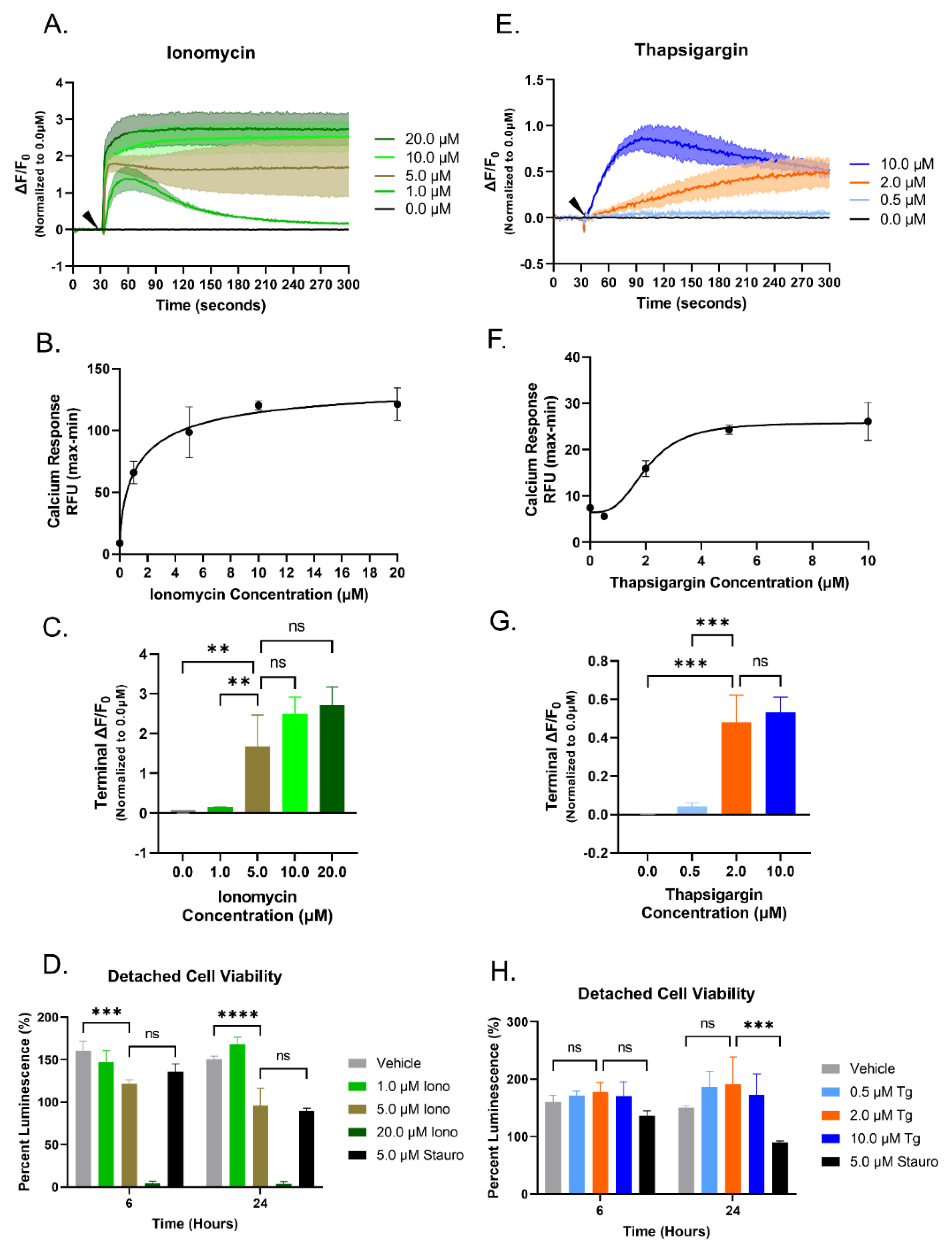
3.1. Increasing Concentrations of Ionomycin or Thapsigargin Induces an Elevation of Cytoplasmic Calcium in Breast Cancer Cells
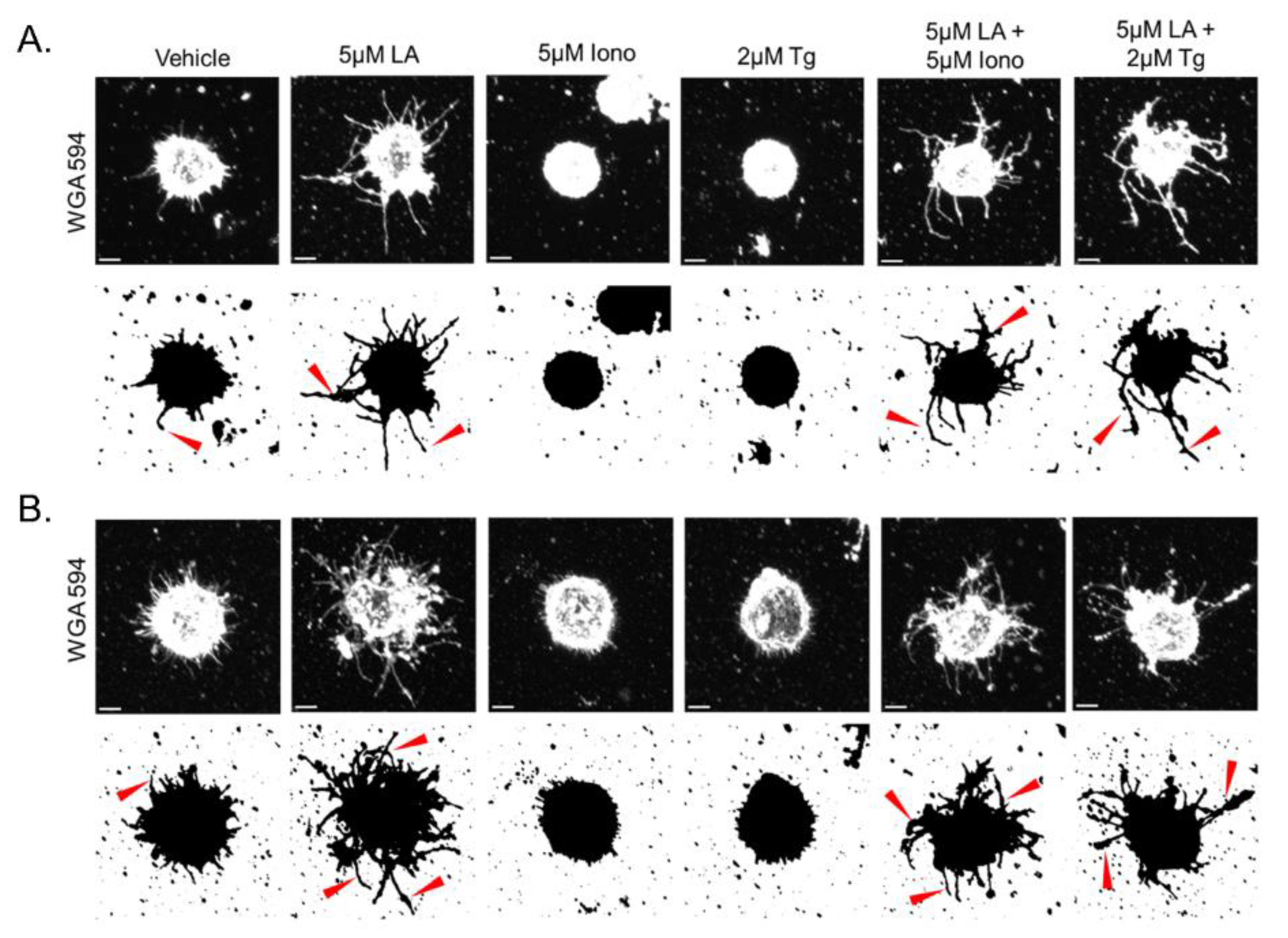
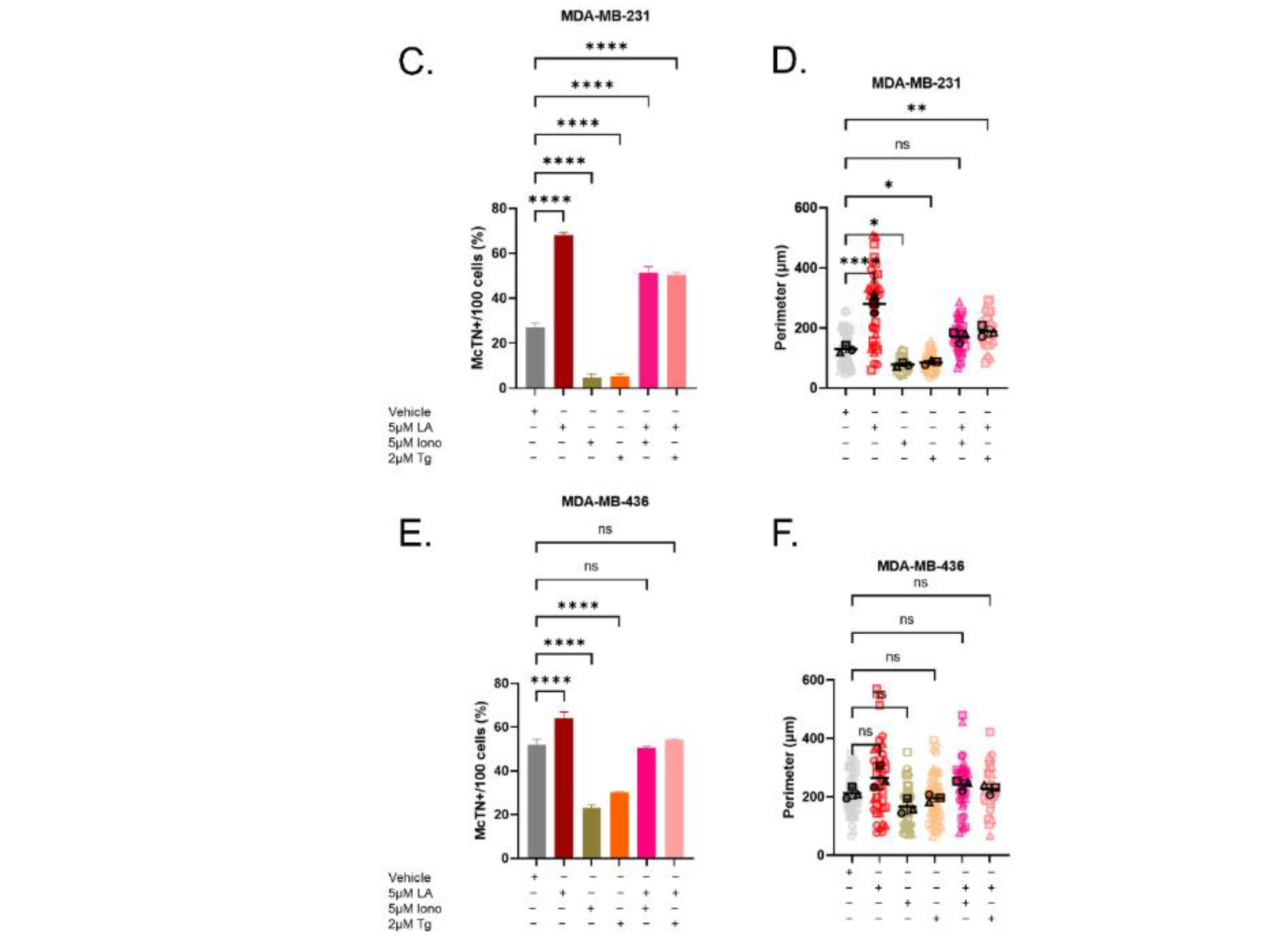
3.2. Elevated Cytoplasmic Calcium Suppresses Microtentacle Formation
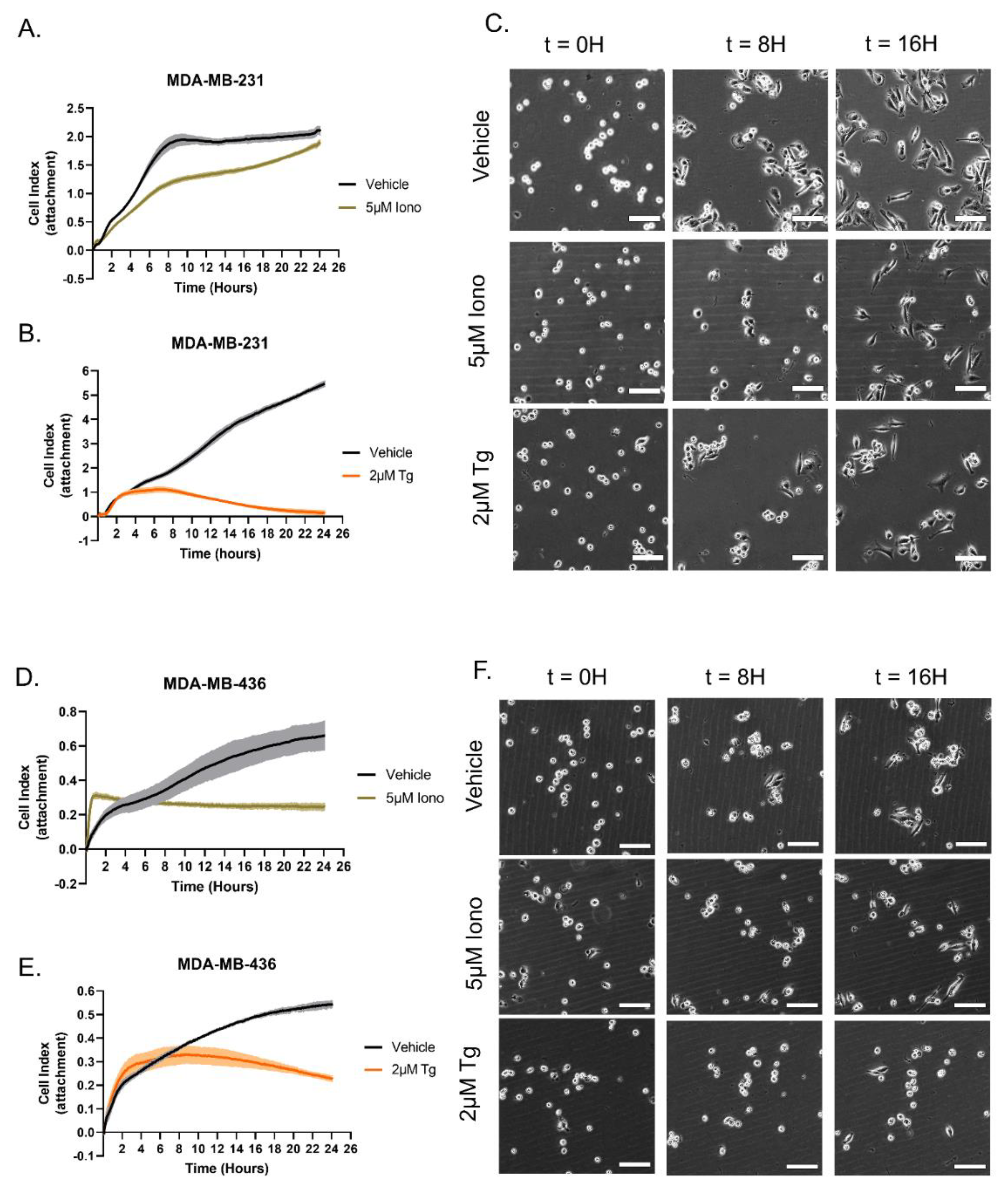
3.3. Distinct Calcium Entry Sources Result in an Inhibited Reattachment Response to Surfaces
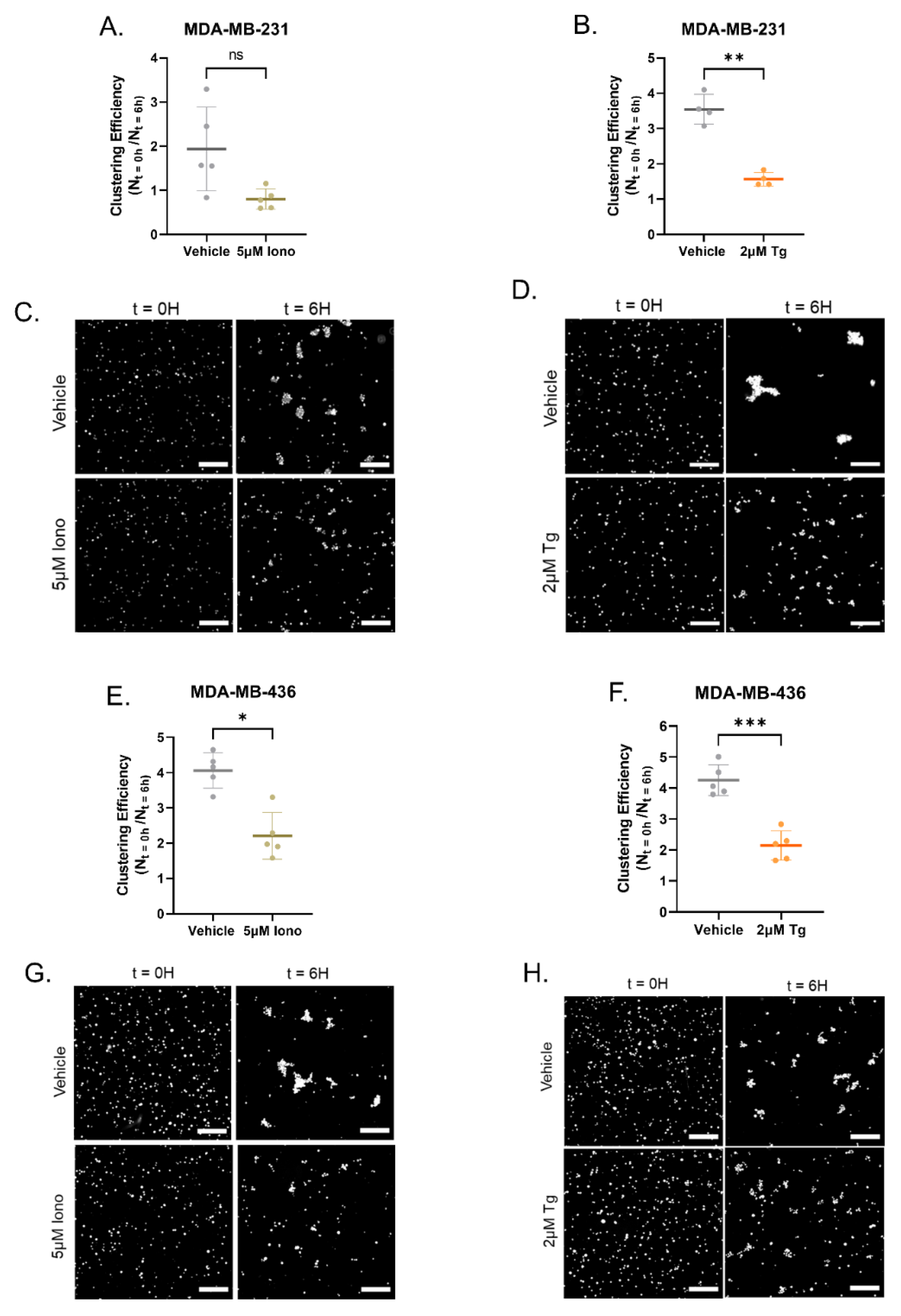
3.4. Increasing Cytoplasmic Calcium Decreases Homotypic Cellular Clustering
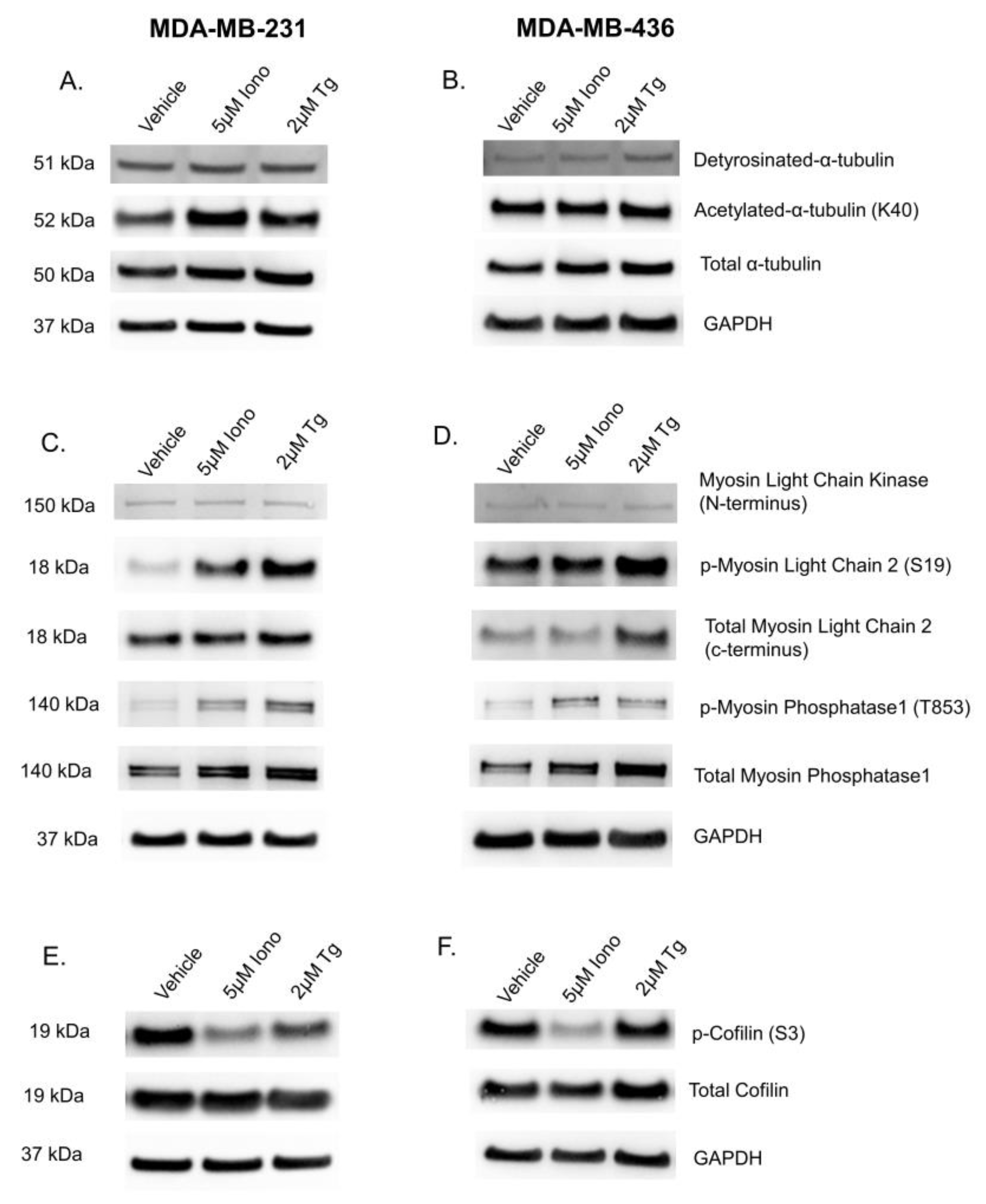
3.5. Elevated Cytoplasmic Calcium Concentration Induces Actin Contraction and Rearrangement
3.6. Calcium-Induced Microtentacle Suppression Requires Actin Polymerization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Cooper, J.A. Actin, a central player in cell shape and movement. Science 2009, 326, 1208–1212. [Google Scholar] [CrossRef] [PubMed]
- Aseervatham, J. Cytoskeletal Remodeling in Cancer. Biology 2020, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Y.; Peng, Y.; Zhang, Y.; Zhou, H.; Chen, X.; Li, T.; Li, S.; Yang, H.; Wu, C.; et al. Notch-1 signaling promotes reattachment of suspended cancer cells by cdc42-dependent microtentacles formation. Cancer Sci. 2021, 112, 4894–4908. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, K.R.; Hessler, L.; Bhandary, L.; Martin, S.S. Molecular Pathways: New Signaling Considerations When Targeting Cytoskeletal Balance to Reduce Tumor Growth. Clin. Cancer Res. 2015, 21, 5209–5214. [Google Scholar] [CrossRef]
- Matrone, M.A.; Whipple, R.A.; Balzer, E.M.; Martin, S.S. Microtentacles tip the balance of cytoskeletal forces in circulating tumor cells. Cancer Res. 2010, 70, 7737–7741. [Google Scholar] [CrossRef]
- Whipple, R.A.; Cheung, A.M.; Martin, S.S. Detyrosinated microtubule protrusions in suspended mammary epithelial cells promote reattachment. Exp. Cell Res. 2007, 313, 1326–1336. [Google Scholar] [CrossRef]
