Simple Summary
The implementation of immunotherapy in the therapeutic landscape of colorectal carcinoma has been difficult due to the inefficacy reported in early clinical trials. Nevertheless, a small subset of tumors deficient in DNA mismatch repair proteins show excellent responses to immune checkpoint inhibitors, with long-lasting results. Most of our patients do not have these alterations and, therefore, immunotherapy appears not to be effective. In the current review, we attempt to describe the main mechanisms of resistance to immunotherapy presented by these tumors, how to cope with them, and the potential use of other biomarkers of response to immunotherapy.
Abstract
Colorectal cancer (CRC) is the third most frequent cancer and the second most common cause of cancer-related death in Europe. High microsatellite instability (MSI-H) due to a deficient DNA mismatch repair (dMMR) system can be found in 5% of metastatic CRC (mCRC) and has been established as a biomarker of response to immunotherapy in these tumors. Therefore, immune checkpoint inhibitors (ICIs) in mCRC with these characteristics were evaluated with results showing remarkable response rates and durations of response. The majority of mCRC cases have high levels of DNA mismatch repair proteins (pMMR) with consequent microsatellite stability or low instability (MSS or MSI-low), associated with an inherent resistance to ICIs. This review aims to provide a comprehensive analysis of the possible approaches to overcome the mechanisms of resistance and evaluates potential biomarkers to establish the role of ICIs in pMMR/MSS/MSI-L (MSS) mCRC.
1. Introduction
According to the latest GLOBOCAN data, colorectal cancer (CRC) is the third most frequent cancer in terms of incidence rate in adults and the second most common cause of cancer-related deaths [1,2,3,4]. Approximately 25% of CRC patients are metastatic at diagnosis, and almost half of the patients with early-stage CRC will develop metastatic disease throughout their life.
Most patients with metastatic colorectal cancer (mCRC) have an incurable disease, and treatment is based on systemic therapy with a palliative purpose. The prognosis for patients with mCRC remains poor, with an overall survival (OS) of approximately 30 months (m) [5].
Several target molecular biomarkers have changed the landscape of treatment of mCRC. KRAS and NRAS mutations are associated with primary resistance to anti-EGFR therapies, so cetuximab and panitumumab are only indicated for RAS wild-type mCRC. Other targeted therapies for mCRC that have the approval of the regulatory agencies are encorafenib and cetuximab in v-raf murine sarcoma viral oncogene homolog B1 (BRAF) V600E mutations, and larotrectinib or entrectinib in NTRK fusions [6].
Immune checkpoint inhibitors (ICI) have changed the prognosis of several cancers, leading to improvements in survival along with remarkable responses [7]. In mCRC, ICIs have demonstrated their efficacy for the treatment of tumors that are mismatch-repair-deficient (dMMR) with high levels of microsatellite instability (MSI-H) (termed dMMR/MSI-H or MSI tumors) [8,9]. By contrast, ICIs are ineffective in tumors that are mismatch-repair-proficient (pMMR) and are microsatellite-stable (MSS) or have low levels of microsatellite instability (MSI-L) (termed pMMR/MSS/MSI-L or pMMR/non-MSI-H tumors, also known as MSS) [8]. However, fewer than 5% of all mCRCs are MSI, with higher rates presented in earlier stages [10]. Therefore, combinations of chemotherapeutic (CT) agents with anti-vascular endothelial growth factor (VEGF) or anti-epidermal growth factor receptor (EGFR), depending on the RAS status, are still the standard treatment on first and successive lines in MSS mCRC [6,11]. For tumors with BRAFV600E mutation, the combination of encorafenib with cetuximab has been proved successful. [12,13].
Overall, this article aims to analyze the possible mechanisms of resistance to immunotherapy presented by MSS mCRC and how to overcome them. Additionally, we describe potential biomarkers of response to ICIs for consideration in this subgroup of patients.
2. Current Landscape of ICIs in MSI mCRC
2.1. Rationale for the Use of ICIs
As mentioned before, mCRC is subclassified into two groups based on mutation patterns and ability to repair DNA microsatellite damage: tumors with an MSI signature that generally has a higher mutation burden (>12 mutations per 106 DNA bases) and tumors with an MSS signature commonly expressing a lower mutation burden (<8.24 mutations per 106 DNA bases) [14].
The development of MSI tumors is a sporadic event in 70–85% of patients with mCRC, but MSI-H is the hallmark of tumors in patients with Lynch syndrome. Nowadays, MMR testing is compulsory because the presence of MSI has prognostic, predictive, and therapeutic implications, but represents only 5% of all mCRC and 10–15% of early-stage CRC [6,11]. Testing for MMR includes a multiplex polymerase chain reaction (PCR) assay, the “Bethesda Panel”, or a multiplex immunohistochemistry (IHC) assay, in order to demonstrate the absence of one of four MMR enzymes (MLH1, MSH2, MSH6, and PMS2). [15].
The deficiency of DNA MMR proteins results in an accumulation of mutations due to the absence of reparation of insertions and deletions. This is especially remarkable in the microsatellite regions of DNA, leading to the hypermutated phenotype and known microsatellite instability [16]. The tumor microenvironment (TME) of MSI CRC tumors includes a higher number of tumor-infiltrating lymphocytes (TILs), including T helper 1 (Th1) CD4+ and cytotoxic CD8+ T cells, as well as antigen-presenting cells (APCs). The number of T cells included is typically high and, in some cases, larger than the number of tumoral cells, and there is an upregulation of the expression of immune inhibitory ligands and receptors including CTLA-4, PD-1, PD-L1, LAG-3, and IDO [17]. The high mutational burden and immune infiltration produce a unique phenotype of MSI tumors with higher immunogenicity than MSS tumors, supporting the response to ICIs [18,19].
2.2. Current Treatments Approved
Several clinical trials have investigated the therapeutic role of PD-1 inhibitors in MSI mCRC. Table 1 summarizes FDA-approved clinical trials that have evaluated ICIs in MSI mCRC.

Table 1.
Clinical trials that evaluated ICIs in MSI mCRC and got the approval from FDA/EMA.
The phase II trial KEYNOTE-016 tested pembrolizumab, an antiPD-1, in three cohorts of patients: MSI mCRC, MSS mCRC, and MSI non-mCRC. The objective response rate (ORR) was 50% in MSI mCRC and 0% in MSS mCRC. The disease control rate (DCR) was 89% in the first group and decreased to 14% in MSS mCRC. The progression-free survival (PFS) and overall survival (OS) rates at 24 m were 61% and 66% in MSI mCRC, respectively [20]. Based on the results of this trial, in 2017 the Food and Drug Administration (FDA) and European Medicines Agency (EMA) approved pembrolizumab for patients with MSI mCRC after prior treatment with CT agents.
The phase III KEYNOTE-177 trial compared pembrolizumab to standard treatment of mCRC (5-fluorouracil-based CT with or without bevacizumab (anti-VEGF) or anti-EGFR) as the first line of treatment in MSI mCRC. After a median follow-up of 32.4 m, PFS was 16.5 m in patients treated with pembrolizumab compared with 8.2 m in patients receiving standard treatment (hazard ratio (HR):0.6; p = 0.0002). An ORR of 43.8% was observed in the pembrolizumab arm, higher than the ORR of 33.1% of the standard treatment arm. Amongst the patients who experienced response in the pembrolizumab group, 83% had an ongoing response at 24 m [21].
The final analysis of KEYNOTE-177 showed a median PFS (mPFS) of 16.5 m vs. 8.2 m (HR = 0.59) with an ORR of 45.1% vs. 33.1% between the pembrolizumab and CT groups, respectively. Although the OS was better in patients treated with pembrolizumab, the improvement did not reach statistical significance, probably due to the 60% crossover rate reported in the intention-to-treat (ITT) population [23].
Based on the results of the KEYNOTE-177 trial, in June 2020 and December 2020 FDA and EMA respectively approved pembrolizumab as first-line treatment for patients with MSI mCRC. According to the results of KEYNOTE-158 trial, pembrolizumab has an agnostic indication for the treatment of refractory patients with unresectable or metastatic solid tumors with high mutational burden (TMB-H) [≥10 mutations per megabase (mut/Mb)] [24].
The CheckMate 142 phase II trial evaluated nivolumab (antiPD-1) and/or ipilimumab (antiCTLA-4) in pre-treated and treatment-naïve MSI mCRC patients. This trial is also evaluating other combinations such as adding daratumumab, an anti-CD38, or relatlimab, an anti-LAG-3, to nivolumab, or combining ipilimumab/nivolumab with cobimetinib. Previously treated patients were assigned to receive either nivolumab as a single agent or a combination of nivolumab plus ipilimumab. The nivolumab arm had an ORR of 31.1%, with a DCR of 69%. The mPFS was 14.3 m, with a mOS not reached. The combination of ipilimumab/nivolumab in previously treated MSI mCRC patients showed an ORR of 65%, including 13% of complete responses (CR), and a DCR of 85%. The mPFS and OS were not reached, with 48 m PFS and OS rates of 53% and 71%, respectively [22].
CheckMate 142 also studied the combination of nivolumab plus ipilimumab as the first line of treatment in MSI mCRC, with a similar ORR to the pre-treated patients’ cohort (69%), including also 13% CR, and a DCR of 84%. The PFS and OS were not reached, with 24 m rates of 74% and 79%, respectively [25]. In July 2017, the FDA also approved nivolumab, either alone or in combination with ipilimumab, in second or further lines for patients with MSI mCRC, based on the results of these cohort studies. These alternatives have not been approved by the EMA.
The CheckMate 8 HW phase III trial (NCT04008030) compares nivolumab, nivolumab, and ipilimumab versus CT as treatment for MSI mCRC [26]. The primary endpoint is PFS for nivolumab plus ipilimumab vs. nivolumab across all lines, and nivolumab plus ipilimumab vs. CT in the first line of treatment.
The NRG-GI004/SWOG-S1610 COMMIT phase III trial (NCT02997228) investigated atezolizumab either alone or in combination with CT plus bevacizumab compared with CT plus bevacizumab as the first line of treatment for MSI mCRC [27]. However, the FOLFOX-bevacizumab arm was closed to enrolment due to emerging data [21]. The redesigned COMMIT trial was reactivated on January 2021 comparing either atezolizumab alone or in combination with FOLFOX-bevacizumab [28].
A phase II trial has investigated the activity of dostarlimab, an antiPD-1, in patients with MSI or POLE mutation in gastrointestinal tumors. The ORR of MSI patients was 38.7% (46% if considering only CRC), with 80.9% of responses ongoing after 18 m [29].
3. Current Landscape and Challenges of ICIs in MSS mCRC
3.1. Does ICIs Work in MSS mCRC?
About 95% of patients with mCRC present with pMMR and consequent MSS or MSI-L status (non-MSI-H). [30]. The use of ICIs in monotherapy or combination has obtained poor results in various clinical trials with MSS mCRC.
The inherent resistance to ICIs in the majority of MSS mCRC can be explained by a considerably lower TMB and an immune-desert TME with absent or inactive cytotoxic T lymphocytes and a low expression of checkpoint proteins (PD-1, PD-L1, CTLA-4, LAG-3) [31,32]. More than 10-fold lower mutation load with a subsequent low number of neoantigens is expressed compared to MSI [33]. This was evaluated in the KEYNOTE-016 study where genomic analysis demonstrated a mean of 1782 somatic mutations in MSI mCRC versus 73 mutations in MSS mCRC [34].
Another theory exposed is the immunoediting hypothesis by which better responses are achieved in early stage MSS CRC treated with ICIs. Cancer cells at these stages lose antigens to APCs and progressively avoid the immune system, so they proliferate and metastasize. There are other theories for explaining the higher absence of response when CRC is presented in the advanced stage: a higher degree of systemic immunosuppression is observed in metastatic tumors with downregulation of HLA molecules and a subsequent reduction of antigen presentation [35,36].
3.2. Challenges
For MSS mCRC, ICIs are being actively explored in combination with treatments that aim to increase intra-tumoral immune response and render the tumor “immune-reactive”. It is a priority to confirm the intrinsic mechanisms of resistance to immunotherapy in this subgroup, and furthermore necessary to identify relevant new biomarkers.
Effective immune response against tumor cells is a complex system, from the activation of the immune cells to their recruitment within the TME to execute their actions. Therefore, promoting the knowledge of the diverse mechanisms that avoid the activation of the immune system in response to ICIs in MSS mCRC is a keystone for construction novel combination therapies [30]. The scarce mutational load can be overcome by using different approaches: on the one hand, new therapies are emerging such as neoantigen vaccines or adoptive T-cell therapy which are specifically directed to the scarce tumor antigens expressed. On the other hand, the mutation burden can hypothetically be increased by different methods to make these tumors more sensitive to ICIs [37,38,39]. Other strategies aim to transform the immunosuppressive TME into an immune-responsive state with a working immune infiltrate, or to influence the interferon-γ (IFN-γ) signature through inhibiting the expression of immunosuppressive ligands [30].
We illustrate the challenges in the field of combination strategies with ICIs in MSS mCRC (Figure 1).
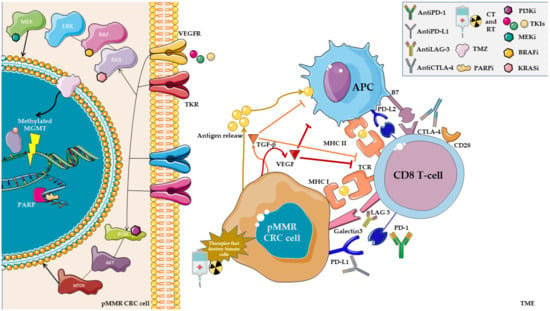
Figure 1.
