Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas
Abstract
Simple Summary
Abstract
1. Introduction
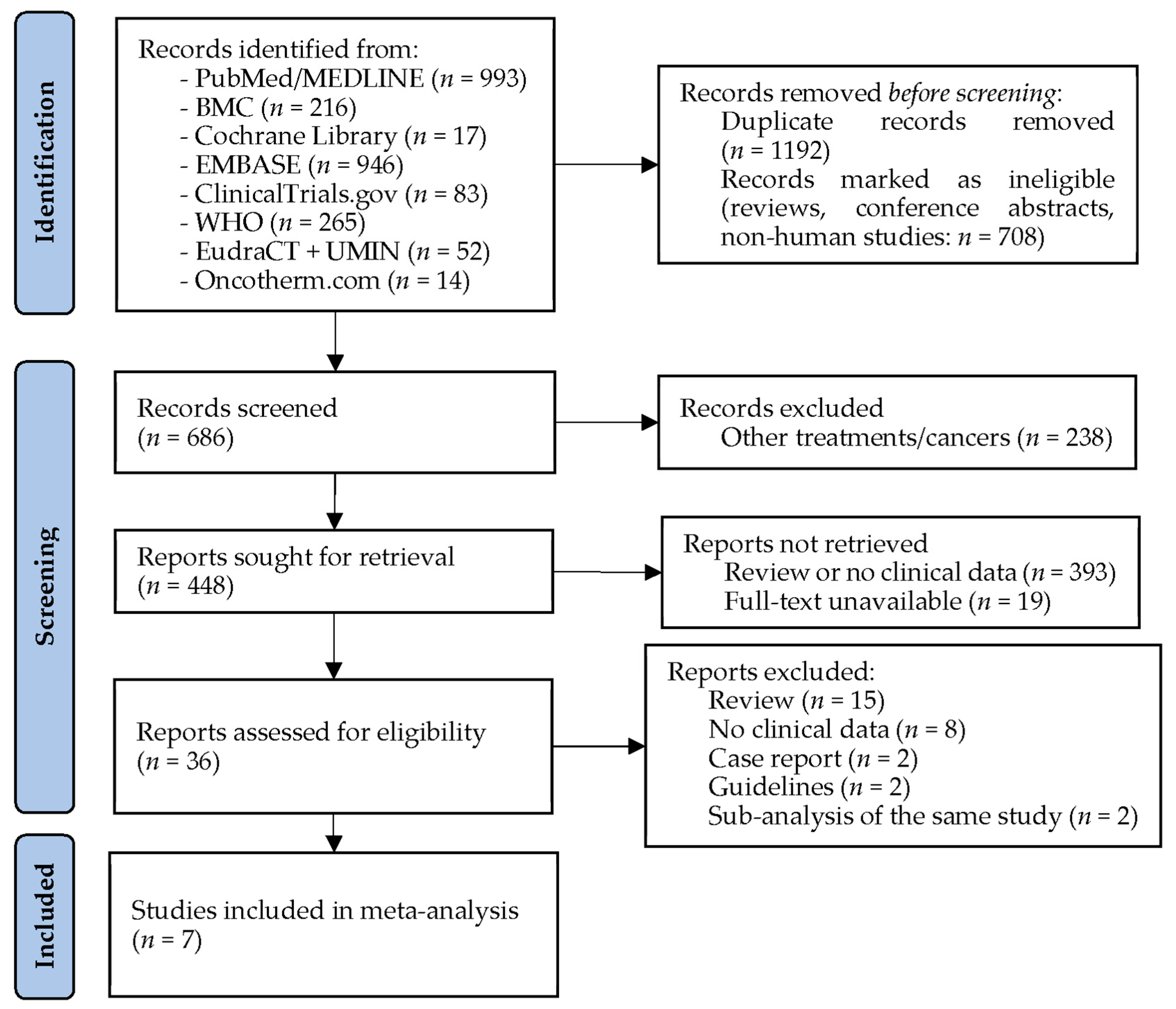
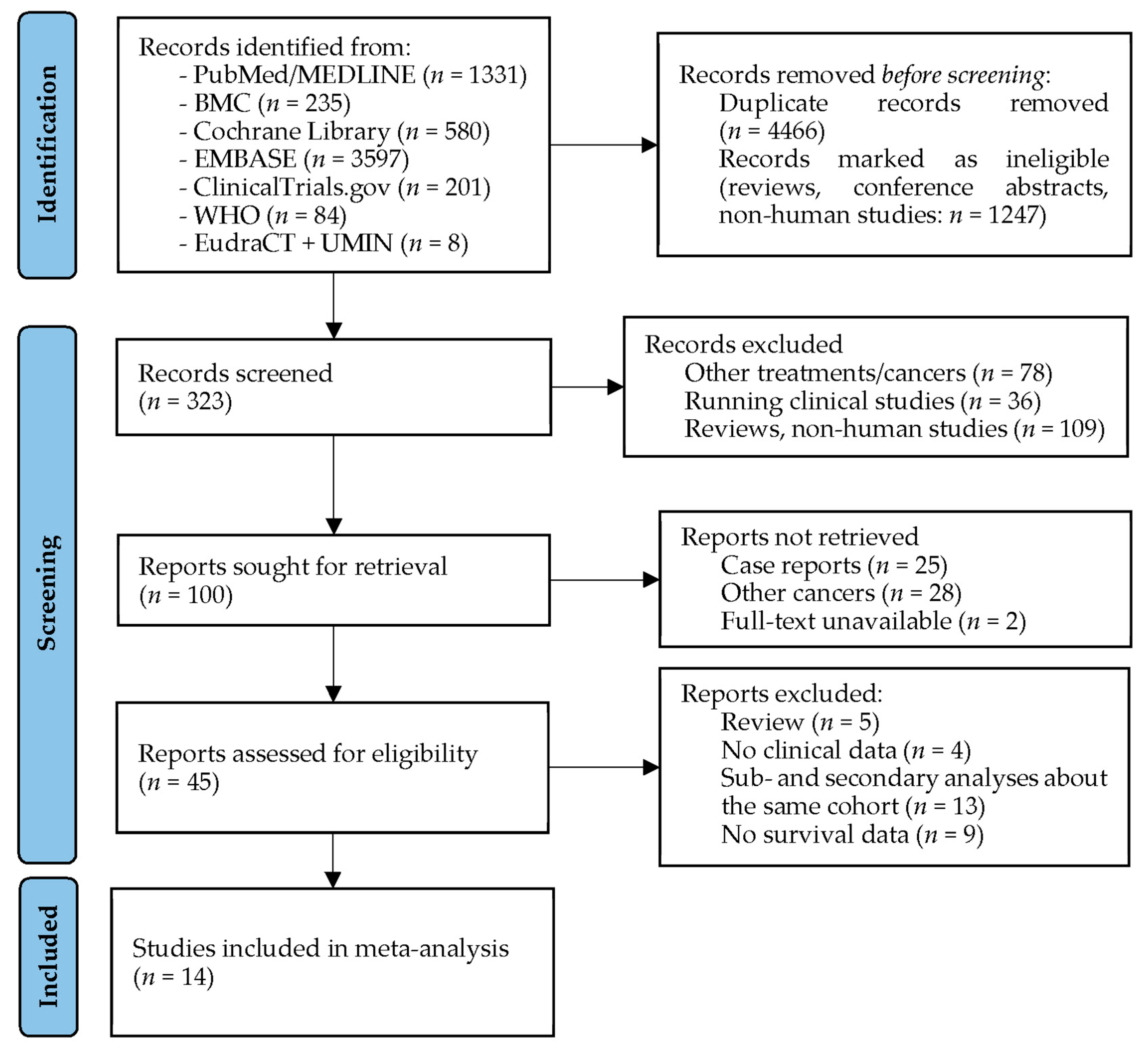
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Statistical Analysis