- Ingber, D.E.; Wang, N.; Stamenović, D. Tensegrity, cellular biophysics and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef]
- Killilea, A.N.; Csencsits, R.; Le, E.B.N.T.; Patel, A.M.; Kenny, S.J.; Xu, K.; Downing, K.H. Cytoskeletal organization in microtentacles. Exp. Cell Res. 2017, 357, 291–298. [Google Scholar] [CrossRef]
- So, C.L.; Saunus, J.M.; Roberts-Thomson, S.J.; Monteith, G.R. Calcium signalling and breast cancer. Semin. Cell Dev. Biol. 2019, 94, 74–83. [Google Scholar] [CrossRef]
- Pratt, S.J.P.; Hernández-Ochoa, E.; Martin, S.S. Calcium signaling: Breast cancer’s approach to manipulation of cellular circuitry. Biophys. Rev. 2020, 12, 1343–1359. [Google Scholar] [CrossRef]
- Lee, D.; Hong, J. Ca2+ Signaling as the Untact Mode during Signaling in Metastatic Breast Cancer. Cancers 2021, 13, 1473. [Google Scholar] [CrossRef]
- Joseph, N.; Reicher, B.; Barda-Saad, M. The calcium feedback loop and T cell activation: How cytoskeleton networks control intracellular calcium flux. Biochim. Biophys. Acta 2014, 1838, 557–568. [Google Scholar] [CrossRef]
- Mo, P.; Yang, S. The store-operated calcium channels in cancer metastasis: From cell migration, invasion to metastatic colonization. Front. Biosci. 2018, 23, 1241–1256. [Google Scholar]
- Bassett, J.J.; Robitaille, M.; Peters, A.A.; Bong, A.H.L.; Taing, M.; Wood, I.A.; Sadras, F.; Roberts-Thomson, S.J.; Monteith, G.R. ORAI1 regulates sustained cytosolic free calcium fluctuations during breast cancer cell apoptosis and apoptotic resistance via a STIM1 independent pathway. FASEB J. 2022, 36, e22108. [Google Scholar] [CrossRef]
- Peters, A.A.; Simpson, P.T.; Bassett, J.J.; Lee, J.M.; Da Silva, L.; Reid, L.E.; Song, S.; Parat, M.-O.; Lakhani, S.R.; Kenny, P.A.; et al. Calcium channel TRPV6 as a potential therapeutic target in estrogen receptor-negative breast cancer. Mol. Cancer Ther. 2012, 11, 2158–2168. [Google Scholar] [CrossRef]