Among the current challenges described in combination with ICIs, we can distinguish the use of therapies that aim to release neoantigens to activate the immune system, such as chemotherapy (CT), targeted therapy, or radiotherapy (RT). Other methods to cope with the inherent resistance to ICIs, such as temozolomide (TMZ) or PARP inhibitors (PARPi), operate by creating a temporary MSI status. The use of tyrosine-kinase inhibitors (TKIs) that target VEGFR in combination with ICIs has had favorable results in other malignancies, with modulation over the TME in addition to the known antitumoral effect. Additionally, blocking TGF- β and VEGF may have a role in promoting ICI activity in these tumors. Meanwhile, there are rationales for combination with inhibitors of the main steps of the MAPK or PIC3KA pathways.
3.2.1. Combination of Anti-VEGF Agents and Chemotherapy with ICIs
VEGF contributes to tumor angiogenesis and upregulates immune checkpoint molecules (PD-1, PD-L1, CTLA4 and LAG-3). It also downregulates antigen-presentation molecules and inhibits dendritic cell maturation [40,41]. Oxaliplatin in combination with an anti-VEGF drug enhanced the anti-tumor activity of PD-1 pathway blockade in mCRC [42]. Therefore, the combination of an anti-VEGF agent with ICIs may induce immune-stimulatory effects. The combination of CT, anti-VEGF, and ICIs may be effective due to the potential synergistic mechanisms [43,44]. The relevant clinical trials evaluating the use of anti-VEGF and CT plus ICIs are summarized in Table 2.

Table 2.
Currently ongoing and completed clinical trials of anti-VEGF, CT, and ICIs in MSS mCRC.
The MODUL phase II trial (NCT02291289) evaluated maintenance treatment with combined atezolizumab/bevacizumab/fluoropyrimidine after first-line induction with FOLFOX/bevacizumab in BRAF wild-type (BRAFwt) MSS mCRC. No differences were demonstrated in terms of PFS or OS compared with bevacizumab in combination with fluoropyrimidine alone [45].
The BACCI phase II trial (NCT02873195) assessed the effect in refractory mCRC of adding atezolizumab to capecitabine and bevacizumab. The majority of tumors (86.7% of mCRC in the atezolizumab and 85.7% in the control group) were MSS. A modest statistically significant improvement in PFS was detected (4.4 m vs. 3.3 m, p = 0.051) [46]. A phase II trial focusing only on MSS CRC patients used bevacizumab, capecitabine, and pembrolizumab (NCT03396926) [47]. The ORR in 40 evaluable patients was 5%, with an mPFS of 4.3 m and mOS of 9.6 m [47]. The CheckMate 9 × 8 phase II trial (NCT03414983) compared the addition of nivolumab to FOLFOX and bevacizumab as the first-line treatment in mCRC. ORR was 60% in the experimental group and 46% in the standard of care control with an mPFS of 11.9 m overall (p = 0.3) and a mOS of 29.2 m in patients treated with nivolumab and not reached in the standard of care group [48].
The AtezoTRIBE phase II study (NCT03721653) randomized mCRC patients to receive standard (FOLFOXIRI and bevacizumab) or experimental (FOLFOXIRI, bevacizumab and atezolizumab) first-line treatment, regardless of tumor microsatellite status [49]. A PFS benefit was attained with the addition of atezolizumab in the ITT population, but pMMR patients did not have a significative improvement in mPFS (12.9 m vs. 11.4 m, p = 0.072).
In conclusion, the combination of anti-VEGF, CT, and ICIs may be a useful strategy of treatment in MSS mCRC, but more clinical trials must demonstrate its effectiveness.
3.2.2. Combination of Anti-EGFR Agents and Chemotherapy with ICIs
Cetuximab, a chimeric IgG1 antibody against EGFR, can evoke a T-cell-mediated anti-tumor immune response and stimulate NK-mediated antibody-dependent cellular cytotoxicity, independently of RAS mutational status [50]. However, panitumumab, the fully human IgG2 antibody anti-EGFR, has not demonstrated the same skills in mobilizing immune cells against tumor cells [51]. Table 3 summarizes clinical trials that have studied anti-EGFR, CT, and ICIs in MSS mCRC.
The AVETUX phase II trial (NCT03174405) studied avelumab, anantiPD-L1, cetuximab, and FOLFOX as the first line of treatment in RAS/BRAFwt mCRC patients, independently of microsatellite status, with 95% of patients being MSS. An 80% ORR was detected and the mPFS was 11.1 m [52].
The AVETUXIRI phase II trial (NCT03608046) evaluated avelumab, cetuximab, and irinotecan in refractory mCRC patients [53]. The DCR was 60.0% and 61.5% in RASwt and RASm, respectively, with an mPFS and mOS of 4.2 and 12.7 m in RASwt patients and 3.8 m and 14 m in RASm patients. These differences were statistically significant.
The CAVE colon phase II trial (NCT04561336) tested avelumab plus cetuximab as a rechallenge in previously treated RASwt mCRC patients. [54]. Patients were not selected for microsatellite status. The mOS and mPFS were 11.6 m and 3.6 m, respectively [55].
A phase II trial (NCT03442569) evaluated the combination of nivolumab, ipilimumab, and panitumumab in pretreated MSS RASwt mCRC. Patients were excluded if they had been treated with prior anti-EGFR therapy. The 12 w ORR was 35% and the mPFS was 5.7 m [56]. A phase I/II single-arm trial (NCT04017650) of nivolumab, cetuximab, and encorafenib in BRAF V600E mutant MSS mCRC patients who had progressed to at least one prior line of treatment showed an ORR of 45%, mPFS of 7.3 m, and mOS of 11.4 m [57].
The combination of anti-EGFR, avelumab, and triplet CT is under evaluation in the AVETRIC phase II study (NCT04513951). FOLFOXIRI plus cetuximab and avelumab is administered as first-line therapy in RASwt mCRC, followed by maintenance with 5-FU plus cetuximab and avelumab, regardless of microsatellite status [58].
Interpretation of the results of trials combining CT and targeted therapy is complicated because a great part of the outcomes could be due to the activity of the standard treatment, with the combination arms having shown scarce and non-statistically significant improvements.

Table 3.
Currently ongoing and completed clinical trials of anti-EGFR, CT, and ICIs in MSS mCRC.
Table 3.
Currently ongoing and completed clinical trials of anti-EGFR, CT, and ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| AVETUX (NCT03174405) [52] | Avelumab + cetuximab + irinotecan | II | PFS | 1st line mCRC | 80% | 11.1 | NA | Completed |
| AVETUXIRI (NCT03608046) [53] | Avelumab + cetuximab + irinotecan | II | PFS | Refractory mCRC | 60.0% (RASwt), 11.5% (RASm) | 4.2 (RASwt), 3.8 (RASm) | 12.7 (RASwt), 14 (RASm) | Completed |
| CAVE Colon (NCT04561336) [55] | Avelumab + cetuximab | II | OS | Refractory RASwt mCRC | NA | 3.6 | 11.6 | Completed |
| NCT04017650 [57] | Nivolumab + cetuximab + encorafenib | I/II | ORR | BRAF V600E mutant pMMR mCRC | 45% | 7.3 | 11.4 | Active, not recruiting |
| AVETRIC (NCT04513951) [58] | FOLFOXIRI + cetuximab + avelumab | II | PFS | 1st line mCRC | NA | NA | NA | Recruiting |
Abbreviations: ORR: objective response rate; OS: overall survival; PFS: progression-free survival; BOR: best objective response; NR: not reached; NA: not available.
3.2.3. Combination of Temozolomide with ICIs
Temozolomide (TMZ) is an oral alkylating drug that acts by methylating DNA strands at the O6 position of guanine, which damages the DNA and inhibits its replication. Its activity is linked with the O6-methylguanine methyltransferase (MGMT) enzyme, which reduces the therapeutic efficacy of TMZ [59]. The epigenetic silencing of MGMT, mediated by the methylation of its promoter region, is involved in low DNA repair of O6-alkylguanine adducts, thereby improving the sensitivity of cancer cells to TMZ [60].
MGMT methylation is detected in 30–40% of CRC and is strongly associated with RAS mutations [61]. Several phase II trials have demonstrated that TMZ is an active option in pretreated mCRC patients [59,62,63,64]. TMZ is not yet a standard treatment, because diverse drug schedules were used in the trials and the definition of MGMT methylation remains unsettled [65,66].
TMZ can induce somatic mutations in MMR genes in solid tumors [67,68] and produce depletion of T-regulator lymphocytes and activation of cytotoxic T lymphocytes [69]. A hypermutated phenotype and a high load of neoantigens were observed in mCRC cells initially responsive to TMZ at the time of acquired resistance [70]. The rationale to explore combining TMZ and ICIs in MSS mCRC patients is the activation of effective immune surveillance by TMZ, so TMZ would change a cold tumor into a hot one.
Clinical trials evaluating combinations of TMZ and ICIs are summarized in Table 4. The MAYA phase II trial (NCT03832621) studied the combination of nivolumab, ipilimumab, and TMZ in patients with MSS MGMT-silenced mCRC who had not progressed after two cycles of TMZ, independently of RAS mutational status. Among 716 pre-screened patients, 204 (29%) were molecularly eligible and 135 of them started the first part of the treatment. Among these, 102 (76%) had to discontinue because of death or disease progression in the TMZ priming phase, whereas 33 patients (24%) achieved disease control and started the second part of the treatment. These patients represented the final study population with mPFS and mOS of 7.0 m and 18.4 m, respectively, and an ORR of 45% [71].

Table 4.
Currently ongoing and completed clinical trials of TMZ and ICIs in MSS mCRC.
The ARETHUSA phase II trial (NCT03519412) is a non-randomized study with two cohorts in which patients with refractory MSI mCRC are treated with pembrolizumab until progression, and patients with MSS, RASm and MGMT-silenced mCRC receive TMZ until progression [72]. A biopsy is performed when disease progression is confirmed in the MSS cohort in order to determine TMB, and patients with TMB > 20 mut/Mb receive pembrolizumab. The primary endpoint is ORR in the MSS cohort treated with pembrolizumab, with the MSI cohort used for indirect comparison. The estimated study completion date is December 2023.
Another phase II clinical trial (NCT04457284) is evaluating the combination of TMZ, cisplatin, and nivolumab in MSS mCRC refractory to prior lines of treatment [73].
3.2.4. Combination of ICIs with DNA Damage Response (DDR) Inhibitors
The DNA damage response (DDR) is a complex mechanism that recognizes an error within the genome and triggers several pathways to activate cell-cycle checkpoints in order to repair the DNA before continuing with the division. When the DNA is irreparably damaged, DDR directs these cells to apoptosis or permanent anergy status [74]. Poly-ADP-ribose polymerase 1 (PARP1) mRNA overexpression was observed in 70.3% of a series of CRC analyzed, most at early stages. A hypothesis of the role of PARP1 in CRC oncogenesis has been postulated from these results [75]
By inhibiting PARP with inhibitors (PARPi) the single-strand DNA cannot be repaired, and consequently an accumulation of DNA damage generates release of neoantigen load which increases TMB and PD-L1 expression, with a potential synergistic effect in combination with ICIs [76]. There have been promising results with the use of PARPi with ICIs in various solid tumors, including MSS mCRC (Table 5). The DAPPER phase II basket trial evaluates the combination of durvalumab with olaparib (PARPi) or cediranib (VEGFR inhibitor) in refractory MSS mCRC, pancreatic carcinoma, or leiomyosarcoma. The DAPPER trial examines the changes in the genomic and immune biomarkers in the baseline biopsy and first treatment biopsy, as well as in peripheral blood and stool samples [77].
Another phase I/II basket study is testing the same drugs as DAPPER and also the combination of the three (NCT02484404). The primary endpoints are ORR and determining the tolerability of the combinations [77]. In ovarian cancer, the preliminary results show a DCR of 67% with 44% partial responses and acceptable tolerability [78]. Other phase I and II trials are also evaluating the combination of PARPi with ICIs in refractory patients with mutations in the homologous recombination repair genes (NCT04123366, NCT03842228) and patients without them (NCT03772561) [79,80,81].
Other molecules involved in DDR include ATR, which also acts on single-strand damage recognition and prevents the cell cycle from progressing to the G2 phase from the S phase by activating the checkpoint kinase 2. A phase I/II trial (NCT04266912) is evaluating the safety of combining an ATR inhibitor berzosertib with avelumab in solid tumors with actionable aberrations in one or more of the DNA DDR genes, including ARID1A, ATM, or ATR, among others. [82]. Another potential target is WEE1, which is involved in preventing the transition from G2 to M by negatively regulating the checkpoint kinase 1. A WEE1 inhibitor, adavosertib, is being tested with durvalumab in a phase I trial, with results pending [82].

Table 5.
Currently ongoing and completed clinical trials of DDR inhibitors and ICIs in MSS mCRC.
Table 5.
Currently ongoing and completed clinical trials of DDR inhibitors and ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| DAPPER (NCT03851614) [77] | Durvalumab + Olaparib/Cediranib | II | Changes in genomic and immune biomarkers | A refractory solid tumor (only pMMR CCR) | NA | NA | NA | Active, not recruiting |
| NCT02484404 [77] | Durvalumab + Olaparib +/o Cediranib | I/II | ORR Safety and tolerability, MTD | Refractory solid tumors | NA | NA | NA | Recruiting |
| NCT04123366 [81] | Olaparib + Pembrolizumab | II | ORR | Refractory solid tumor + mutation in HRR/HRD | NA | NA | NA | Recruiting |
| NCT03842228 [80] | Copanlisib + Olaparib + durvalumab | I | MTD | Refractory solid tumor + germline or somatic mutations in DDR genes | NA | NA | NA | Recruiting |
| NCT03772561 [79] | AZD5363 + Olaparib + Durvalumab | I | ORR/BOR | Refractory solid tumors | NA | NA | NA | Recruiting |
| NCT04266912 [83] | Avelumab and Berzosertib | I/II | Safety and tolerability, MTD | Refractory solid tumors with a mutation in DDR genes | NA | NA | NA | Recruiting |
| NCT02617277 [82] | Adavosertib + Durvalumab | I | DLTs | Refractory solid tumors | NA | NA | NA | Active, not recruiting |
Abbreviations: DLT: dose-limiting toxicity; MTD: maximum tolerable dose; DCR: disease control rate; IDCR: immune disease control rate; RD: recommended dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; nr: not reached; NA: not available.