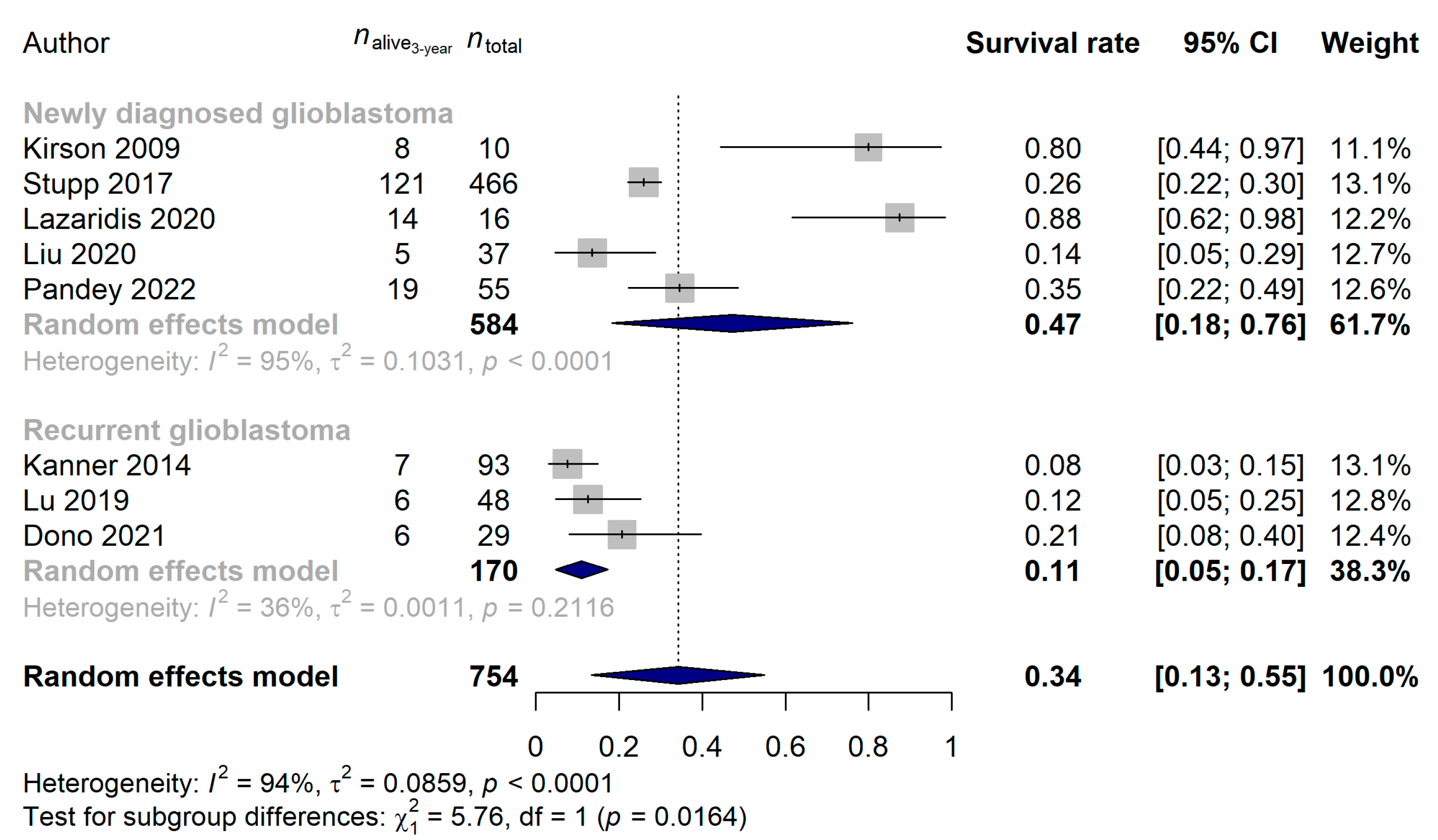
3. Results
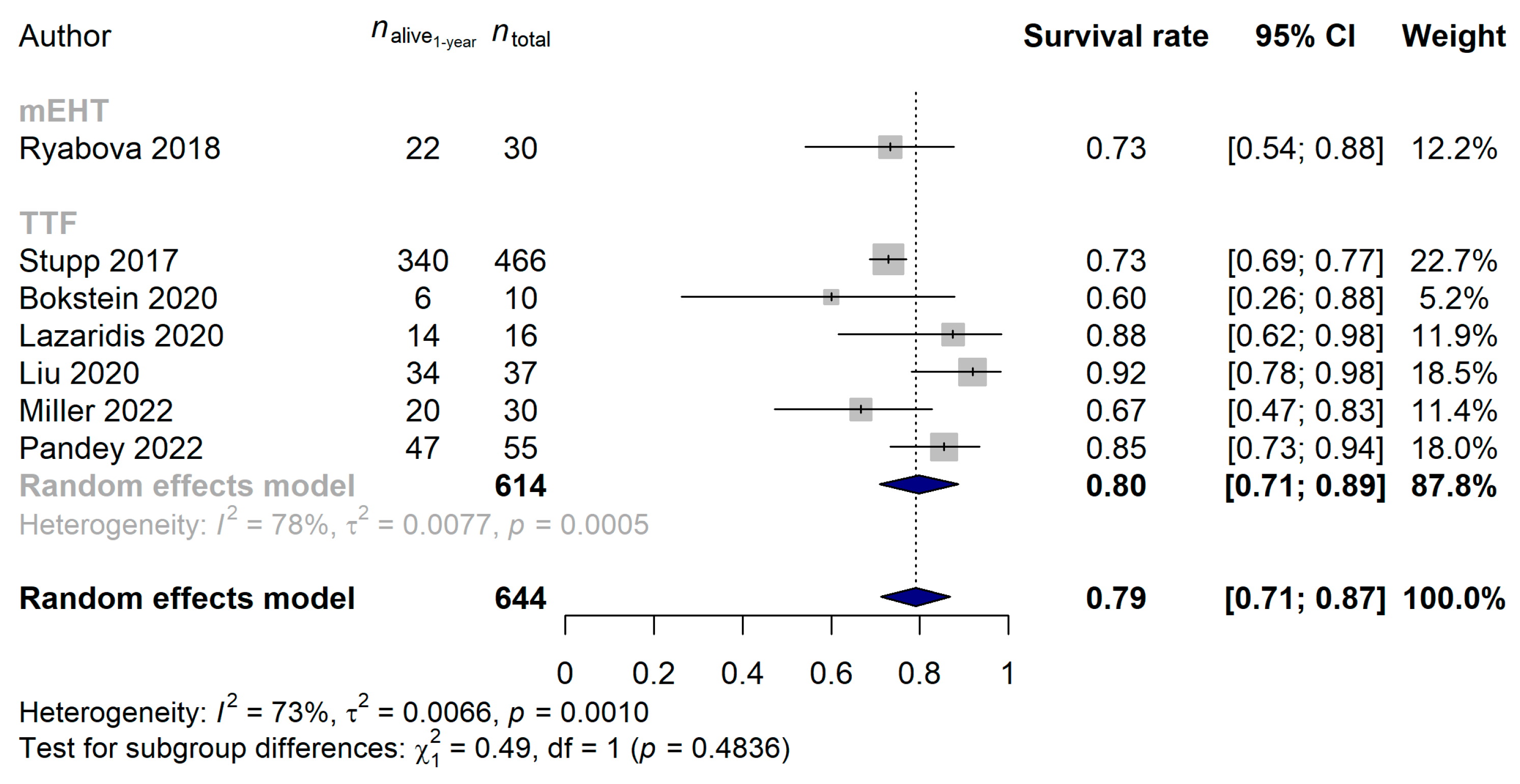
3.1. Studies Investigating Modulated Electro-Hyperthermia in Glioblastoma
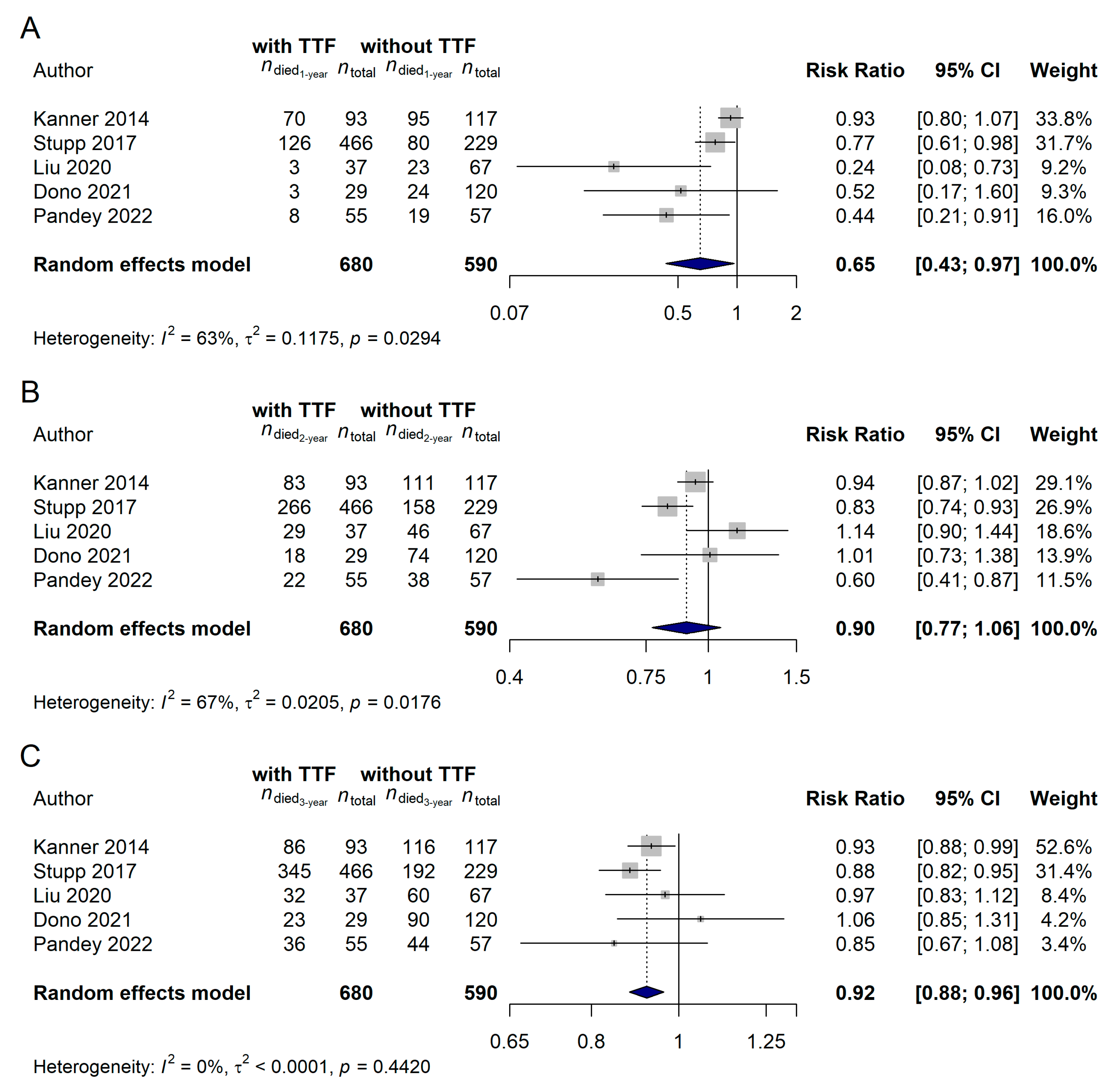
3.2. Studies Investigating Tumor Treating Flields in Glioblastoma
3.3. The Direct Comparison of Modulated Electro-Hyperthermia and Tumor Treating Flields Studies
4. Discussion
Strength and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today (accessed on 22 October 2022).