- Azimi, I.; Robitaille, M.; Armitage, K.; So, C.; Milevskiy, M.; Northwood, K.; Lim, H.; Thompson, E.; Roberts-Thomson, S.; Monteith, G. Activation of the Ion Channel TRPV4 Induces Epithelial to Mesenchymal Transition in Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9417. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Wang, Z.; Zhou, J.; Jia, Y.; He, X.; Zhao, L.; Dong, Y.; Fan, Y.; Yang, X.; et al. Calcium and TRPV4 promote metastasis by regulating cytoskeleton through the RhoA/ROCK1 pathway in endometrial cancer. Cell Death Dis. 2020, 11, 1009. [Google Scholar] [CrossRef]
- Wales, P.; Schuberth, C.E.; Aufschnaiter, R.; Fels, J.; García-Aguilar, I.; Janning, A.; Dlugos, C.P.; Schäfer-Herte, M.; Klingner, C.; Wälte, M.; et al. Calcium-mediated actin reset (CaAR) mediates acute cell adaptations. Elife 2016, 5, e19850. [Google Scholar] [CrossRef]
- Pratt, S.J.; Hernández-Ochoa, E.O.; Lee, R.M.; Ory, E.C.; Lyons, J.S.; Joca, H.C.; Johnson, A.; Thompson, K.; Bailey, P.; Lee, C.J.; et al. Real-time scratch assay reveals mechanisms of early calcium signaling in breast cancer cells in response to wounding. Oncotarget 2018, 9, 25008–25024. [Google Scholar] [CrossRef]
- Pratt, S.J.P.; Lee, R.M.; Chang, K.T.; Martin, S.S. Mechanoactivation of NOX2-generated ROS elicits persistent TRPM8 Ca2+ signals that are inhibited by oncogenic KRas. Proc. Natl. Acad. Sci. USA 2020, 117, 26008–26019. [Google Scholar] [CrossRef]
- Curry, M.; Roberts-Thomson, S.J.; Monteith, G.R. PMCA2 silencing potentiates MDA-MB-231 breast cancer cell death initiated with the Bcl-2 inhibitor ABT-263. Biochem. Biophys. Res. Commun. 2016, 478, 1792–1797. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin-From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Bhandary, L.; Bailey, P.C.; Chang, K.T.; Underwood, K.F.; Lee, C.J.; Whipple, R.A.; Jewell, C.M.; Ory, E.; Thompson, K.N.; Ju, J.A.; et al. Lipid tethering of breast tumor cells reduces cell aggregation during mammosphere formation. Sci. Rep. 2021, 11, 3214. [Google Scholar] [CrossRef] [PubMed]