3.2.5. Combination of Multikinase Inhibitors (Anti-VEGFR) with ICIs
The combination of ICIs with vascular endothelial growth factor receptors (VEGFR) inhibitors may represent a useful approach due to the immunosuppressive role that VEGF has in the TME. VEGF promotes angiogenesis within the tumor with an expansion of tumor-suppressing immune cells and tumor-associated macrophages (TAMs) that supports the characteristic immune evasion. Furthermore, it decreases T cell activity by promoting the expression of the immune checkpoints PD-1, CTLA-4, TIM3, and LAG3 on their surface. Therefore, there is a demonstrable basis for combining inhibitors of VEGF/VEGFR with ICIs [84,85].
Regorafenib is a multikinase inhibitor (MKI) against VEGFR1, VEGFR2, VEGFR3, epidermal growth factor homology domain 2 (TIE-2), platelet-derived growth factor receptor-β (PDGFR β), c-kit, RET, RAF-1, colony-stimulating factor-1 (CSF-1R), and BRAFwt and V600E mutation. [86,87] Its potential synergistic role with ICIs lies in its immunomodulatory properties through the inhibition of CSF1R, a tyrosine kinase receptor that is involved in TAM proliferation and differentiation. These cells may be presented as M1 macrophages that promote antitumor actions, or M2 macrophages which are involved in immune evasion favoring oncogenesis. The latter are downregulated with the inhibition of CSFR1 in the presence of regorafenib: M2 TAMs express CD206 on their membrane and have been shown to be considerably reduced in CRC murine models, with a significant increase of M1 TAMs [87,88]. The effects on tumor reduction as well as on reducing intratumoral macrophages are synergistically enhanced with antiPD-1 treatment, as shown in preclinical models. Additionally, a reduction of regulatory T cells was observed with the addition of regorafenib to antiPD-1 treatment, promoting an antitumor effect [88,89]. Interestingly, the benefits obtained from regorafenib are maintained by the presence of antiPD-1 treatment when the treatment is discontinued [89]. Table 6 describes the clinical trials that have studied the activity of MKI plus ICIs in MSS mCRC.
The REGONIVO phase Ib trial combining regorafenib and nivolumab included patients with metastatic gastric and mCRC on progression to two or more lines of treatment. The primary endpoint (ORR) was encouraging (33%) in the subset of MSS mCRC patients (n = 24) [90]. Nonetheless, REGNIVO, the phase II study from America, failed to produce similar results (ORR = 7%) [91]. Other clinical trials have also published poor results: in REGOMUNE, a single-arm phase II study testing regorafenib in combination with avelumab in pretreated MSS mCRC patients, no objective response was achieved, with stable disease as the best outcome (54% of patients). However, considering the results of regorafenib as a single agent, the addition of the antiPD-1 improved the PFS and OS results (median PFS and OS of 3.6 and 10.8 m, respectively, vs. 1.9 m and 6.4 m observed in the CORRECT trial) [92,93]. Another phase I/Ib study also tested regorafenib and nivolumab in refractory MSS mCRC patients, with only 10% of patients achieving partial response, but >50% of patients had stable disease as the best response, with considerable control of the disease [94]. The combination with pembrolizumab in a phase I/II clinical trial on refractory patients also showed no objective response, with stable disease in 49% of the patients. Although it did not meet its primary endpoint (PFS), the OS was 10.9 m, similar to the value observed in the REGOMUNE trial. [95] Further studies are required to establish the role of regorafenib in this subgroup of patients.
Lenvatinib is an MKI that targets VEGFR 1–3, RET, FGFR 1–4, c-KIT, and PDGFRα. Due to its immunomodulatory activity, it has been assessed in combination with pembrolizumab in other malignancies including renal cell carcinoma, with remarkable results which positioned it as part of the standard of care in first-line treatment [96]. In the single-arm phase II study LEAP-005, MSS mCRC patients on progression to standard lines of therapy received lenvatinib in combination with pembrolizumab, and a substantial ORR of 22% was reported. [97] The phase III trial (LEAP-017) comparing lenvatinib–pembrolizumab with the standard of care regorafenib or TAS-102 is currently ongoing, with pending results [98]. As with regorafenib, the antitumor activity of lenvatinib and pembrolizumab is enhanced by the combination of both drugs, with an increase in the percentage of CD8+ T cells and a reduction of M2 TAMs demonstrated in preclinical models. It is hypothesized that the dual inhibition of VEGF and FGFR is involved not only in inhibiting tumor angiogenesis but also in converting the immunosuppressive TME to a more immunogenic status by increasing IFN-γ production by CD8+ T cells [99].
The use of other MKIs that target VEGFR, such as cabozantinib, has not demonstrated improvement on previous results but reinforces the idea of the synergy between antiVEGFR and ICI; the phase II CAMILLA trial assessed the combination of cabozantinib with durvalumab in MSS mCRC patients who had progressed from two or more prior therapies, with a considerable ORR of 27.6% and similar rates of PFS and OS (3.8 m and 9.1 m, respectively) [100,100]. The phase Ib COSMIC-021 study evaluated the efficacy of combining cabozantinib and atezolizumab, an antiPD-L1, in pretreated solid tumors; the ORR was 9.7% in MSS patients, considerably lower than that reported in the CAMILLA trial. However, in the subgroup analysis, the patients with RASwt tumors had an ORR of 25%, coinciding with the higher ORR presented in this subgroup in the CAMILLA trial (ORR of 50%) [100,101], which may indicate a higher response rate in subgroups of patients within MSS mCRC; a retrospective study of patients with MSI CRC tumors revealed a less immunogenic TME in RASm [102]. Nonetheless, the results of the combinations were much better than those observed with MKI in monotherapy, suggesting a synergistic mechanism with ICIs.
Fruquintinib is a highly selective oral inhibitor of VEGFR 1–3 that recently proved its benefits on OS in pre-treated mCRC. A retrospective study comparing the combination of fruquintinib with antiPD-1 vs. regorafenib with antiPD-1 agents, including toripalimab, nivolumab, sintilimab, or camrelizumab, was performed by Sun et al., reporting better results in the first group in terms of PFS (6.4 m vs. 3.9 m), although the ORR was slightly better in the second group (7.1% vs. 8.7%). Curiously, as described previously, the presence of RAS mutations, as well as other factors such as the presence of liver metastases or a right colon localization, were associated with worse responses, although no significant differences in PFS were observed between the subgroup of patients with and without these characteristics [103]. Other studies have not obtained the same results comparing regorafenib and fruquintinib in combination with an antiPD1, although favorable results make them both a possible approach in refractory MSS mCRC, with studies currently ongoing (NCT04577963 and NCT04483219) [104,105,106].

Table 6.
Currently ongoing and completed clinical trials of multikinase inhibitors (Anti-VEGFR) and ICIs in MSS mCRC.
Table 6.
Currently ongoing and completed clinical trials of multikinase inhibitors (Anti-VEGFR) and ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| REGONIVO (NCT03406871) [90] | Nivolumab + Regorafenib MSS mCRC cohort | I/II | MTD and RD | Refractory to standard treatments (≥3rd line) | 33.3% (MSS patients) | 7.9 | NR | Completed |
| REGNIVO (NCT04126733) [91] | Nivolumab + Regorafenib | II | ORR | Refractory to standard treatment | 7.1% | 2 | 13 | Completed |
| REGOMUNE (NCT03475953) [92] | Avelumab+Regorafenib MSS mCRC cohort | I/II | I: Safety and tolerability II: DCR | Refractory to standard treatment | NA | 3.6 | 10.8 | Recruiting |
| NCT03712943 [94] | Nivolumab + Regorafenib | I | DLT and MTD | Refractory to standard treatment | 10% | 4.3 | 11.1 | Active, not recruiting |
| NCT03657641 [95] | Pembrolizumab + Regorafenib | I/II | DLT, PFS, and OS | Refractory to standard treatment | 0% | 2 | 10.9 | Active, not recruiting |
| LEAP-005 (NCT03797326) [97] | Pembrolizumab + Lenvatinib MSS mCRC cohort | II | ORR | Refractory to standard treatments (≥3rd line) | 22% | 2.3 | 7.5 | Active, not recruiting |
| LEAP-017 (NCT04776148) [98] | Pembrolizumab + Lenvatinib vs. TAS-102/Regorafenib | III | OS | Refractory to standard treatment | NA | NA | NA | Active, not recruiting |
| CAMILLA (NCT03539822) [100] | Cabozantinib + Durvalumab mCRC cohort (all MSS) | I/II | MTD and ORR | Refractory to standard treatments (≥3rd line) | 27.6% | 4.4 | 9.1 | Recruiting |
| COSMIC-O21 (NCT03170960) [101] | Cabozantinib + Atezolizumab | I/II | MTD and ORR | Refractory to standard treatment | 9.7% | 3 | 14 | Active, not recruiting |
| NCT04483219 [106] | TKI (fruquintinib or regorafenib) + Toripalimab | II | 9-month PFS | Refractory to standard treatment | NA | NA | NA | Recruiting |
| NCT04577963 [105] | Fruquintinib+ tislelizumab | I/II | ORR | IO-Naïve | NA | NA | NA | Recruiting |
Abbreviations: DLT: dose-limiting toxicity; MTD: maximum tolerable dose; DCR: disease control rate; iDCR: immune disease control rate; RD: Recommended dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; BOR: best objective response; NR: not reached; NA: not available.
3.2.6. Targeting MAPK Pathway in Combination with ICIs
It is known that the mitogen-activated protein kinase (MAPK) pathway, composed of the RAS/RAF/MEK/ERK signaling cascade, plays an essential role in cellular proliferation and survival. RAS is one of the most frequently mutated oncogenes in numerous neoplasms including CRC [107]. One of the hypotheses put forward is that mutations on the MAPK axis result in the upregulation of expression of immune suppressive checkpoints such as PD-L1 along with downregulation of class I MHC on the tumor surface, with a correlated decrease of intratumoral T-cell infiltration [108,109,110]. Therefore, this rationale is based on the inhibition of that axis in combination with the effects of ICIs. The main clinical trials are summarized in Table 7.
The role of MEK inhibitors in combination with ICIs has been researched in preclinical studies, with an observed increase of the CD8+ T-cell population and class I MHC expression, resulting in better responses in comparison with MEK inhibitors in monotherapy [110,111]. Hence, the phase III IMblaze 370 trial, which involved up to only 5% MSI mCRC cases, assessed the combination of encorafenib, a MEK1 and MEK2 inhibitor, plus atezolizumab vs. atezolizumab vs. regorafenib in a third or subsequent line of treatment. The primary endpoint was OS, but no benefit was observed in the combination group in comparison with regorafenib (8.87 m vs. 8.51 m) or for atezolizumab and regorafenib (7.1 m vs. 8.51 m), nor were there significant differences in PFS or ORR [112]. Possible explanations for these results were proposed: on the one hand, the outcomes from the regorafenib group were better than those from the CORRECT trial so the possible benefit may have been masked. On the other hand, MEK inhibitors may not be effective enough to achieve the necessary immunomodulation to enhance the ICIs’ effect, as immunosuppressive actions associated with MEK inhibitors such as inhibiting T-cell proliferation have been described in other preclinical studies. Meanwhile, MAPK gene expression may be involved: in the phase I study of atezolizumab plus cobimetinib, cases with MAPK gene expression >50% had better mPFS and mOS in comparison with lower expressions, which could represent a biomarker of response, although further research is required [113,114].
Preliminary results from a phase II study combining pembrolizumab, binimetinib, a MEK1 and MEK2 inhibitor, and bevacizumab in third or subsequent treatment lines reported scarce activity with an ORR of 13% [115]. A phase I dose-escalation study in refractory solid tumors testing selumetinib, another MEK1 and 2 inhibitor, plus durvalumab has advanced to a phase II where a cohort of MSS mCRC has been enrolled [116,117].
The inhibition of other steps of the cascade signaling has also been investigated in the MSS setting. Approximately 60% of the somatic inactivation of MMR genes is co-presented with a BRAF mutation (BRAFm). The tumorigenesis of BRAFm tumors is related to extensive DNA methylation of the CpG islands and MLH1 promoter methylation that causes an MSI phenotype [118,119]. Only 5–10% of MSS tumors co-occur with a BRAF mutation and the addition of ICIs to the targeted therapy is based on the increase of T cell infiltration caused by inhibiting BRAF with BRAF, MEK, and EGFR inhibitors. The potential synergistic action of the combination has been tested in vivo with a benefit in response compared with their separate use. A phase I trial is testing the combination of dabrafenib and trametinib plus spartalizumab, an antiPD-1, in BRAFV600E mCRC patients. An ORR of 42 has been reported, with one complete response notified. Biopsies at baseline and day 15 were analyzed, with an increase in T-cell infiltration and expression of cytotoxic genes observed [120,121]. Another phase I study is assessing the combination of dabrafenib with LTT462, an ERK inhibitor, plus spartalizumab/tislelizumab, both antiPD-1, with results pending [122].