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee Sh, U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Mohile, N.A.; Messersmith, H.; Gatson, N.T.; Hottinger, A.F.; Lassman, A.; Morton, J.; Ney, D.; Nghiemphu, P.L.; Olar, A.; Olson, J.; et al. Therapy for Diffuse Astrocytic and Oligodendroglial Tumors in Adults: ASCO-SNO Guideline. J. Clin. Oncol. 2022, 40, 403–426. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Herrlinger, U.; Tzaridis, T.; Mack, F.; Steinbach, J.P.; Schlegel, U.; Sabel, M.; Hau, P.; Kortmann, R.D.; Krex, D.; Grauer, O.; et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): A randomised, open-label, phase 3 trial. Lancet 2019, 393, 678–688. [Google Scholar] [CrossRef]
- Delgado-Lopez, P.D.; Corrales-Garcia, E.M. Survival in glioblastoma: A review on the impact of treatment modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma Multiforme: An Overview of Emerging Therapeutic Targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef]
- Kanner, A.A.; Wong, E.T.; Villano, J.L.; Ram, Z.; on behalf of EF-11 Investigators. Post Hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A system versus best physician’s choice chemotherapy. Semin. Oncol. 2014, 41 (Suppl. S6), S25–S34. [Google Scholar] [CrossRef]
- Vymazal, J.; Wong, E.T. Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin. Oncol. 2014, 41 (Suppl. S6), S14–S24. [Google Scholar] [CrossRef]
- Ballo, M.T.; Urman, N.; Lavy-Shahaf, G.; Grewal, J.; Bomzon, Z.; Toms, S. Correlation of Tumor Treating Fields Dosimetry to Survival Outcomes in Newly Diagnosed Glioblastoma: A Large-Scale Numerical Simulation-Based Analysis of Data from the Phase 3 EF-14 Randomized Trial. Int. J. Radiat Oncol. Biol. Phys. 2019, 104, 1106–1113. [Google Scholar] [CrossRef]
- Toms, S.A.; Kim, C.Y.; Nicholas, G.; Ram, Z. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: A subgroup analysis of the EF-14 phase III trial. J. NeuroOncol. 2019, 141, 467–473. [Google Scholar] [CrossRef]
- Ram, Z.; Kim, C.Y.; Hottinger, A.F.; Idbaih, A.; Nicholas, G.; Zhu, J.J. Efficacy and Safety of Tumor Treating Fields (TTFields) in Elderly Patients with Newly Diagnosed Glioblastoma: Subgroup Analysis of the Phase 3 EF-14 Clinical Trial. Front. Oncol. 2021, 11, 671972. [Google Scholar] [CrossRef] [PubMed]
- Onken, J.; Staub-Bartelt, F.; Vajkoczy, P.; Misch, M. Acceptance and compliance of TTFields treatment among high grade glioma patients. J. NeuroOncol. 2018, 139, 177–184. [Google Scholar] [CrossRef]
- Pandey, M.; Xiu, J.; Mittal, S.; Zeng, J.; Saul, M.; Kesari, S.; Azadi, A.; Newton, H.; Deniz, K.; Ladner, K.; et al. Molecular alterations associated with improved outcome in patients with glioblastoma treated with Tumor-Treating Fields. NeuroOncol. Adv. 2022, 4, vdac096. [Google Scholar] [CrossRef]
- Mrugala, M.M.; Engelhard, H.H.; Dinh Tran, D.; Kew, Y.; Cavaliere, R.; Villano, J.L.; Annenelie Bota, D.; Rudnick, J.; Love Sumrall, A.; Zhu, J.J.; et al. Clinical practice experience with NovoTTF-100A system for glioblastoma: The Patient Registry Dataset (PRiDe). Semin. Oncol. 2014, 41 (Suppl. S6), S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Wismeth, C.; Dudel, C.; Pascher, C.; Ramm, P.; Pietsch, T.; Hirschmann, B.; Reinert, C.; Proescholdt, M.; Rummele, P.; Schuierer, G.; et al. Transcranial electro-hyperthermia combined with alkylating chemotherapy in patients with relapsed high-grade gliomas: Phase I clinical results. J. NeuroOncol. 2010, 98, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kirson, E.D.; Gurvich, Z.; Schneiderman, R.; Dekel, E.; Itzhaki, A.; Wasserman, Y.; Schatzberger, R.; Palti, Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004, 64, 3288–3295. [Google Scholar] [CrossRef] [PubMed]
- Blatt, R.; Davidi, S.; Munster, M.; Shteingauz, A.; Cahal, S.; Zeidan, A.; Marciano, T.; Bomzon, Z.; Haber, A.; Giladi, M.; et al. In Vivo Safety of Tumor Treating Fields (TTFields) Applied to the Torso. Front. Oncol. 2021, 11, 670809. [Google Scholar] [CrossRef]