- Whipple, R.A.; Balzer, E.M.; Cho, E.H.; Matrone, M.A.; Yoon, J.R.; Martin, S.S. Vimentin filaments support extension of tubulin-based microtentacles in detached breast tumor cells. Cancer Res. 2008, 68, 5678–5688. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.A.; Lee, C.J.; Thompson, K.N.; Ory, E.C.; Lee, R.M.; Mathias, T.J.; Pratt, S.J.P.; Vitolo, M.I.; Jewell, C.M.; Martin, S.S. Partial thermal imidization of polyelectrolyte multilayer cell tethering surfaces (TetherChip) enables efficient cell capture and microtentacle fixation for circulating tumor cell analysis. Lab Chip 2020, 20, 2872–2888. [Google Scholar] [CrossRef]
- Thompson, K.N.; Ju, J.A.; Ory, E.C.; Pratt, S.J.P.; Lee, R.M.; Mathias, T.J.; Chang, K.T.; Lee, C.J.; Goloubeva, O.G.; Bailey, P.C.; et al. Microtubule disruption reduces metastasis more effectively than primary tumor growth. Breast Cancer Res. 2022, 24, 13. [Google Scholar] [CrossRef]
- Bhandary, L.; Whipple, R.A.; Vitolo, M.I.; Charpentier, M.S.; Boggs, A.E.; Chakrabarti, K.R.; Thompson, K.N.; Martin, S.S. ROCK inhibition promotes microtentacles that enhance reattachment of breast cancer cells. Oncotarget 2015, 6, 6251–6266. [Google Scholar] [CrossRef]
- Dedkova, E.N.; Sigova, A.A.; Zinchenko, V.P. Mechanism of action of calcium ionophores on intact cells: Ionophore-resistant cells. Membr. Cell Biol. 2000, 13, 357–368. [Google Scholar]
- Boggs, A.E.; Vitolo, M.I.; Whipple, R.A.; Charpentier, M.S.; Goloubeva, O.G.; Ioffe, O.B.; Tuttle, K.C.; Slovic, J.; Lu, Y.; Mills, G.B.; et al. α-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration. Cancer Res. 2015, 75, 203–215. [Google Scholar] [CrossRef]
- Butler, T.P.; Gullino, P.M. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer Res. 1975, 35, 512–516. [Google Scholar]