Approximately 45% of CRC has a KRAS mutation (KRASm), leading to constant MAPK activation and T-cell exclusion. Recently, sotorasib, a KRAS inhibitor against KRAS p.G12C, has emerged with promising results in pretreated non-small cell lung cancer, with an ORR of 37.1% and DCR of 80.6%. However, the outcomes were poorer in pretreated mCRC, with an ORR of 9.7%; this inconsistency may be explained by the presence of other oncogenic drivers in CRC, and combination strategies should be explored [123,124,125]. As mentioned earlier, KRAS mutations are linked to upregulation of PD-L1 and combination treatment with antiPD-1/L1 is being explored in the CodeBreaK100 trial [126]. A phase I/II study (KontRASt-01) is currently evaluating the combination of tislelizumab, an antiPD-1, with JDQ443, another KRAS G12C inhibitor, with or without TNO155, an SHP2 inhibitor [127]. SHP2 is a tyrosine phosphatase that plays a key role in the carcinogenesis of KRASm CRC, with an antitumor effect demonstrated both in vitro and in vivo [128].
3.2.7. Targeting PIK3CA/AKT/mTOR Pathway
Derived either from mutations in PIK3CA (PIK3CAm) or from the loss of PTEN, PI3K pathway activation can be found in approximately 20% of CRC. Preclinical studies have shown that tumoral cells with activations on this pathway are associated with increased immune evasion and resistance to immunotherapy due to an upregulation of CD4+ T cells as well as reprogramming of TAMs to their immunotolerant subtype (M2) [129]. Consequently, to cope with this mechanism of primary resistance, a combination of an inhibitor of PI3K (copanlisib) with nivolumab has been proposed in a phase I/II study in pretreated patients, with a second phase in which the primary endpoint is a 6-month ORR in MSS CRC patients, either PIK3CA wild-type or PIK3CAm [130,131].

Table 7.
Currently ongoing and completed clinical trials of inhibitors of MAPK/PIK3CA and ICIs in MSS mCRC.
Table 7.
Currently ongoing and completed clinical trials of inhibitors of MAPK/PIK3CA and ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| NCT01988896 [114] | Atezolizumab + Cobimetinib | I | Safety and tolerability, MTD | Refractory solid tumors | 8% | 1.9 | 9.8 | Completed |
| IMblaze 370 (NCT02788279) [112] | Atezolizumab + Cobimetinib vs. Atezolizumab vs. Regorafenib | III | OS | Refractory to standard treatments (≥3rd line) | 3% | 1.9 vs. 1.9 vs. 2 | 8.9 vs. 7.1 vs. 8.5 | Completed |
| NCT02586987 [117] | Selumetinib + Durvalumab +/- Tremelimumab | I | DLT Safety and tolerability | Refractory solid tumors | NA | NA | NA | Completed |
| NCT03668431 [120] MSS patients | Dabrafenib + Trametinib + PDR001 (Spartalizumab) | II | ORR | First or subsequent lines BRAFV600E | 42% | NA | NA | Recruiting |
| NCT04294160 [122] | Dabrafenib + LTT462 (ERK inhibitor) + PDR001 (Spartalizumab)/Tislelizumab [122] | I | DLT Safety and tolerability | ≥Second line BRAFV600 | NA | NA | NA | Recruiting |
| CodeBreaK 100 (NCT03600883) [126] | Sotorasib + AntiPD-1/L1 | I/II | DLT Safety and tolerability ORR | Refractory KRAS G12C | NA | NA | NA | Active, not recruiting |
| KontRASt-01 (NCT04699188) [127] | Tislelizumab + JDQ443 +/- TNO155 | I/II | DLT Safety and tolerability | Refractory to standard treatments | NA | NA | NA | Recruiting |
| NCT03711058 [130] | Copanlisib + Nivolumab | I/II | MTD ORR | Refractory to standard treatments (≥third line) | NA | NA | NA | Active, not recruiting |
Abbreviations: DLT: dose-limiting toxicity; MTD: maximum tolerable dose; DCR: disease control rate; iDCR: immune disease control rate; RD: recommended dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; NR: not reached; NA: not available.
3.2.8. Combination with Other ICIs
As previously mentioned, monotherapy with antiPD-1 failed to prove efficacy in MSS tumors [34,132]. Therefore, the dual blockade with antiCTLA4 was tested due to the better results obtained in other neoplasms such as melanoma or renal cell carcinoma, attributed to the CTLA4 expression on regulatory T-cell surfaces [133]. A phase II trial assessed the combination of durvalumab and tremelimumab vs. BSC in both MSS and MSI mCRC refractory to all standard treatments. A disappointing global ORR of 0.8% was observed, although the OS had a trend in favor of the combination (6.6 m vs. 4.1 m). In the subgroup analysis, MSS patients had significantly greater OS and those who possessed high TMB (defined as ≥28 Mut/Mb), accounting for 21% of the patients, experienced the best results in terms of OS (HR: 0.34; p = 0.004) [134]. Following these findings, further clinical trials have tried to demonstrate the role of TMB in responding to ICI: the phase II basket study TAPUR analyzed the effect of nivolumab plus ipilimumab in pretreated mCRC with high TMB, defined as ≥9 Mut/Mb: DCR and ORR were both 10% and the trial closed due to futility [25,135].
An expanded phase I clinical trial evaluated the combination of botensilimab (next-generation antiCTLA-4) and balstilimab (antiPD-1) in pretreated MSS mCRC. A total of 41 patients were evaluated with an ORR of 24%, higher (42%) in patients with no liver metastases or locally-treated with resection or ablation techniques [136,137]. These results contribute to the theory that the presence of liver metastases does influence the response to immunotherapy; these lesions are thought to diminish tumor-specific CD8+ T-cell recount, lessening the efficacy of immunotherapy. This has been studied in preclinical models, and CD11b+F4/80+ macrophages presented in the liver are believed to be the main factor responsible for CD8+ T-cell apoptosis by inducing the Fas–FasL pathway. Hence, these liver macrophages induce systemic immunosuppression, and the hypothesis is postulated that treating these lesions with local therapies may help increase the response to immunotherapy [138].
Combinations with other ICIs have also been tested, also with poor results. The association of favezelimab, an antiLAG-3 antibody, with pembrolizumab in a phase I study in pre-treated mCRC reported four partial responses and one complete response (ORR 6.3%). Curiously, patients with CPS ≥1 presented a mOS of 12.7 m, almost twice that of patients with CPS < 1 (6.7 m) [139]. The main clinical trials are reviewed in Table 8.

Table 8.
Currently ongoing and completed clinical trials of combinations with other ICIs in MSS mCRC.
3.2.9. Combination of ICIs with Radiotherapy
As radiotherapy is predominantly a local therapy, its combination with other systemic therapies such as CT or immunotherapy aims to benefit from their systemic effects following radiation of a localized area. The destruction of tumoral cells liberates neoantigens and activates tumor-infiltrating dendritic cells, which triggers STING-mediated type I interferon production. A local immune response is activated, with a subsequent activation elsewhere, aimed at achieving systemic control by the so-called abscopal effect. The addition of immunotherapy can help trigger and increase the abscopal effect by blocking the checkpoint inhibitors [85]. This has been demonstrated in murine models of melanoma and non-small-cell lung cancer, with a benefit obtained from the use of antiCTLA4 in monotherapy, which is usually the most effective treatment for these kinds of tumors [140]. A case of tumor regression of non-irradiated distant lesions with the use of pembrolizumab and radiotherapy was described in a non-randomized phase II study involving pre-treated patients (Table 9). However, that was the only response achieved by the abscopal effect with this combination (ORR 9%) [141]. Similarly, the combination of ipilimumab and nivolumab with radiotherapy during the second cycle presented an ORR of 7.5% and a DCR of 17.5%. [142]. Another phase II study assessed the combination of durvalumab and tremelimumab with concurrent radiotherapy. Even though abscopal responses were observed (ORR = 8.3%), the study did not meet the prespecified endpoint criteria necessary to carry out a phase III follow-up [143].
The lack of effective response may be explained by the fact that the TME of MSS mCRC is characteristically cold, and may require further strategies between radiotherapy and ICI, such as the use of vaccines including the most immunodominant antigen in order to make the TME more immunogenic and hence overcome the intrinsic resistance to ICIs expressed by MSS mCRC [144]. An ongoing clinical trial aims to demonstrate the efficacy of using hypofractionated stereotactic ablative radiotherapy, as this seems to increase the effectiveness of ICIs [145].
Where the use of radiotherapy or ICIs in monotherapy would not obtain any response, a synergic effect is presented. Identifying additional biomarkers to identify the patients that could benefit from these strategies could be useful for the development of new clinical trials. As mentioned earlier, the presence of liver metastases is thought to lessen the response to immunotherapy, due to the specific microenvironment that collects and inactivates CD8+ T cells. Hence, the use of radiotherapy on liver metastases may overcome this acquired resistance mechanism and induce immune sensitivity [138].

Table 9.
Currently ongoing and completed clinical trials of RT and ICIs in MSS mCRC.
Table 9.
Currently ongoing and completed clinical trials of RT and ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03122509 [143] | Radiotherapy + Durvalumab + Tremelimumab | II | ORR | Refractory to standard treatments (≥3rd line) | 8.3% | 1.8 | 11.4 | Completed |
| NCT03104439 [142] | Radiotherapy + Nivolumab + Ipilimumab | II | DCR (17.5%) | Refractory to standard treatments (≥3rd line) | 7.5% | NA | NA | Recruiting |
| NCT02992912 [145] | Atezolizumab With Stereotactic Ablative Radiotherapy | II | PFS | Refractory | NA | NA | NA | Unknown |
Abbreviations: DCR: disease control rate; iDCR: immune disease control rate; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; NA: not available.
3.2.10. Blocking TGF-β and Wnt Pathway + ICIs
Mutations in several signaling pathways have been associated with CRC proliferation and invasion, including transforming growth factor-beta (TGF-β), wingless-type MMTV integration site family (Wnt), EGFR, and p53. [146]
Genomic alterations in the TGF-β signaling pathway can be found in up to 27% of the non-hypermutated CRC. [14] TGF-β is a cytokine secreted by CRC cells and as it occurs with VEGF acts as a promoter of the tumorigenesis through multiple mechanisms, including promoting angiogenesis and immune evasion within the TME through direct and indirect actions on T cells, dendritic cells, and/or through regulating certain cytokines and extracellular matrix proteins. It impacts on T-cell differentiation towards a Th1 effector phenotype by repressing T-bet, the essential transcription factor to achieve this phenotype, and inhibits CD8+ T-cell responses. Therefore, by blocking TGF-β we can confer a more immunogenic TME that may allow ICIs to function. In preclinical models with mutations in three or the four of the pathways described, prominent T-cell exclusion and elevated TGF-β activity were demonstrated and the use of Galunisertib, a potent and selective TGF-β receptor I kinase inhibitor, reduced tumor size and prevented the appearance of liver metastases [146]. Vactosertib is another TGF-β receptor I kinase inhibitor that is being tested along with pembrolizumab in MSS mCRC refractory to all treatments. An ORR of 15.2% including five partial responses has been described, with the median duration of response not reached among the patients [147]. However, the use of bispecific antibody Bintrafusp-α (TGFβ-trap and anti-PD-L1) in MSS liver-limited mCRC treated with surgery and standard perioperative therapy failed to demonstrate its primary endpoint (ctDNA clearance), with associated large-volume recurrence [148].
Another hypothesis supported by the results from a study on squamous cell carcinoma tumor cell lines is that antiPD-1 therapy induces the known cytotoxic T-cell activity and also a competitive TGFβ-driven immunosuppressive program, and therefore there is a benefit to be gained from using a TGF-β inhibitor [149]. Following these findings, a phase I/Ib study is evaluating the use of NIS793, a fully human monoclonal antibody that binds with high affinity to TGFβ1 and TGFβ2, and with lower affinity to TGFβ3, as a single agent and in combination with spartalizumab, an antiPD-1 [150].
Other TGF-β inhibitors and monoclonal antibodies that bind to TGFβ are currently under investigation, with results pending (NCT03192345, NCT02423343, NCT03821935). Clinical trials underway or completed are listed in Table 10.
The activation of the Wnt pathway occurs in over 90% of MSS CRC patients and is associated with lower recruitment of T-cells. It may be caused by mutations in β-catenin or by adenomatous polyposis coli (APC) or R-spondin (RSPO) gene fusions, which are mutually exclusive [151]. As with KRAS mutations, Wnt pathway activation has been shown to confer T-cell exclusion, and a combination of Wnt-signalling inhibitors or small-molecule β-catenin inhibitors with ICIs may therefore have therapeutical potential. E7386 is a selective inhibitor of the interaction between β-catenin and CREB binding protein and is essential in the Wnt/β-catenin signaling pathway, with synergistic activity with antiPD-1 demonstrated in cell lines. This combination is being tested in refractory MSS CRC in a phase I/II study, with results pending (NCT05091346) [152,153].

Table 10.
Clinical trials including blocking TGF-β, Wnt pathway plus ICIs in MSS mCRC.
Table 10.