- Kirson, E.D.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Soustiel, J.F.; Itzhaki, A.; Mordechovich, D.; Steinberg-Shapira, S.; Gurvich, Z.; Schneiderman, R.; et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. USA 2007, 104, 10152–10157. [Google Scholar] [CrossRef]
- Rominiyi, O.; Vanderlinden, A.; Clenton, S.J.; Bridgewater, C.; Al-Tamimi, Y.; Collis, S.J. Tumour treating fields therapy for glioblastoma: Current advances and future directions. Br. J. Cancer 2021, 124, 697–709. [Google Scholar] [CrossRef]
- Chang, E.; Patel, C.B.; Pohling, C.; Young, C.; Song, J.; Flores, T.A.; Zeng, Y.; Joubert, L.M.; Arami, H.; Natarajan, A.; et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018, 4, 113. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.; Szasz, N.; Szasz, O. Oncothermia: Principles and Practices; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Szasz, O.; Szasz, A. Heating, Efficacy and Dose of Local Hyperthermia. Open J. Biophys. 2016, 6, 10–18. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef] [PubMed]
- Alshaibi, H.F.; Al-Shehri, B.; Hassan, B.; Al-Zahrani, R.; Assiss, T. Modulated Electrohyperthermia: A New Hope for Cancer Patients. BioMed. Res. Int. 2020, 2020, 8814878. [Google Scholar] [CrossRef]
- Andocs, G.; Rehman, M.U.; Zhao, Q.L.; Tabuchi, Y.; Kanamori, M.; Kondo, T. Comparison of biological effects of modulated electro-hyperthermia and conventional heat treatment in human lymphoma U937 cells. Cell Death Discov. 2016, 2, 16039. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.M.; Minnaar, C.A.; Szentmartoni, G.; Szigeti, G.P.; Dank, M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front. Oncol. 2019, 9, 1012. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, S.; Rucker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid Based Ment Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Viechtbauer, W. Bias and Efficiency of Meta-Analytic Variance Estimators in the Random-Effects Model. J. Educ. Behav. Stat. 2005, 30, 261–293. [Google Scholar] [CrossRef]
- Harrer, M.; Cuijpers, P.; Furukawa, T.A.; Ebert, D.D. Doing Meta-Analysis with R: A Hands-On Guide, 1st ed.; Chapman & Hall/CRC Press: Boca Raton, FL, USA; London, UK, 2021. [Google Scholar]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Robins, J.; Greenland, S.; Breslow, N.E. A general estimator for the variance of the Mantel-Haenszel odds ratio. Am. J. Epidemiol. 1986, 124, 719–723. [Google Scholar] [CrossRef]
- Sahinbas, H.; Grönemeyer, D.H.W.; Böcher, E.; Szasz, A. Retrospective clinical study of adjuvant electro-hyperthermia treatment for advanced brain-gliomas. Dtsch. Z. Für Onkol. 2007, 39, 154–160. [Google Scholar] [CrossRef]
- Roussakow, S.V. Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: A retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis. BMJ Open 2017, 7, e017387. [Google Scholar] [CrossRef] [PubMed]
- Fiorentini, G.; Sarti, D.; Milandri, C.; Dentico, P.; Mambrini, A.; Guadagni, S. Retrospective observational Clinical Study on Relapsed Malignant Gliomas Treated with Electro-hyperthermia. Oncothermia J. 2018, 22, 32–45. [Google Scholar]
- Fiorentini, G.; Sarti, D.; Milandri, C.; Dentico, P.; Mambrini, A.; Fiorentini, C.; Mattioli, G.; Casadei, V.; Guadagni, S. Modulated Electrohyperthermia in Integrative Cancer Treatment for Relapsed Malignant Glioblastoma and Astrocytoma: Retrospective Multicenter Controlled Study. Integr. Cancer 2019, 18, 1534735418812691. [Google Scholar] [CrossRef]
- Douwes, F.; Douwes, O.; Migeod, F.; Grote, C.; Bogovic, J. Hyperthermia in Combination with ACNU Chemotherapy in theTreatment of Recurrent Glioblastoma. Available online: https://www.klinik-st-georg.de/wp-content/downloads/Professional-Articles/hyperthermia_in_combination_with_ACNU_chemotherapy_in_the_treatment_of_recurrent_glioblastoma.pdf (accessed on 1 July 2022).
- Fiorentini, G.; Giovanis, P.; Rossi, S.; Dentico, P.; Paola, R.; Turrisi, G.; Bernardeschi, P. A phase II clinical study on relapsed malignant gliomas treated with electro-hyperthermia. Vivo 2006, 20, 721–724. [Google Scholar]