- Jakabova, A.; Bielcikova, Z.; Pospisilova, E.; Petruzelka, L.; Blasiaket, P.; Bobek, V.; Kolostova, K. Characterization of circulating tumor cells in early breast cancer patients receiving neoadjuvant chemotherapy. Ther. Adv. Med. Oncol. 2021, 13, 17588359211028492. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; You, J.; Zhang, H.; Zhang, X.; Ye, L. Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2. Cancer Lett. 2008, 270, 312–327. [Google Scholar] [CrossRef]
- Ortiz-Otero, N.; Marshall, J.R.; Lash, B.; King, M.R. Chemotherapy-induced release of circulating-tumor cells into the bloodstream in collective migration units with cancer-associated fibroblasts in metastatic cancer patients. BMC Cancer 2020, 20, 873. [Google Scholar] [CrossRef]
- Brito, C.; Sousa, S. Non-Muscle Myosin 2A (NM2A): Structure, Regulation and Function. Cells 2020, 9, 1590. [Google Scholar] [CrossRef]
- Vitolo, M.I.; Boggs, A.E.; A Whipple, R.; Yoon, J.R.; Thompson, K.; A Matrone, M.; Cho, E.H.; Balzer, E.M.; Martin, S.S. Loss of PTEN induces microtentacles through PI3K-independent activation of cofilin. Oncogene 2013, 32, 2200–2210. [Google Scholar] [CrossRef]
- Hall, C.; Karhade, M.; Laubacher, B.; Anderson, A.; Kuerer, H.; DeSynder, S.; Lucci, A. Circulating Tumor Cells after Neoadjuvant Chemotherapy in Stage I-III Triple-Negative Breast Cancer. Ann. Surg. Oncol. 2015, 22, S552–S558. [Google Scholar] [CrossRef]
- Mahalingam, D.; Wilding, G.; Denmeade, S.; Sarantopoulas, J.; Cosgrove, D.; Cetnar, J.; Azad, N.; Bruce, J.; Kurman, M.; Allgood, V.E.; et al. Mipsagargin, a novel thapsigargin-based PSMA-activated prodrug: Results of a first-in-man phase I clinical trial in patients with refractory, advanced or metastatic solid tumours. Br. J. Cancer 2016, 114, 986–994. [Google Scholar] [CrossRef]
- Mahalingam, D.; Peguero, J.; Cen, P.; Arora, S.P.; Sarantopoulos, J.; Rowe, J.; Allgood, V.; Tubb, B.; Campos, L. A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers 2019, 11, 833. [Google Scholar] [CrossRef]
- Weitz, J.; Kienle, P.; Lacroix, J.; Willeke, F.; Benner, A.; Lehnert, T.; Herfarth, C.; von Knebel Doeberitz, M. Dissemination of tumor cells in patients undergoing surgery for colorectal cancer. Clin. Cancer Res. 1998, 4, 343–348. [Google Scholar]