Clinical trials including blocking TGF-β, Wnt pathway plus ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | mPFS (Months) | mOS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03436563 [154]—cOHORT D | Anti-PD-L1/TGFbetaRII Fusion Protein M7824 (Bintrafusp-α) | Ib/II | Clearance of ctDNA | Completion of standard-of-care perioperative therapy | 0% | NA | NA | Completed (other cohorts active, not recruiting) |
| NCT03724851 [147] | TEW-7197 (Vactosertib) + Pembrolizumab | I/II | MTD | Refractory to standard treatment | 15.2% | NA | NA | Active, not recruiting |
| NCT02947165 [150]—Group 4 | NIS793 +/- PDR001 (spartalizumab) | I/Ib | Incidence of DLTs and safety and tolerability | Refractory to standard treatment | NA | NA | NA | Completed |
| NCT03192345 [155] | SAR439459 + Cemiplimab | I | DLT and ORR | Refractory to standard treatment | NA | NA | NA | Terminated (competitive landscape and toxicity) |
| NCT02423343 [156] | Galunisertib +/- Nivolumab | I/II | MTD | Refractory solid tumors | NA | NA | NA | Completed |
Abbreviations: DLT: dose-limiting toxicity; MTD: maximum tolerable dose; DCR: disease control rate; iDCR: immune disease control rate; RD: recommended dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; NR: not reached; NA: not available.
3.2.11. Other Combinations: Epigenetic Therapy and Novel Agents
Other strategies are being developed trying to turn these tumors into hot tumors (Table 11): the hypermethylation of DNA silences genes associated with the expression of cancer testis antigens and endogenous retroviruses, which enable T-cell and B-cell immunoreactivity by stimulating a state of viral mimicry. When not silenced, this results in an innate immune response that produces type I and type III interferons and other cytokines that attract CD8+ T cells to the TME [157]. Hence, a DNA methyltransferase inhibitor (DNAMi) can re-express these genes and make the tumor more sensitive to immunotherapy. Azacitidine is a DNAMi that has been tested with pembrolizumab in chemotherapy-refractory MSS Mcrc in a phase II trial, with ORR as the primary endpoint: a modest ORR of 3% was achieved, with an Mpfs of 1.9 m [158]. Another mechanism to prevent silencing these genes involves histone-deacetylase inhibitors (HDAi), enzymes which remove the acetyl groups on histones and reduce gene expression [157]. A combination of zabadinostat and nivolumab is being tested in refractory MSS Mcrc in a phase II trial; Mos of 7 m was reported with a DCR of 48% in the 3-year data [159,160]. Another HDAi, entinostat, has been assessed with pembrolizumab in a phase II study with ORR as the primary endpoint: only one partial response has been published, with five patients with stable disease (DCR 37.5%) [161].
Studies are investigating the therapeutic effect on enhancing immunity within the TME of Pmmr CRC that may be attained by acting on epigenetic factors such as the bromo- and extraterminal domain (BET). JQ1 is a BET inhibitor that regulates PD-L1 expression, boosts MHC-I mediated cytotoxicity, and downregulates T-reg cell infiltration in TME. Hence, its combination with antiPD-1 exerts a synergic action on CRC cell lines and animal models [162]. The use of other novel agents with atezolizumab is being tested, such as the T-cell bispecific antibody cibisatamab, which acts against carcinoembryonic antigen (CEA) and T-cell CD3. The goal of these types of therapies is to target both a tumor-enriched antigen (such as CEA) and immune cells, and the combination with ICIs may enhance their activity. Cibisatamab has shown promising antitumor activity in preclinical studies by increasing intratumoral T-cell infiltration and upregulating PD-1/PD-L1. In the phase I trial, a partial response was observed in one patient with a Pmmr tumor (10%) [163].
The Inhibition of C-C motif chemokine receptor 5 (CCR5) with maraviroc can polarize macrophages towards the M1 phenotype, which boosts the immune response. Its combination with pembrolizumab in heavily pretreated PmmR tumors was assessed in a phase I trial, with prolonged disease stabilization although a modest 5.3% ORR was observed [164,165].
The combination of ibrutinib and pembrolizumab was tested in a phase I/II trial; ibrutinib is a Bruton’s tyrosine kinase (BTK) inhibitor, which is essential in B-lymphocyte development. This kinase is expressed in up to 90% of CRCs and using ibrutinib in cell lines proved its cytotoxic effect, with a presumed synergistic effect in combination with ICIs. Nonetheless, an ORR of 0% was observed [166].

Table 11.
Clinical trials involving novel blocking therapies plus ICIs in MSS mCRC.
Table 11.
Clinical trials involving novel blocking therapies plus ICIs in MSS mCRC.
| Study | Treatment | Phase | Endpoint 1 | Setting | ORR (%) | MpfS (Months) | MoS (Months) | Status |
|---|---|---|---|---|---|---|---|---|
| NCT03821935 [167] | ABBV-151 +/- budigalimab (ABBV-181) | I | Dose escalation and dose expansion | Progression on two prior chemotherapy regimens. | NA | NA | NA | Recruiting |
| NCT02260440 [158] | Azacitidine + Pembrolizumab | II | ORR | Refractory to standard treatment | 3% | 1.9 | 6.3 | Completed |
| CAROSELL (NCT03993626) [159] | Zabadinostat + Nivolumab | I/II | IdcR | Refractory to standard treatments (≥3rd line) | NA | NA | 7 | Unknown |
| ENCORE 601 (NCT02437136) [161] | Entinostat + Pembrolizumab MSS McrC cohort | II | MTD and ORR | Refractory to standard treatments | 6% | NA | NA | Active, not recruiting |
| PICCASSO (NCT03274804) [165] | Pembrolizumab + maraviroc | I | Feasibility Rate of a Combined Therapy | Refractory to standard treatments | 5.3% | 2.1 | 9.83 | Completed |
| NCT02650713 [163] | Cibisatamab (RO6958688) + atezolizumab | I | DLT, MTD Safety and tolerability | Refractory to standard treatments | 10% | NA | NA | Completed |
| NCT03332498 [166] | Ibrutinib + Pembrolizumab | I/II | DCR 4 months | Refractory to standard treatments | 0% | 1.4 | 6.6 | Completed |
Abbreviations: DLT: dose-limiting toxicity; MTD: maximum tolerable dose; DCR: disease control rate; IdcR: immune disease control rate; RD: recommended dose; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; NR: not reached; NA: not available.
3.3. Biomarkers in MSS CRC
There is a strong need to identify new mechanisms that will make MSS tumors sensitive to immunotherapy, in order to achieve the benefits associated with these therapies. Therefore, apart from the combination strategies mentioned, the detection of new biomarkers beyond the MMR status is essential to differentiate the MSS populations for whom development of new strategies using ICIs and other types of immunotherapies may be helpful.
3.3.1. PD-L1
PD-1 is expressed on the surface of T cells, B cells, and natural killer cells and binds to its ligand, PD-L1, which is expressed on the surface of tumor cells to a higher or lower degree. PD-L1 expression or combined positive score (CPS) are used in other malignancies to establish a cutoff from which ICIs may be useful in clinical practice.
In mCRC, regardless of the MMR status, PD-L1 expression has not proved to be predictive of response to ICIs; in the CheckMate 142 clinical trial, a subgroup analysis was performed in the nivolumab group, with no significant differences observed in ORR between PD-L1 <1 and ≥1% (28% vs. 29%, respectively) [168]. In CRC cell lines, observed PD-L1 expression is scarce and below the detection level of IHC techniques, whereas it is predominantly seen in the surround ding myeloid cells; this presentation is unusual in comparison with other immunogenic tumors such as renal or lung cancer, which may explain why PD-L1 is not a trustworthy biomarker for CRC [17].
3.3.2. POLE and POLD1 Mutations
Somatic or germline mutations in the POLE and POLD1 genes are found in 1–2% of all CRC tumors. They lead to alterations in encoding the DNA polymerase epsilon (POLε) and delta (POLδ1), respectively, which play a key role in DNA proofreading and replication. POLε synthesizes the leading strand in the replication process, while POLδ1 oversees the lagging strand. They both enhance the accuracy of replication by recognizing and deleting mismatched base pairs, due in the case of POLε to its exonuclease domain. Therefore, somatic or germline mutations especially in that domain lead to a hypermutated phenotype without dMMR expression [169,170]. Thus, an increase of TILs is found in these tumors, with an average TMB of 158 Mut/Mb, and acquired MMR mutations may develop. In an analysis of 499 CRC tumors, POLE and POLD1 mutated tumors had higher rates of TILs (82%) in comparison with MSI-H (68%) and non-MSI-H CRC (4.5%) [171]
A recent research study reported a greater DCR associated with pathogenic mutations in comparison with the DCR linked to non-actionable variants (82.4% vs. 30.0%; p = 0.013). Although most pathogenic mutations are found in the exonuclease domain, others outside this domain have shown similar results in terms of response to ICIs. Increases in mPFS, mOS, and duration of therapy were observed in patients with pathogenic mutations in comparison with those with benign variants, which remained significant when MSI status was considered. Therefore, POLE and POLD1 pathogenic mutations represent a biomarker of response to ICIs regardless of MMR status [172].
In a recent phase II single-arm study, an ORR of 50% was observed in pretreated POLE mutated tumors treated with nivolumab, including MSS CRC [173]. Other studies are currently ongoing to assess the efficacy of nivolumab vs. nivolumab plus ipilimumab in the same setting [174]. In terms of response rate, comparison with MSI status is under evaluation in a phase II study for MSI or POLE mutated mCRC treated with avelumab, with ORR as the primary endpoint [175].
3.3.3. Tumor Mutational Burden
The tumor mutational burden (TMB) refers to the quantity of somatic mutations in a tumor specimen. It is correlated to an elevated neoantigen expression with an associated higher response to ICIs. Its determination is controversial, since it can vary depending on the method used to calculate it. Currently, next-generation sequencing (NGS) techniques are implemented, which differ in their region of study. The process may range from a genome-wide analysis (whole genome sequencing [WGS]) to whole exome sequencing (WES) that includes the entire coding regions and has been considered the reference standard. However, the appearance of large, targeted gene panels such as Foundation One and MSK-IMPACT represents an improvement on prior techniques, and both have been approved by the FDA for profiling solid tumors. Non-MSI tumors with high TMB are estimated to be present in ~3% of cases [176,177,178].
There is no consensus about the TMB cutoff [179] and currently, based on the results from the phase II basket study KEYNOTE-158, pembrolizumab can be used in pre-treated metastatic tumors with TMB ≥ 10 mut/Mb according to the FoundationOne CDx assay, since an ORR of 29.4% was observed with this cutoff vs. 6.3% in TMB < 10 mut/Mb. This correlated with retrospective WES analyses, corresponding to a WES TMB ≥175 mut/exome, in which an ORR of 31.4% was reported (vs. 9.5% when <175 mut/exome) [24,176]. Although mCRC was not included within the spectrum of tumors of this clinical trial, this was not specifically noted in the approval from the FDA [180].
In MyPathway, a phase IIa multibasket study, atezolizumab was tested in advanced solid tumors with TMB ≥10 mut/Mb. The primary endpoint was ORR in patients with TMB ≥16 mut/Mb. A total of 19 tumor types including mCRC were evaluated, with an ORR of 38.1% and DCR of 61.9% vs. 2.1% and 22.9%in TMB <16 mut/Mb, respectively. Focusing on mCRC, 21 patients were assessed, including MSI and MSS tumors, with a better response observed in the former when TMB ≥16 mut/Mb (n = 10). An objective response was achieved in three out of five patients with MSS tumors and TMB ≥ 16 mut/Mb. Therefore, TMB may play a role in defining the MSS patients that might respond to ICIs and can also predict patients with MSI mCRC who may not benefit from ICIs [181].
3.3.4. Immunoscore
A subgroup of MSS patients presents a prominent immune gene expression similar to that presented by MSI, leading to higher quantities of CD4+ and CD8+ T cells. These immune infiltrates are associated with better outcomes and a decreased probability of developing metastases. The quantities of cytotoxic (CD8+) and memory (CD3+) T cells are measured in the core and margins of the tumor and can be evaluated using the immunoscore system. Its utility has been proved as a prognostic and response biomarker to ICI; higher immunoscores (I3 or I4) are associated with better disease-specific survival (DSS), which was observed mainly in MSI but also in MSS tumors. Among the MSS tumors analyzed, the risk of relapse in tumors with high Th1 CD4+ and cytotoxic CD8+ T-cell gene expression presented no difference in comparison with MSI but was associated with a significantly lower risk of relapse in comparison with MSS with low gene expression (HR = 2.1; p = 0.03) [182,183].
Thus, immunoscore seems a reliable method to predict which patients, including MSS patients, may benefit from immunotherapy, including ICIs and adoptive T-cell therapy, and has already been validated in early-stage (I-III) CRC as a prognostic scale [184].
3.3.5. Microbiome
The human gut microbiome is one of the factors contributing to CRC tumorigenesis, and is formed by the symbiotic microbial cells that form the microbiota. It can be altered by multiple factors that range from genetics to diet or the use of antibiotics. The interaction of the microbiome with the host immunity that determines the homeostasis within the gastrointestinal environment is regulated by microRNAs, which are small non-coding RNAs that participate in post-transcriptional gene regulation. They are secreted by intestinal epithelial cells and released into the lumen, where they modulate the microbiome and establish crosstalk between the microbiome and the host immunity. The interaction is bidirectional, so the gut microbiome may also regulate the expression of microRNAs, as do exogenic dietary microRNAs [185]. Bacterial small RNAs (bsRNAs) also seem to play a role in this crosstalk, although they have been less studied. In a study performed by Tarallo et al., 80 stool specimens from healthy people and patients with CRC or adenomas were collected and analyzed, with higher levers of Escherichia coli bsRNAs and DNA in CRC patients in comparison with the other two groups, which may help establish diagnostic models [186].