- Hager, E.D.; Sahinbas, H.; Groenemeyer, D.H.; Migeod, F. Prospective phase II trial for recurrent high-grade gliomas with capacitive coupled low radiofrequency (LRF) hyperthermia. J. Clin. Oncol. 2008, 26, 2047. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.H.; Oh, Y.T.; Chun, M.; Noh, O.K. Concurrent hyperthermia and re-irradiation for recurrent high-grade gliomas. Neoplasma 2017, 64, 803–808. [Google Scholar] [CrossRef]
- Ryabova, A.I.; Novikov, V.A.; Gribova, O.V.; Choynzonov, E.L.; Startseva, Z.A.; Grigoryev, E.G.; Miloichikova, I.A.; Turgunova, N.D.; Surkova, P.V. Concurrent Thermochemoradiotherapy in Glioblastoma Treatment: Preliminary Results. In Glioma; Ibrahim, O., Kenan, A., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Song, A.; Bar-Ad, V.; Martinez, N.; Glass, J.; Andrews, D.W.; Judy, K.; Evans, J.J.; Farrell, C.J.; Werner-Wasik, M.; Chervoneva, I.; et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J. NeuroOncol. 2020, 147, 653–661. [Google Scholar] [CrossRef]
- Miller, R.; Song, A.; Ali, A.; Niazi, M.; Bar-Ad, V.; Martinez, N.; Glass, J.; Alnahhas, I.; Andrews, D.; Judy, K.; et al. Scalp-Sparing Radiation With Concurrent Temozolomide and Tumor Treating Fields (SPARE) for Patients With Newly Diagnosed Glioblastoma. Front. Oncol. 2022, 12, 896246. [Google Scholar] [CrossRef]
- Stupp, R.; Wong, E.T.; Kanner, A.A.; Steinberg, D.; Engelhard, H.; Heidecke, V.; Kirson, E.D.; Taillibert, S.; Liebermann, F.; Dbaly, V.; et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur. J. Cancer 2012, 48, 2192–2202. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Lok, E.; Swanson, K.D.; Gautam, S.; Engelhard, H.H.; Lieberman, F.; Taillibert, S.; Ram, Z.; Villano, J.L. Response assessment of NovoTTF-100A versus best physician’s choice chemotherapy in recurrent glioblastoma. Cancer Med. 2014, 3, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Lok, E.; Gautam, S.; Swanson, K.D. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br. J. Cancer 2015, 113, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Kesari, S.; Ram, Z.; on behalf of EF-14 Trial Investigators. Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: A post hoc analysis of the EF-14 trial. CNS Oncol. 2017, 6, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Demireva, P.; Kanner, A.A.; Pannullo, S.; Mehdorn, M.; Avgeropoulos, N.; Salmaggi, A.; Silvani, A.; David, C.; et al.; on behalf of the EF-14 Trial Investigators Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J. NeuroOncol. 2017, 135, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Taphoorn, M.J.B.; Dirven, L.; Kanner, A.A.; Lavy-Shahaf, G.; Weinberg, U.; Taillibert, S.; Toms, S.A.; Honnorat, J.; Chen, T.C.; Sroubek, J.; et al. Influence of Treatment With Tumor-Treating Fields on Health-Related Quality of Life of Patients With Newly Diagnosed Glioblastoma: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2018, 4, 495–504. [Google Scholar] [CrossRef]
- Kim, C.Y.; Paek, S.H.; Nam, D.H.; Chang, J.H.; Hong, Y.K.; Kim, J.H.; Kim, O.L.; Kim, S.H. Tumor treating fields plus temozolomide for newly diagnosed glioblastoma: A sub-group analysis of Korean patients in the EF-14 phase 3 trial. J. NeuroOncol. 2020, 146, 399–406. [Google Scholar] [CrossRef]
- Kirson, E.D.; Schneiderman, R.S.; Dbaly, V.; Tovarys, F.; Vymazal, J.; Itzhaki, A.; Mordechovich, D.; Gurvich, Z.; Shmueli, E.; Goldsher, D.; et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 2009, 9, 1. [Google Scholar] [CrossRef]
- Wong, E.T.; Lok, E.; Swanson, K.D. Clinical benefit in recurrent glioblastoma from adjuvant NovoTTF-100A and TCCC after temozolomide and bevacizumab failure: A preliminary observation. Cancer Med. 2015, 4, 383–391. [Google Scholar] [CrossRef]
- Lu, G.; Rao, M.; Zhu, P.; Liang, B.; El-Nazer, R.T.; Fonkem, E.; Bhattacharjee, M.B.; Zhu, J.J. Triple-drug Therapy With Bevacizumab, Irinotecan, and Temozolomide Plus Tumor Treating Fields for Recurrent Glioblastoma: A Retrospective Study. Front. Neurol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Bokstein, F.; Blumenthal, D.; Limon, D.; Harosh, C.B.; Ram, Z.; Grossman, R. Concurrent Tumor Treating Fields (TTFields) and Radiation Therapy for Newly Diagnosed Glioblastoma: A Prospective Safety and Feasibility Study. Front. Oncol. 2020, 10, 411. [Google Scholar] [CrossRef]