- Polascik, T.J.; Wang, Z.-P.; Shue, M.; DI, S.; Gurganus, R.T.; Hortopan, S.C.; Ts’O, P.O.; Partin, A.W. Influence of sextant prostate needle biopsy or surgery on the detection and harvest of intact circulating prostate cancer cells. J. Urol. 1999, 162, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Boudot, C.; Abdoune, R.; Petit, L.; Brazier, M.; Mentaverri, R.; Kamel, S. Extracellular calcium promotes the migration of breast cancer cells through the activation of the calcium sensing receptor. Exp. Cell Res. 2009, 315, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Orduña-Castillo, L.B.; Eduardo del-Río-Robles, J.; García-Jiménez, I.; Zavala-Barrera, C.; Beltrán-Navarro, Y.M.; Hidalgo-Moyle, J.J.; Ramírez-Rangel, I.; Hernández-Bedolla, M.A.; Reyes-Ibarra, A.P.; Valadez-Sánchez, M.; et al. Calcium sensing receptor stimulates breast cancer cell migration via the Gβγ-AKT-mTORC2 signaling pathway. J. Cell Commun. Signal. 2022, 16, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Helfman, D.M. Loss of MLCK leads to disruption of cell-cell adhesion and invasive behavior of breast epithelial cells via increased expression of EGFR and ERK/JNK signaling. Oncogene 2016, 35, 4495–4508. [Google Scholar] [CrossRef]
- Lin, J.; Chen, L.; Chen, X.; Zang, S. MYLK promotes hepatocellular carcinoma progression through regulating cytoskeleton to enhance epithelial-mesenchymal transition. Clin. Exp. Med. 2018, 18, 523–533. [Google Scholar] [CrossRef]
- Connell, L.E.; Helfman, D.M. Myosin light chain kinase plays a role in the regulation of epithelial cell survival. J. Cell Sci. 2006, 119, 2269–2281. [Google Scholar] [CrossRef]
- Jackisch, C.; Hahm, H.A.; Tombal, B.; McCloskey, D.; Butash, K.; Davidson, N.E.; Denmeade, S.R. Delayed micromolar elevation in intracellular calcium precedes induction of apoptosis in thapsigargin-treated breast cancer cells. Clin. Cancer Res. 2000, 6, 2844–2850. [Google Scholar]