Interventions to target the gut microbiome can prevent the development of inflammatory or malignant diseases in preclinical models: administration of Lactobacillus acidophilus avoided the development of CRC in mice carrying a germline APC mutation [187]. Use of the microbiota to boost the efficacy of treatments is also under investigation: administrating L. acidophilus has been demonstrated to modulate tumor growth in preclinical models, reducing oncogenic microRNAs and increasing tumor-suppressor microRNAs in rectal cancer patients [188,189]. The administration of IL-10 and CpG oligodeoxynucleotide proved successful in treating early CRC in mice, due to the release of tumor necrosis factor alpha (TNFα) by Alistipes shahii bacterial species, which are over-represented in the colon of mice with CRC. This effect was lost with the use of broad-spectrum antibiotics or germ-free mice.
The effect of local flora on responses to ICIs has been studied in mice, where the use of ipilimumab reduced the growth of tumoral cells in animals with normal microflora. This is believed to depend partly on non-enterotoxin-producing strains of Bacteroides fragilis. Curiously, transferring B. fragilis-specific CD4+ T cells or transplanting faecal microbiome including Bacteroides species have proved to be effective in reinstating sensitivity to ipilimumab. In cases of anti-PD1 or anti-PDL1, the antitumoral effectiveness is believed to be helped by Bifidobacterium longum, B. adolescentis, and B. breve species. Therefore, the use of a faecal transplant containing the species mentioned may have a role in overcoming the intrinsic resistance in MSS patients [8,190,191,192]. Other species that seem to be favorable for an immune response include Akkermansia muciniphila, Ruminococcaceae, and Faecalibacterium, which were represented in basal samples of patients with superior responses and improved outcomes. The addition of these species by faecal transplant into mice colonized by non-responding patients’ feces showed amelioration of responses to anti-PD(L)1, along with a higher infiltration of immune cells in TME. All the species involved in favorable responses are implicated in decreasing peripheric regulatory T cells and increasing the numbers of dendritic cells and cytotoxic T cells [8,193,194]. Furthermore, the presence of Lachnospiraceae and the already mentioned Ruminococcaceae have been tested in clinical CRC samples. They were correlated with a higher expression of CCR5 and CXCR3 chemokines, which are involved in the recruitment of T cells and hence may boost the response to ICIs [195].
Currently, clinical trials are testing these hypotheses (NCT04264975, NCT04130763, and NCT05279677), with promising results in refractory to ICIs melanoma patients, since three out of ten refractory patients achieved either PR or CR after faecal transplant [194,196,197,198,199].
In conclusion, further studies are required for to understand how the composition of the microbiota may guide us to predict responses to ICIs and how its modulation may have a role in clinical practice. Faecal transplant might change the immune cell infiltrates and gene expression profiles, which could help establish new treatment strategies for MSS CRC.
4. Discussion
CRC represents the third most frequent tumor worldwide and 95% of mCRC cases are MSS [3,6,11]. The use of ICIs has been demonstrated useful in MSI mCRC, with a current indication of pembrolizumab as the standard of care by EMA and FDA, and nivolumab and its combination with ipilimumab by FDA in second or further lines [20,22,29]. However, the use of monotherapy or a combination of ICIs may be less effective in MSS mCRC [134,200].
The immune landscape of mCRC is heterogenous and complex [33]. The differences in TME composition and immune cell infiltrate that exist between MSS and MSI CRC may explain the diverse responses to ICIs observed between these subgroups [201]. The inherent resistance to ICIs in MSS mCRC can be justified by the absence of an effective immune infiltrate for them to work, along with a considerably lower mutation load. Due to these reasons, MSS mCRC tumors require the use of combination strategies and novel agents to increase the antigen load and force the immune system to activate [30,39,202].
The main strategy lies in using combination treatments in order to make these tumors more sensitive to ICIs, either by modulating the immunosuppressant TME and/or by increasing the mutational load. Different strategies are currently under investigation: the combination with CT or RT tries to modulate the surrounding immune infiltrate and obtain from tumoral cell death neoantigens to be presented to the surrounding APC and T cells, which can be enhanced by adding anti-EGFR or anti-VEGF when using CT [203,204]. However, the efficacy of these treatments has not yet been proved, although scant improvements were observed in the combination groups. Furthermore, TMZ can induce a transient MSI status in silenced MGMT MSS mCRC that may bring about sensitization to ICIs [205].
PARP1 is believed to be part of the carcinogenesis of CRC and its role in repairing single-strand DNA damage may be involved in increasing the neoantigen load. Apart from PARP1, other molecules involved in DDR are also being investigated as possible targets to enhance ICIs activity [74,75,76].
Combination strategies with MKI have proved their synergistic effect on other malignancies including renal carcinoma, due to the immune modulator role that these agents present. Although combinations with ICIs have shown better responses than in monotherapy, demonstrating synergistic effects, further studies are required to establish their position in the therapeutic landscape of MSS mCRC [97,98,100,103].
Due to the role of the MAPK pathway in T-cell expansion and immune evasion, MEK inhibitors were first tested in combination with ICIs in these tumors, with unimpressive results. However, promising preliminary results have been observed by targeting BRAF or KRAS when there is a BRAF mutation associated with an MSI phenotype or a KRAS G12C mutation is present, respectively [120,125]. Novel strategies are being assessed including inhibiting DNA methyltransferase or histone deacetylase, which are involved in silencing genes related to the activation of the immune system, with modest responses but considerable DCR achieved [158,159,161].
The combination with other ICIs has not to date provided a clear amelioration of the outcomes presented in monotherapy, although these seem to be improved in cases where no liver metastases are presented or CPS ≥1 [134,135,138,139].
Other signaling pathways involved in the tumorigenesis of MSS CRC include TGF-β and Wnt, due to their roles in promoting angiogenesis and immune evasion in the former and decreasing the recruitment of T cells in the latter. The combination of ICIs with TGF-β inhibitors or monoclonal antibodies has not shown remarkable results so far, but pending clinical trials will clarify their potential role in these tumors [147,148,153].
As previously mentioned, there is a clear necessity of finding robust biomarkers of response beyond the MMR status [206]. PD-L1 expression has not proved its efficacy as a trusty biomarker in CRC, unlike in other malignancies [168]. The analysis of POLE/POLD1 mutations and high TMB might be relevant for finding MSS patients who could benefit from ICIs [173,181]. At the moment, immunoscore seems another promising biomarker, but its utility has only been proved in early-stage CRC [184]. The role that the composition of the microbiome may have in predicting a patient’s responder status, including the possible benefit of using faecal transplant in boosting the response to ICIs in MSS CRC tumors, requires further research [196].
5. Conclusions
In conclusion, there is a clear need to improve therapeutic strategies in MSS mCRC to benefit these patients in terms of long-lasting response and the reduced toxicity of ICIs in comparison with standard chemotherapy. ICIs have not yet been approved in this subgroup due to the scarce results presented so far. Hence, combination treatments and the use of novel therapeutic agents are being considered, although modest outcomes have been observed to date. Thus, translational research focused on novel actionable targets and biomarker selection is constantly needed.
Author Contributions
Conceptualization, M.S.-R.-G. and R.F.-M. (Raquel Fuentes-Mateos); methodology, M.S.-R.-G. and J.T.-J.; software, M.S.-R.-G., P.Á.-B. and J.E.-V.; validation, J.T.-J., D.R.-R., Í.M.-D. and V.A.-F.; formal analysis, M.S.-R.-G., J.T.-J., J.P., J.C.-P.; investigation, R.F.-M. (Raquel Fuentes-Mateos), J.T.-J. and M.S.-R.-G.; resources, M.S.-R.-G. and I.O.-M.; data curation, P.R.-P. and R.F.-M. (Raquel Fuentes-Mateos); writing—original draft preparation, M.S.-R.-G. and J.T.-J.; writing—review and editing, M.S.-R.-G. and J.T.-J.; visualization, M.S.-R.-G., Í.M.-D. and J.T.-J.; supervision, R.F.-M. (Reyes Ferreiro-Monteagudo); project administration, M.S.-R.-G. and J.T.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the patients who participated and are currently participating in the studies mentioned, and their families.
Conflicts of Interest
Reyes Ferreiro-Monteagudo: Amgen, Roche Pharma, Pierre Fabre (C/A), Amgen, Servier, Merck, Roche Pharma, Bayer Hispania, Pierre Fabre (SH) The rest of the authors declares no conflict of interest. (C/A): Consulting/Advisory relationship; (SH) Speaker Honorarium.
References
- Keum, N.; Giovannucci, E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of Colorectal Cancer: Incidence, Mortality, Survival, and Risk Factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669. [Google Scholar] [CrossRef]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2022, 34, S0923753422041928. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The Evolving Landscape of Biomarkers for Checkpoint Inhibitor Immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Arrichiello, G.; Poliero, L.; Borrelli, C.; Paragliola, F.; Nacca, V.; Napolitano, S.; Corte, C.M.D.; Martini, G.; Martinelli, E. Immunotherapy in Colorectal Cancer: Is the Long-Awaited Revolution Finally Happening? Cancer Treat. Res. Commun. 2021, 28, 100442. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in Colorectal Cancer: Current Achievements and Future Perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- Venderbosch, S.; Nagtegaal, I.D.; Maughan, T.S.; Smith, C.G.; Cheadle, J.P.; Fisher, D.; Kaplan, R.; Quirke, P.; Seymour, M.T.; Richman, S.D.; et al. Mismatch Repair Status and BRAF Mutation Status in Metastatic Colorectal Cancer Patients: A Pooled Analysis of the CAIRO, CAIRO2, COIN, and FOCUS Studies. Clin. Cancer Res. 2014, 20, 5322–5330. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Cohen, R.; Pudlarz, T.; Delattre, J.-F.; Colle, R.; André, T. Molecular Targets for the Treatment of Metastatic Colorectal Cancer. Cancers 2020, 12, 2350. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nature 2012, 487, 330–337. [CrossRef]
- Kawakami, H.; Zaanan, A.; Sinicrope, F.A. Microsatellite Instability Testing and Its Role in the Management of Colorectal Cancer. Curr. Treat. Options Oncol. 2015, 16, 30. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in Cancer Immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The Vigorous Immune Microenvironment of Microsatellite Instable Colon Cancer Is Balanced by Multiple Counter-Inhibitory Checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Chan, T.A.; Yarchoan, M.; Jaffee, E.; Swanton, C.; Quezada, S.A.; Stenzinger, A.; Peters, S. Development of Tumor Mutation Burden as an Immunotherapy Biomarker: Utility for the Oncology Clinic. Ann. Oncol. 2019, 30, 44–56. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.; Kemberling, H.; Eyring, A.; Azad, N.S.; Laheru, D.; Donehower, R.C.; Crocenzi, T.S.; et al. Programmed Death-1 Blockade in Mismatch Repair Deficient Colorectal Cancer. J. Clin. Oncol. 2016, 34, 103. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability–High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Lonardi, S.; Wong, M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.; Van Cutsem, E.; McDermott, R.S.; Hill, A.G.; et al. Nivolumab + Ipilimumab Combination in Patients with DNA Mismatch Repair-Deficient/Microsatellite Instability-High (DMMR/MSI-H) Metastatic Colorectal Cancer (MCRC): First Report of the Full Cohort from CheckMate-142. J. Clin. Oncol. 2018, 36, 553. [Google Scholar] [CrossRef]
- Andre, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.M.; Garcia-Carbonero, R.; Alcaide, J.; Gibbs, P.; et al. Final Overall Survival for the Phase III KN177 Study: Pembrolizumab versus Chemotherapy in Microsatellite Instability-High/Mismatch Repair Deficient (MSI-H/DMMR) Metastatic Colorectal Cancer (MCRC). J. Clin. Oncol. 2021, 39, 3500. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair–Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.-J.; Lonardi, S.; Zagonel, V.; Van Cutsem, E.; Limon, M.L.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; Garcia-Alfonso, P.; Neyns, B.; et al. Nivolumab plus Low-Dose Ipilimumab as First-Line Therapy in Microsatellite Instability-High/DNA Mismatch Repair Deficient Metastatic Colorectal Cancer: Clinical Update. J. Clin. Oncol. 2020, 38, 11. [Google Scholar] [CrossRef]
- André, T.; Van Cutsem, E.; Elez, E.; Bennouna, J.; de la Fouchardière, C.; Yoshino, T.; Jensen, L.; Mendez, G.; Li, J.; Goekkurt, E.; et al. P-12 A Phase 3 Study of Nivolumab (NIVO), NIVO + Ipilimumab (IPI), or Chemotherapy for Microsatellite Instability-High (MSI-H)/Mismatch Repair-Deficient (DMMR) Metastatic Colorectal Cancer (MCRC): CheckMate 8HW. Ann. Oncol. 2022, 33, S250. [Google Scholar] [CrossRef]
- Lee, J.J.; Yothers, G.; Jacobs, S.A.; Sanoff, H.K.; Cohen, D.J.; Guthrie, K.A.; Henry, N.L.; Ganz, P.A.; Kopetz, S.; Lucas, P.C.; et al. Colorectal Cancer Metastatic DMMR Immuno-Therapy (COMMIT) Study (NRG- GI004/SWOG-S1610): A Randomized Phase III Study of MFOLFOX6/Bevacizumab Combination Chemotherapy with or without Atezolizumab or Atezolizumab Monotherapy in the First-Line Treatment of Patients with Deficient DNA Mismatch Repair (DMMR) Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, TPS3615. [Google Scholar] [CrossRef]