- Korshoej, A.R.; Lukacova, S.; Lassen-Ramshad, Y.; Rahbek, C.; Severinsen, K.E.; Guldberg, T.L.; Mikic, N.; Jensen, M.H.; Cortnum, S.O.S.; von Oettingen, G.; et al. OptimalTTF-1: Enhancing tumor treating fields therapy with skull remodeling surgery. A clinical phase I trial in adult recurrent glioblastoma. NeuroOncol. Adv. 2020, 2, vdaa121. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, L.; Schafer, N.; Teuber-Hanselmann, S.; Blau, T.; Schmidt, T.; Oster, C.; Weller, J.; Tzaridis, T.; Pierscianek, D.; Keyvani, K.; et al. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J. Cancer Res. Clin. Oncol. 2020, 146, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Strawderman, M.S.; Warren, K.T.; Richardson, M.; Serventi, J.N.; Mohile, N.A.; Milano, M.T.; Walter, K.A. Clinical Efficacy of Tumor Treating Fields for Newly Diagnosed Glioblastoma. Anticancer Res. 2020, 40, 5801–5806. [Google Scholar] [CrossRef]
- Dono, A.; Mitra, S.; Shah, M.; Takayasu, T.; Zhu, J.J.; Tandon, N.; Patel, C.B.; Esquenazi, Y.; Ballester, L.Y. PTEN mutations predict benefit from tumor treating fields (TTFields) therapy in patients with recurrent glioblastoma. J. NeuroOncol. 2021, 153, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Poon, M.T.C.; Sudlow, C.L.M.; Figueroa, J.D.; Brennan, P.M. Longer-term (>/= 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 11622. [Google Scholar] [CrossRef] [PubMed]
- Oronsky, B.; Reid, T.R.; Oronsky, A.; Sandhu, N.; Knox, S.J. A Review of Newly Diagnosed Glioblastoma. Front. Oncol. 2020, 10, 574012. [Google Scholar] [CrossRef]
- Stupp, R.; Brada, M.; van den Bent, M.J.; Tonn, J.C.; Pentheroudakis, G. ESMO Guidelines Working Group. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. S3), iii93–iii101. [Google Scholar] [CrossRef]
- Raposo, C.; Vitorino-Araujo, J.L.; Barreto, N. Molecular Markers of Gliomas to Predict Treatment and Prognosis: Current State and Future Directions. In Gliomas; Debinski, W., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fiorentini, G.; Szasz, A.M.; Szigeti, G.; Szasz, A.; Minnaar, C.A. Quo Vadis Oncological Hyperthermia (2020)? Front. Oncol. 2020, 10, 1690. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Talukdar, R.; Kannan, S.; Dasgupta, A.; Chatterjee, A.; Patil, V. Efficacy and safety of extended adjuvant temozolomide compared to standard adjuvant temozolomide in glioblastoma: Updated systematic review and meta-analysis. NeuroOncol. Pr. 2022, 9, 354–363. [Google Scholar] [CrossRef]
- Lacouture, M.E.; Davis, M.E.; Elzinga, G.; Butowski, N.; Tran, D.; Villano, J.L.; DiMeglio, L.; Davies, A.M.; Wong, E.T. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin. Oncol. 2014, 41 (Suppl. S4), S1–S14. [Google Scholar] [CrossRef]
- Krigers, A.; Pinggera, D.; Demetz, M.; Kornberger, L.M.; Kerschbaumer, J.; Thome, C.; Freyschlag, C.F. The Routine Application of Tumor-Treating Fields in the Treatment of Glioblastoma WHO degrees IV. Front. Neurol. 2022, 13, 900377. [Google Scholar] [CrossRef]
- Regev, O.; Merkin, V.; Blumenthal, D.T.; Melamed, I.; Kaisman-Elbaz, T. Tumor-Treating Fields for the treatment of glioblastoma: A systematic review and meta-analysis. NeuroOncol. Pr. 2021, 8, 426–440. [Google Scholar] [CrossRef]
- Li, X.; Jia, Z.; Yan, Y. Efficacy and safety of tumor-treating fields in recurrent glioblastoma: A systematic review and meta-analysis. Acta Neurochir. 2022, 164, 1985–1993. [Google Scholar] [CrossRef] [PubMed]
- Dongpo, S.; Zhengyao, Z.; Xiaozhuo, L.; Qing, W.; Mingming, F.; Fengqun, M.; Mei, L.; Qian, H.; Tong, C. Efficacy and Safety of Bevacizumab Combined with Other Therapeutic Regimens for Treatment of Recurrent Glioblastoma: A Network Meta-analysis. World Neurosurg. 2022, 160, e61–e79. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yang, X.; Wu, J.; Yang, H.; Li, Y.; Li, J.; Liu, Q.; Wu, C.; Xing, H.; Liu, P.; et al. Tumor-Treating Fields in Glioblastomas: Past, Present, and Future. Cancers 2022, 14, 3669. [Google Scholar] [CrossRef]