- Wang, C.; Li, T.; Tang, S.; Zhao, D.; Zhang, C.; Zhang, S.; Deng, S.; Zhou, Y.; Xiao, X. Thapsigargin induces apoptosis when autophagy is inhibited in HepG2 cells and both processes are regulated by ROS-dependent pathway. Environ. Toxicol. Pharmacol. 2016, 41, 167–179. [Google Scholar] [CrossRef]
- Gkountela, S.; Castro-Giner, F.; Szczerba, B.M.; Vetter, M.; Landin, J.; Scherrer, R.; Krol, I.; Scheidmann, M.C.; Beisel, C.; Stirnimann, C.U.; et al. Circulating Tumor Cell Clustering Shapes DNA Methylation to Enable Metastasis Seeding. Cell 2019, 176, 98–112.e14. [Google Scholar] [CrossRef]
- Aceto, N. Bring along your friends: Homotypic and heterotypic circulating tumor cell clustering to accelerate metastasis. Biomed. J. 2020, 43, 18–23. [Google Scholar] [CrossRef]
- Peralta, M.; Osmani, N.; Goetz, J.G. Circulating tumor cells: Towards mechanical phenotyping of metastasis. iScience 2022, 25, 103969. [Google Scholar] [CrossRef]
- Hansen, E.; Wolff, N.; Knuechel, R.; Ruschoff, J.; Hofstaedter, F.; Taeger, K. Tumor cells in blood shed from the surgical field. Arch. Surg. 1995, 130, 387–393. [Google Scholar] [CrossRef]
- Einbond, L.S.; Wu, H.-A.; Sandu, C.; Ford, M.; Mighty, J.; Antonetti, V.; Redenti, S.; Ma, H. Digitoxin enhances the growth inhibitory effects of thapsigargin and simvastatin on ER negative human breast cancer cells. Fitoterapia 2016, 109, 146–154. [Google Scholar] [CrossRef]
- Kusukawa, J.; Suefuji, Y.; Ryu, F.; Noguchi, R.; Iwamoto, O.; Kameyama, T. Dissemination of cancer cells into circulation occurs by incisional biopsy of oral squamous cell carcinoma. J. Oral Pathol. Med. 2000, 29, 303–307. [Google Scholar]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Karagiannis, G.S.; Pastoriza, J.M.; Wang, Y.; Harney, A.S.; Entenberg, D.; Pignatelli, J.; Sharma, V.P.; Xue, E.A.; Cheng, E.; D’Alfonso, T.M.; et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 2017, 9, eaan0026. [Google Scholar] [CrossRef]