- Overman, M.J.; Yothers, G.; Jacobs, S.A.; Sanoff, H.K.; Cohen, D.J.; Guthrie, K.A.; Henry, N.L.; Ganz, P.A.; Kopetz, S.; Lucas, P.C.; et al. Colorectal Cancer Metastatic DMMR Immuno-Therapy (COMMIT) Study: A Randomized Phase III Study of Atezolizumab (Atezo) Monotherapy versus MFOLFOX6/Bevacizumab/Atezo in the First-Line Treatment of Patients (Pts) with Deficient DNA Mismatch Repair (DMMR) or Microsatellite Instability High (MSI-H) Metastatic Colorectal Cancer (MCRC)—NRG-GI004/SWOG-S1610. J. Clin. Oncol. 2021, 39, TPS3618. [Google Scholar] [CrossRef]
- Andre, T.; Berton, D.; Curigliano, G.; Ellard, S.; Trigo Pérez, J.M.; Arkenau, H.-T.; Abdeddaim, C.; Moreno, V.; Guo, W.; Im, E.; et al. Safety and Efficacy of Anti–PD-1 Antibody Dostarlimab in Patients (Pts) with Mismatch Repair-Deficient (DMMR) Solid Cancers: Results from GARNET Study. J. Clin. Oncol. 2021, 39, 9. [Google Scholar] [CrossRef]
- Marmorino, F.; Boccaccino, A.; Germani, M.M.; Falcone, A.; Cremolini, C. Immune Checkpoint Inhibitors in PMMR Metastatic Colorectal Cancer: A Tough Challenge. Cancers 2020, 12, 2317. [Google Scholar] [CrossRef]
- O’Neil, B.H.; Wallmark, J.; Lorente, D.; Elez, E.; Raimbourg, J.; Gomez-Roca, C.; Ejadi, S.; Piha-Paul, S.A.; Moss, R.A.; Siu, L.L.; et al. 502 Pembrolizumab (MK-3475) for Patients (Pts) with Advanced Colorectal Carcinoma (CRC): Preliminary Results from KEYNOTE-028. Eur. J. Cancer 2015, 51, S103. [Google Scholar] [CrossRef]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; de Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The Consensus Molecular Subtypes of Colorectal Cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Kim, R.; Emi, M.; Tanabe, K. Cancer Immunoediting from Immune Surveillance to Immune Escape. Immunology 2007, 121, 9–27. [Google Scholar] [CrossRef]
- Newey, A.; Griffiths, B.; Michaux, J.; Pak, H.S.; Stevenson, B.J.; Woolston, A.; Semiannikova, M.; Spain, G.; Barber, L.J.; Matthews, N.; et al. Immunopeptidomics of Colorectal Cancer Organoids Reveals a Sparse HLA Class I Neoantigen Landscape and No Increase in Neoantigens with Interferon or MEK-Inhibitor Treatment. J. Immunother. Cancer 2019, 7, 309. [Google Scholar] [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The Future of Cancer Treatment: Immunomodulation, CARs and Combination Immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273–290. [Google Scholar] [CrossRef]
- Grasso, C.S.; Giannakis, M.; Wells, D.K.; Hamada, T.; Mu, X.J.; Quist, M.; Nowak, J.A.; Nishihara, R.; Qian, Z.R.; Inamura, K.; et al. Genetic Mechanisms of Immune Evasion in Colorectal Cancer. Cancer Discov. 2018, 8, 730–749. [Google Scholar] [CrossRef]
- Lote, H.; Starling, N.; Pihlak, R.; Gerlinger, M. Advances in Immunotherapy for MMR Proficient Colorectal Cancer. Cancer Treat. Rev. 2022, 111, 102480. [Google Scholar] [CrossRef]
- Vanneman, M.; Dranoff, G. Combining Immunotherapy and Targeted Therapies in Cancer Treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef]
- Chen, D.S.; Hurwitz, H. Combinations of Bevacizumab with Cancer Immunotherapy. Cancer J. 2018, 24, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Dosset, M.; Vargas, T.R.; Lagrange, A.; Boidot, R.; Végran, F.; Roussey, A.; Chalmin, F.; Dondaine, L.; Paul, C.; Marie-Joseph, E.L.; et al. PD-1/PD-L1 Pathway: An Adaptive Immune Resistance Mechanism to Immunogenic Chemotherapy in Colorectal Cancer. OncoImmunology 2018, 7, e1433981. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR Pathway Blockade Inhibits Tumor-Induced Regulatory T-Cell Proliferation in Colorectal Cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Elamin, Y.Y.; Rafee, S.; Toomey, S.; Hennessy, B.T. Immune Effects of Bevacizumab: Killing Two Birds with One Stone. Cancer Microenviron. 2015, 8, 15–21. [Google Scholar] [CrossRef]
- Grothey, A.; Tabernero, J.; Arnold, D.; De Gramont, A.; Ducreux, M.P.; O’Dwyer, P.J.; Van Cutsem, E.; Bosanac, I.; Srock, S.; Mancao, C.; et al. Fluoropyrimidine (FP) + Bevacizumab (BEV) + Atezolizumab vs. FP/BEV in BRAFwt Metastatic Colorectal Cancer (MCRC): Findings from Cohort 2 of MODUL—A Multicentre, Randomized Trial of Biomarker-Driven Maintenance Treatment Following First-Line Induction Therapy. Ann. Oncol. 2018, 29, viii714–viii715. [Google Scholar] [CrossRef]
- Mettu, N.B.; Niedzwiecki, D.; Boland, P.M.; Fakih, M.; Arrowood, C.; Bolch, E.; Hurwitz, H.; Grothey, A. BACCI: A Phase II Randomized, Double-Blind, Placebo-Controlled Study of Capecitabine Bevacizumab plus Atezolizumab versus Capecitabine Bevacizumab plus Placebo in Patients with Refractory Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, TPS873. [Google Scholar] [CrossRef]
- Bocobo, A.G.; Wang, R.; Behr, S.; Carnevale, J.C.; Cinar, P.; Collisson, E.A.; Fong, L.; Kidder, W.A.; Ko, A.H.; Kolli, K.P.; et al. Phase II Study of Pembrolizumab plus Capecitabine and Bevacizumab in Microsatellite Stable (MSS) Metastatic Colorectal Cancer (MCRC): Interim Analysis. J. Clin. Oncol. 2021, 39, 77. [Google Scholar] [CrossRef]
- Lenz, H.-J.; Parikh, A.R.; Spigel, D.R.; Cohn, A.L.; Yoshino, T.; Kochenderfer, M.D.; Elez, E.; Shao, S.H.; Deming, D.A.; Holdridge, R.C.; et al. Nivolumab (NIVO) + 5-Fluorouracil/Leucovorin/Oxaliplatin (MFOLFOX6)/Bevacizumab (BEV) versus MFOLFOX6/BEV for First-Line (1L) Treatment of Metastatic Colorectal Cancer (MCRC): Phase 2 Results from CheckMate 9X8. J. Clin. Oncol. 2022, 40, 8. [Google Scholar] [CrossRef]
- Antoniotti, C.; Rossini, D.; Pietrantonio, F.; Catteau, A.; Salvatore, L.; Lonardi, S.; Boquet, I.; Tamberi, S.; Marmorino, F.; Moretto, R.; et al. Upfront FOLFOXIRI plus Bevacizumab with or without Atezolizumab in the Treatment of Patients with Metastatic Colorectal Cancer (AtezoTRIBE): A Multicentre, Open-Label, Randomised, Controlled, Phase 2 Trial. Lancet Oncol. 2022, 23, 876–887. [Google Scholar] [CrossRef]
- Van den Eynde, M.; Mlecnik, B.; Bindea, G.; Fredriksen, T.; Church, S.E.; Lafontaine, L.; Haicheur, N.; Marliot, F.; Angelova, M.; Vasaturo, A.; et al. The Link between the Multiverse of Immune Microenvironments in Metastases and the Survival of Colorectal Cancer Patients. Cancer Cell 2018, 34, 1012–1026.e3. [Google Scholar] [CrossRef]
- Trivedi, S.; Srivastava, R.M.; Concha-Benavente, F.; Ferrone, S.; Garcia-Bates, T.M.; Li, J.; Ferris, R.L. Anti-EGFR Targeted Monoclonal Antibody Isotype Influences Antitumor Cellular Immunity in Head and Neck Cancer Patients. Clin. Cancer Res. 2016, 22, 5229–5237. [Google Scholar] [CrossRef]
- Stein, A.; Binder, M.; Goekkurt, E.; Lorenzen, S.; Riera-Knorrenschild, J.; Depenbusch, R.; Ettrich, T.J.; Doerfel, S.; Al-Batran, S.-E.; Karthaus, M.; et al. Avelumab and Cetuximab in Combination with FOLFOX in Patients with Previously Untreated Metastatic Colorectal Cancer (MCRC): Final Results of the Phase II AVETUX Trial (AIO-KRK-0216). J. Clin. Oncol. 2020, 38, 96. [Google Scholar] [CrossRef]
- Van Den Eynde, M.; Huyghe, N.; De Cuyper, A.; Sinapi, I.; Ferrier, M.; Gilet, M.; Van Maanen, A.; Castella, M.-L.; Galon, J.; Carrasco, J. Interim Analysis of the AVETUXIRI Trial: Avelumab Combined with Cetuximab and Irinotecan for Treatment of Refractory Microsatellite Stable (MSS) Metastatic Colorectal Cancer (MCRC)—A Proof of Concept, Open-Label, Nonrandomized Phase IIa Study. J. Clin. Oncol. 2021, 39, 80. [Google Scholar] [CrossRef]
- Martinelli, E.; Martini, G.; Troiani, T.; Pietrantonio, F.; Avallone, A.; Normanno, N.; Nappi, A.; Maiello, E.; Falcone, A.; Santabarbara, G.; et al. 397O Avelumab plus Cetuximab in Pre-Treated RAS Wild Type Metastatic Colorectal Cancer Patients as a Rechallenge Strategy: The Phase II CAVE (Cetuximab-Avelumab) MCRC Study. Ann. Oncol. 2020, 31, S409–S410. [Google Scholar] [CrossRef]
- Napolitano, S.; Martini, G.; Ciardiello, D.; Di Maio, M.; Normanno, N.; Avallone, A.; Martinelli, E.; Maiello, E.; Troiani, T.; Ciardiello, F. CAVE-2 (Cetuximab-AVElumab) MCRC: A Phase II Randomized Clinical Study of the Combination of Avelumab Plus Cetuximab as a Rechallenge Strategy in Pre-Treated RAS/BRAF Wild-Type MCRC Patients. Front. Oncol. 2022, 12, 940523. [Google Scholar] [CrossRef]
- Lee, M.S.; Loehrer, P.J.; Imanirad, I.; Cohen, S.; Ciombor, K.K.; Moore, D.T.; Carlson, C.A.; Sanoff, H.K.; McRee, A.J. Phase II Study of Ipilimumab, Nivolumab, and Panitumumab in Patients with KRAS/NRAS/BRAF Wild-Type (WT) Microsatellite Stable (MSS) Metastatic Colorectal Cancer (MCRC). J. Clin. Oncol. 2021, 39, 7. [Google Scholar] [CrossRef]
- Morris, V.K.; Parseghian, C.M.; Escano, M.; Johnson, B.; Raghav, K.P.S.; Dasari, A.; Huey, R.; Overman, M.J.; Willis, J.; Lee, M.S.; et al. Phase I/II Trial of Encorafenib, Cetuximab, and Nivolumab in Patients with Microsatellite Stable, BRAF V600E Metastatic Colorectal Cancer. JCO 2022, 40, 12. [Google Scholar] [CrossRef]
- Martinelli, E.; Ciardiello, D.; Martini, G.; Troiani, T.; Cardone, C.; Vitiello, P.P.; Normanno, N.; Rachiglio, A.M.; Maiello, E.; Latiano, T.; et al. Implementing Anti-Epidermal Growth Factor Receptor (EGFR) Therapy in Metastatic Colorectal Cancer: Challenges and Future Perspectives. Ann. Oncol. 2020, 31, 30–40. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Lobefaro, R.; Antista, M.; Lonardi, S.; Raimondi, A.; Morano, F.; Mosconi, S.; Rimassa, L.; Murgioni, S.; Sartore-Bianchi, A.; et al. Capecitabine and Temozolomide versus FOLFIRI in RAS-Mutated, MGMT-Methylated Metastatic Colorectal Cancer. Clin. Cancer Res. 2020, 26, 1017–1024. [Google Scholar] [CrossRef]
- Esteller, M.; Sanchez-Cespedes, M.; Rosell, R.; Sidransky, D.; Baylin, S.B.; Herman, J.G. Detection of Aberrant Promoter Hypermethylation of Tumor Suppressor Genes in Serum DNA from Non-Small Cell Lung Cancer Patients. Cancer Res. 1999, 59, 67–70. [Google Scholar]
- Esteller, M.; Herman, J.G. Generating Mutations but Providing Chemosensitivity: The Role of O6-Methylguanine DNA Methyltransferase in Human Cancer. Oncogene 2004, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hochhauser, D.; Glynne-Jones, R.; Potter, V.; Grávalos, C.; Doyle, T.J.; Pathiraja, K.; Zhang, Q.; Zhang, L.; Sausville, E.A. A Phase II Study of Temozolomide in Patients with Advanced Aerodigestive Tract and Colorectal Cancers and Methylation of the O 6-Methylguanine-DNA Methyltransferase Promoter. Mol. Cancer Ther. 2013, 12, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Amatu, A.; Barault, L.; Moutinho, C.; Cassingena, A.; Bencardino, K.; Ghezzi, S.; Palmeri, L.; Bonazzina, E.; Tosi, F.; Ricotta, R.; et al. Tumor MGMT Promoter Hypermethylation Changes over Time Limit Temozolomide Efficacy in a Phase II Trial for Metastatic Colorectal Cancer. Ann. Oncol. 2016, 27, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Calegari, M.A.; Inno, A.; Monterisi, S.; Orlandi, A.; Santini, D.; Basso, M.; Cassano, A.; Martini, M.; Cenci, T.; de Pascalis, I.; et al. A Phase 2 Study of Temozolomide in Pretreated Metastatic Colorectal Cancer with MGMT Promoter Methylation. Br. J. Cancer 2017, 116, 1279–1286. [Google Scholar] [CrossRef]
- Barault, L.; Amatu, A.; Bleeker, F.E.; Moutinho, C.; Falcomatà, C.; Fiano, V.; Cassingena, A.; Siravegna, G.; Milione, M.; Cassoni, P.; et al. Digital PCR Quantification of MGMT Methylation Refines Prediction of Clinical Benefit from Alkylating Agents in Glioblastoma and Metastatic Colorectal Cancer. Ann. Oncol. 2015, 26, 1994–1999. [Google Scholar] [CrossRef]
- Sartore-Bianchi, A.; Pietrantonio, F.; Amatu, A.; Milione, M.; Cassingena, A.; Ghezzi, S.; Caporale, M.; Berenato, R.; Falcomatà, C.; Pellegrinelli, A.; et al. Digital PCR Assessment of MGMT Promoter Methylation Coupled with Reduced Protein Expression Optimises Prediction of Response to Alkylating Agents in Metastatic Colorectal Cancer Patients. Eur. J. Cancer 2017, 71, 43–50. [Google Scholar] [CrossRef]
- Hunter, C.; Smith, R.; Cahill, D.P.; Stephens, P.; Stevens, C.; Teague, J.; Greenman, C.; Edkins, S.; Bignell, G.; Davies, H.; et al. A Hypermutation Phenotype and Somatic MSH6 Mutations in Recurrent Human Malignant Gliomas after Alkylator Chemotherapy. Cancer Res. 2006, 66, 3987–3991. [Google Scholar] [CrossRef]
- Yip, S.; Miao, J.; Cahill, D.P.; Iafrate, A.J.; Aldape, K.; Nutt, C.L.; Louis, D.N. MSH6 Mutations Arise in Glioblastomas during Temozolomide Therapy and Mediate Temozolomide Resistance. Clin. Cancer Res. 2009, 15, 4622–4629. [Google Scholar] [CrossRef]
- Hervieu, A.; Rébé, C.; Végran, F.; Chalmin, F.; Bruchard, M.; Vabres, P.; Apetoh, L.; Ghiringhelli, F.; Mignot, G. Dacarbazine-Mediated Upregulation of NKG2D Ligands on Tumor Cells Activates NK and CD8 T Cells and Restrains Melanoma Growth. J. Investig. Dermatol. 2013, 133, 499–508. [Google Scholar] [CrossRef]
- Germano, G.; Lamba, S.; Rospo, G.; Barault, L.; Magrì, A.; Maione, F.; Russo, M.; Crisafulli, G.; Bartolini, A.; Lerda, G.; et al. Inactivation of DNA Repair Triggers Neoantigen Generation and Impairs Tumour Growth. Nature 2017, 552, 116–120. [Google Scholar] [CrossRef]
- Morano, F.; Raimondi, A.; Pagani, F.; Lonardi, S.; Salvatore, L.; Cremolini, C.; Murgioni, S.; Randon, G.; Palermo, F.; Antonuzzo, L.; et al. Temozolomide Followed by Combination with Low-Dose Ipilimumab and Nivolumab in Patients With Microsatellite-Stable, O 6 -Methylguanine–DNA Methyltransferase–Silenced Metastatic Colorectal Cancer: The MAYA Trial. J. Clin. Oncol. 2022, 40, 1562–1573. [Google Scholar] [CrossRef]
- Siena, S.; Sartore-Bianchi, A.; Personeni, N.; Pietrantonio, F.; Germano, G.; Amatu, A.; Bonoldi, E.; Valtorta, E.; Barault, L.; Di Nicolantonio, F.; et al. Pembrolizumab in MMR-Proficient Metastatic Colorectal Cancer Pharmacologically Primed to Trigger Dynamic Hypermutation Status: The ARETHUSA Trial. J. Clin. Oncol. 2019, 37, TPS2659. [Google Scholar] [CrossRef]
- Temozolomide, Cisplatin, and Nivolumab in People with Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04457284 (accessed on 13 November 2022).