- Jin, L.; Guo, S.; Zhang, X.; Mo, Y.; Ke, S.; Duan, C. Optimal treatment strategy for adult patients with newly diagnosed glioblastoma: A systematic review and network meta-analysis. Neurosurg. Rev. 2021, 44, 1943–1955. [Google Scholar] [CrossRef]
- Michiels, S.; Piedbois, P.; Burdett, S.; Syz, N.; Stewart, L.; Pignon, J.P. Meta-analysis when only the median survival times are known: A comparison with individual patient data results. Int. J. Technol. Assess Health Care 2005, 21, 119–125. [Google Scholar] [CrossRef]

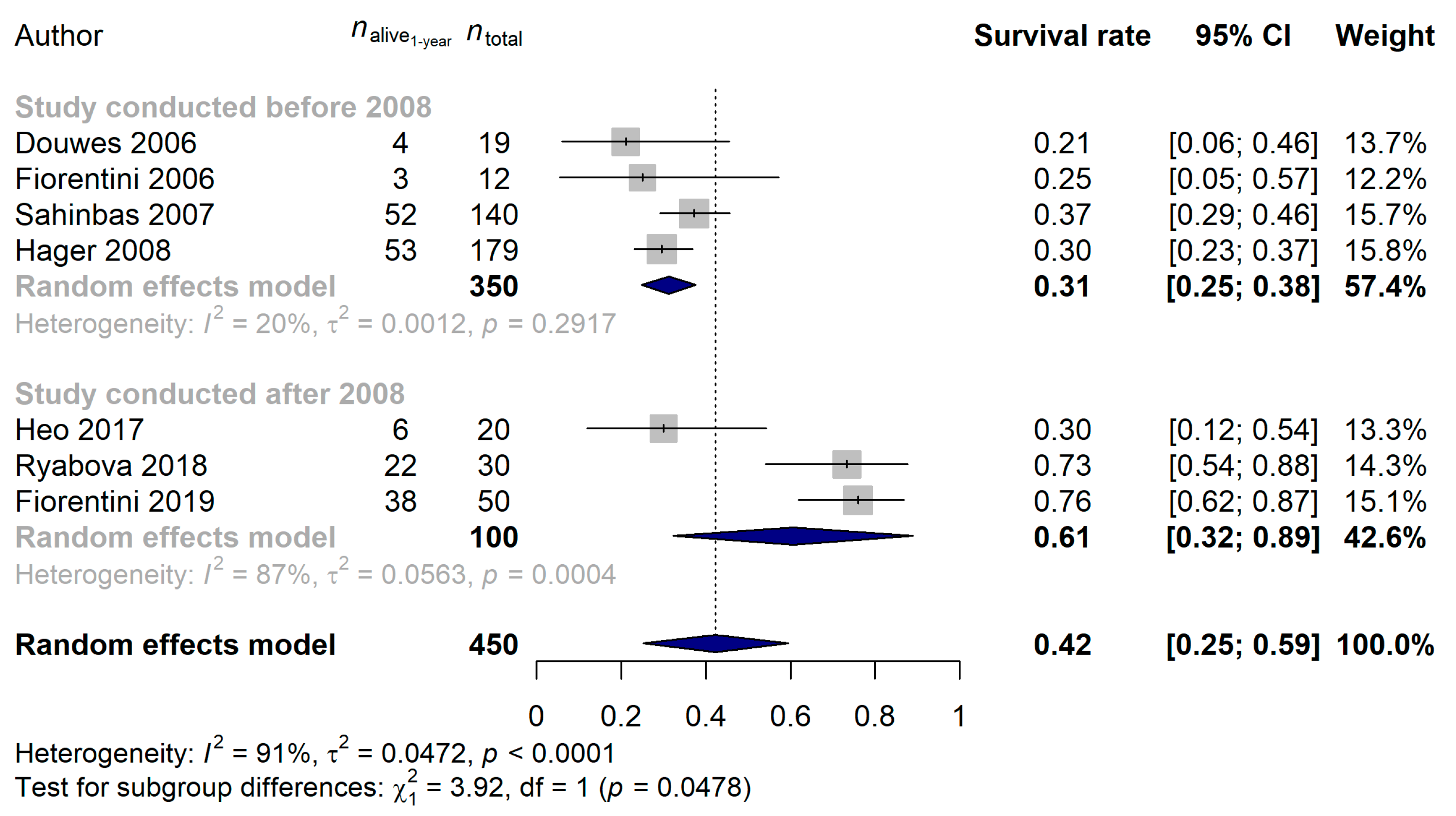
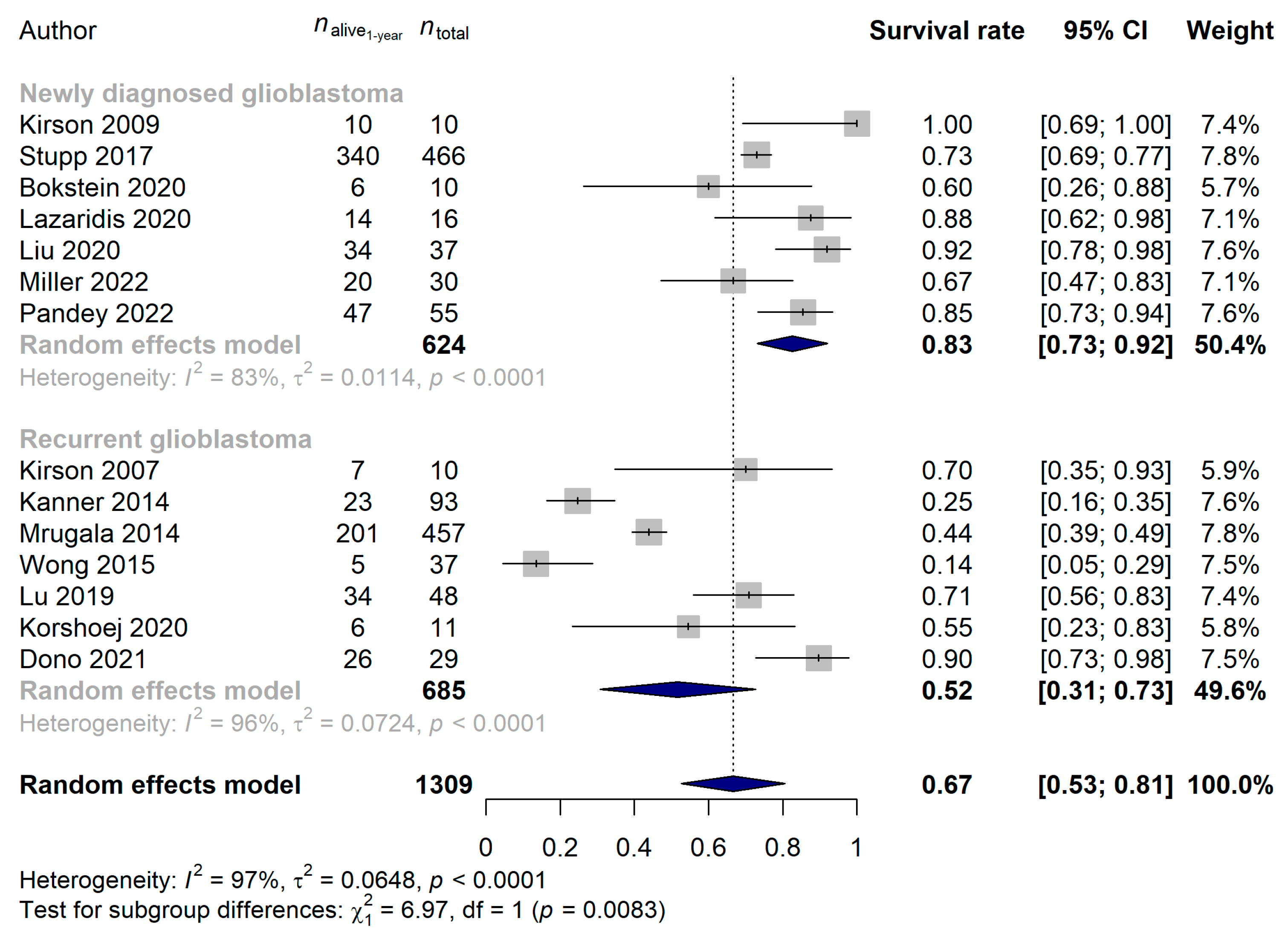


| Author (Year) | Type of Study | Cases (n) | mEHT Device | Additional Therapy | Age (Median) | Females |
|---|---|---|---|---|---|---|
| Douwes et al. (2006) [47] | Prospective | 19 | Oncotherm EHY2000 | nimustine | 55 | – 1 |
| Fiorentini et al. (2006) [48] | Prospective | 12 | Oncotherm EHY2000 | – 1 | – 1 | – 1 |
| Sahinbas et al. (2007) [43] | Retrospective | 140 | Oncotherm EHY2000 | temozolomide and/or herbal medicines and/or irradiation | 44 | 35.7% |
| Hager et al. (2008) [49] | Prospective | 179 | LRF-DHT | – 1 | – 1 | – 1 |
| Heo et al. (2017) [50] | Prospective | 20 | Celsius 42+ | re-irradiation | 56 | 60% |
| Ryabova et al. (2018) [51] | Prospective | 30 | Celsius 42+ | temozolomide + irradiation | 56 | 36.7% |
| Fiorentini et al. (2019) [46] | Retrospective | 50 | Oncotherm EHY2000 | no | 55 | – 1 |
| Author (Year) | Type of Study | Cases (n) | Controls (n) | Additional Therapy | Age (Median) | Females |
|---|---|---|---|---|---|---|
| Kirson et al. (2007) [26] | Prospective | 10 | – | temozolomide | – 1 | – 1 |
| Kirson et al. (2009) [63] | Prospective | 10 | – | temozolomide | – 1 | – 1 |
| Kanner et al. (2014) [15] | RCT | 93 | 117 | no | 54 | – 1 |
| Mrugala et al. (2014) [22] | Prospective | 457 | – | no restriction on any combination therapies, but not detailed | 55 | 32.4% |
| Wong et al. (2015) [64] | Retrospective | 37 | – | bevacizumab with or without 6-thioguanine, lomustine, capecitabine, and celecoxib (TCCC) | 57 | 37.8% |
| Stupp et al. (2017) [57] | RCT | 466 | 229 | temozolomide | 56 | 32.2% |
| Lu et al. (2019) [65] | Retrospective | 48 | – | temozolomide + bevacizumab + irinotecan or bevacizumab-based chemo regimen | 55 | 33.3% |
| Bokstein et al. (2020) [66] | Prospective | 10 | – | temozolomide + irradiation | 60 | 20% |
| Korshoej et al. (2020) [67] | Prospective | 11 | – | chemotherapy after skull remodeling surgery | 57 | 18.2% |
| Lazaridis et al. (2020) [68] | Retrospective | 16 | – | lomustine + temozolomide | 50 | 43.8% |
| Liu et al. (2020) [69] | Retrospective | 37 | 67 | temozolomide + irradiation | 61 | 37.8% |
| Dono et al. (2021) [70] | Retrospective | 29 | 120 | temozolomide + irradiation | 58 | 34.5% |
| Miller et al. (2022) [53] | Prospective | 30 | – | temozolomide + irradiation | 58 | 33.3% |
| Pandey et al. (2022) [21] | Retrospective | 55 | 57 | temozolomide | 59 | 30.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szasz, A.M.; Arrojo Alvarez, E.E.; Fiorentini, G.; Herold, M.; Herold, Z.; Sarti, D.; Dank, M. Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas. Cancers 2023, 15, 880. https://doi.org/10.3390/cancers15030880
Szasz AM, Arrojo Alvarez EE, Fiorentini G, Herold M, Herold Z, Sarti D, Dank M. Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas. Cancers. 2023; 15(3):880. https://doi.org/10.3390/cancers15030880
Chicago/Turabian StyleSzasz, Attila Marcell, Elisabeth Estefanía Arrojo Alvarez, Giammaria Fiorentini, Magdolna Herold, Zoltan Herold, Donatella Sarti, and Magdolna Dank. 2023. "Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas" Cancers 15, no. 3: 880. https://doi.org/10.3390/cancers15030880
APA StyleSzasz, A. M., Arrojo Alvarez, E. E., Fiorentini, G., Herold, M., Herold, Z., Sarti, D., & Dank, M. (2023). Meta-Analysis of Modulated Electro-Hyperthermia and Tumor Treating Fields in the Treatment of Glioblastomas. Cancers, 15(3), 880. https://doi.org/10.3390/cancers15030880