- Yan, W.T.; Cui, X.; Chen, Q.; Li, Y.-F.; Cui, Y.-H.; Wang, Y.; Jiang, J. Circulating tumor cell status monitors the treatment responses in breast cancer patients: A meta-analysis. Sci. Rep. 2017, 7, 43464. [Google Scholar] [CrossRef]
- Regmi, S.; Fu, A.; Luo, K.Q. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci. Rep. 2017, 7, 39975. [Google Scholar] [CrossRef]
- Hope, J.M.; Bersi, M.R.; Dombroski, J.A.; Clinch, A.B.; Pereles, R.S.; Merryman, W.D.; King, M.R. Circulating prostate cancer cells have differential resistance to fluid shear stress-induced cell death. J. Cell Sci. 2021, 134, jcs251470. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.T.; Thompson, K.N.; Pratt, S.J.P.; Ju, J.A.; Lee, R.M.; Mathias, T.J.; Mull, M.L.; Annis, D.A.; Ory, E.C.; Stemberger, M.B.; et al. Elevation of Cytoplasmic Calcium Suppresses Microtentacle Formation and Function in Breast Tumor Cells. Cancers 2023, 15, 884. https://doi.org/10.3390/cancers15030884
Chang KT, Thompson KN, Pratt SJP, Ju JA, Lee RM, Mathias TJ, Mull ML, Annis DA, Ory EC, Stemberger MB, et al. Elevation of Cytoplasmic Calcium Suppresses Microtentacle Formation and Function in Breast Tumor Cells. Cancers. 2023; 15(3):884. https://doi.org/10.3390/cancers15030884
Chicago/Turabian StyleChang, Katarina T., Keyata N. Thompson, Stephen J. P. Pratt, Julia A. Ju, Rachel M. Lee, Trevor J. Mathias, Makenzy L. Mull, David A. Annis, Eleanor C. Ory, Megan B. Stemberger, and et al. 2023. "Elevation of Cytoplasmic Calcium Suppresses Microtentacle Formation and Function in Breast Tumor Cells" Cancers 15, no. 3: 884. https://doi.org/10.3390/cancers15030884
APA StyleChang, K. T., Thompson, K. N., Pratt, S. J. P., Ju, J. A., Lee, R. M., Mathias, T. J., Mull, M. L., Annis, D. A., Ory, E. C., Stemberger, M. B., Vitolo, M. I., & Martin, S. S. (2023). Elevation of Cytoplasmic Calcium Suppresses Microtentacle Formation and Function in Breast Tumor Cells. Cancers, 15(3), 884. https://doi.org/10.3390/cancers15030884