- Deng, S.; Vlatkovic, T.; Li, M.; Zhan, T.; Veldwijk, M.R.; Herskind, C. Targeting the DNA Damage Response and DNA Repair Pathways to Enhance Radiosensitivity in Colorectal Cancer. Cancers 2022, 14, 4874. [Google Scholar] [CrossRef]
- Nosho, K.; Yamamoto, H.; Mikami, M.; Taniguchi, H.; Takahashi, T.; Adachi, Y.; Imamura, A.; Imai, K.; Shinomura, Y. Overexpression of Poly(ADP-Ribose) Polymerase-1 (PARP-1) in the Early Stage of Colorectal Carcinogenesis. Eur. J. Cancer 2006, 42, 2374–2381. [Google Scholar] [CrossRef]
- Vikas, P.; Borcherding, N.; Chennamadhavuni, A.; Garje, R. Therapeutic Potential of Combining PARP Inhibitor and Immunotherapy in Solid Tumors. Front. Oncol. 2020, 10, 570. [Google Scholar] [CrossRef]
- Phase I/II Study of the Anti-Programmed Death Ligand-1 Durvalumab Antibody (MEDI4736) in Combination with Olaparib and/or Cediranib for Advanced Solid Tumors and Advanced or Recurrent Ovarian, Triple Negative Breast, Lung, Prostate and Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02484404 (accessed on 21 November 2022).
- Zimmer, A.S.; Nichols, E.; Cimino-Mathews, A.; Peer, C.; Cao, L.; Lee, M.-J.; Kohn, E.C.; Annunziata, C.M.; Lipkowitz, S.; Trepel, J.B.; et al. A Phase I Study of the PD-L1 Inhibitor, Durvalumab, in Combination with a PARP Inhibitor, Olaparib, and a VEGFR1–3 Inhibitor, Cediranib, in Recurrent Women’s Cancers with Biomarker Analyses. J. Immunother. Cancer 2019, 7, 197. [Google Scholar] [CrossRef]
- Phase I Study of AZD5363 + Olaparib + Durvalumab in Patients with Advanced or Metastatic Solid Tumor Malignancies. Available online: https://clinicaltrials.gov/ct2/show/NCT03772561 (accessed on 21 November 2022).
- Testing the Combination of the Anti-Cancer Drugs Copanlisib, Olaparib, and MEDI4736 (Durvalumab) in Patients with Advanced Solid Tumors with Selected Mutations. Available online: https://clinicaltrials.gov/ct2/show/NCT03842228 (accessed on 21 November 2022).
- Study of Olaparib (MK-7339) in Combination with Pembrolizumab (MK-3475) in the Treatment of Homologous Recombination Repair Mutation (HRRm) and/or Homologous Recombination Deficiency (HRD)-Positive Advanced Cancer (MK-7339-007/KEYLYNK-007. Available online: https://clinicaltrials.gov/ct2/show/NCT04123366 (accessed on 21 November 2022).
- Safety, Tolerability and Pharmacokinetics of AZD1775 (Adavosertib) Plus MEDI4736 (Durvalumab) in Patients with Advanced Solid Tumours. Available online: https://clinicaltrials.gov/ct2/show/NCT02617277 (accessed on 21 November 2022).
- Avelumab and M6620 for the Treatment of DDR Deficient Metastatic or Unresectable Solid Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04266912 (accessed on 21 November 2022).
- Ohm, J.E.; Gabrilovich, D.I.; Sempowski, G.D.; Kisseleva, E.; Parman, K.S.; Nadaf, S.; Carbone, D.P. VEGF Inhibits T-Cell Development and May Contribute to Tumor-Induced Immune Suppression. Blood 2003, 101, 4878–4886. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to Treat Immune Hot, Altered and Cold Tumours with Combination Immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Grothey, A.; Prager, G.; Yoshino, T. The Mechanism of Action of Regorafenib in Colorectal Cancer: A Guide for the Community Physician. Clin. Adv. Hematol. Oncol. 2019, 17 (Suppl. 12), 1–19. [Google Scholar] [PubMed]
- Abou-Elkacem, L.; Arns, S.; Brix, G.; Gremse, F.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib Inhibits Growth, Angiogenesis, and Metastasis in a Highly Aggressive, Orthotopic Colon Cancer Model. Mol. Cancer Ther. 2013, 12, 1322–1331. [Google Scholar] [CrossRef]
- Ou, D.-L.; Chen, C.-W.; Hsu, C.-L.; Chung, C.-H.; Feng, Z.-R.; Lee, B.-S.; Cheng, A.-L.; Yang, M.-H.; Hsu, C. Regorafenib Enhances Antitumor Immunity via Inhibition of P38 Kinase/Creb1/Klf4 Axis in Tumor-Associated Macrophages. J. Immunother. Cancer 2021, 9, e001657. [Google Scholar] [CrossRef] [PubMed]
- Doleschel, D.; Hoff, S.; Koletnik, S.; Rix, A.; Zopf, D.; Kiessling, F.; Lederle, W. Regorafenib Enhances Anti-PD1 Immunotherapy Efficacy in Murine Colorectal Cancers and Their Combination Prevents Tumor Regrowth. J. Exp. Clin. Cancer Res. 2021, 40, 288. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients with Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Fakih, M.; Raghav, K.P.S.; Chang, D.Z.; Bendell, J.C.; Larson, T.; Cohn, A.L.; Huyck, T.K.; Cosgrove, D.; Fiorillo, J.A.; Garbo, L.E.; et al. Single-Arm, Phase 2 Study of Regorafenib plus Nivolumab in Patients with Mismatch Repair-Proficient (PMMR)/Microsatellite Stable (MSS) Colorectal Cancer (CRC). J. Clin. Oncol. 2021, 39, 3560. [Google Scholar] [CrossRef]
- Cousin, S.; Cantarel, C.; Guegan, J.-P.; Gomez-Roca, C.; Metges, J.-P.; Adenis, A.; Pernot, S.; Bellera, C.; Kind, M.; Auzanneau, C.; et al. Regorafenib-Avelumab Combination in Patients with Microsatellite Stable Colorectal Cancer (REGOMUNE): A Single-Arm, Open-Label, Phase II Trial. Clin. Cancer Res. 2021, 27, 2139–2147. [Google Scholar] [CrossRef]
- Grothey, A.; Cutsem, E.V.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Kim, R.D.; Kovari, B.P.; Martinez, M.; Xie, H.; Sahin, I.H.; Mehta, R.; Strosberg, J.; Imanirad, I.; Ghayouri, M.; Kim, Y.; et al. A Phase I/Ib Study of Regorafenib and Nivolumab in Mismatch Repair Proficient Advanced Refractory Colorectal Cancer. Eur. J. Cancer 2022, 169, 93–102. [Google Scholar] [CrossRef]
- Barzi, A.; Azad, N.S.; Yang, Y.; Tsao-Wei, D.; Rehman, R.; Fakih, M.; Iqbal, S.; El-Khoueiry, A.B.; Millstein, J.; Jayachandran, P.; et al. Phase I/II Study of Regorafenib (Rego) and Pembrolizumab (Pembro) in Refractory Microsatellite Stable Colorectal Cancer (MSSCRC). J. Clin. Oncol. 2022, 40, 15. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.H.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Gomez-Roca, C.; Yanez, E.; Im, S.-A.; Castanon Alvarez, E.; Senellart, H.; Doherty, M.; García-Corbacho, J.; Lopez, J.S.; Basu, B.; Maurice-Dror, C.; et al. LEAP-005: A Phase II Multicohort Study of Lenvatinib plus Pembrolizumab in Patients with Previously Treated Selected Solid Tumors—Results from the Colorectal Cancer Cohort. J. Clin. Oncol. 2021, 39, 94. [Google Scholar] [CrossRef]
- Merck Sharp & Dohme LLC A Phase 3 Randomized Study of Lenvatinib in Combination with Pembrolizumab Versus Standard of Care in Participants with Metastatic Colorectal Cancer Who Have Received and Progressed On or After or Became Intolerant to Prior Treatment. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04776148 (accessed on 21 November 2022).
- Kato, Y.; Tabata, K.; Kimura, T.; Yachie-Kinoshita, A.; Ozawa, Y.; Yamada, K.; Ito, J.; Tachino, S.; Hori, Y.; Matsuki, M.; et al. Lenvatinib plus Anti-PD-1 Antibody Combination Treatment Activates CD8+ T Cells through Reduction of Tumor-Associated Macrophage and Activation of the Interferon Pathway. PLoS ONE 2019, 14, e0212513. [Google Scholar] [CrossRef]
- Saeed, A.; Park, R.; Dai, J.; Al-Rajabi, R.M.T.; Kasi, A.; Saeed, A.; Collins, Z.; Thompson, K.; Barbosa, L.; Mulvaney, K.; et al. Phase II Trial of Cabozantinib (Cabo) plus Durvalumab (Durva) in Chemotherapy Refractory Patients with Advanced Mismatch Repair Proficient/Microsatellite Stable (PMMR/MSS) Colorectal Cancer (CRC): CAMILLA CRC Cohort Results. J. Clin. Oncol. 2022, 40, 135. [Google Scholar] [CrossRef]
- Abrams, T.A.; Kazmi, S.M.A.; Winer, I.S.; Subbiah, V.; Falchook, G.S.; Reilley, M.; Kunk, P.R.; Goel, S.; Garrido-Laguna, I.; Kochenderfer, M.D.; et al. A Phase 1b Multitumor Cohort Study of Cabozantinib plus Atezolizumab in Advanced Solid Tumors (COSMIC-021): Results of the Colorectal Cancer Cohort. J. Clin. Oncol. 2022, 40, 121. [Google Scholar] [CrossRef]
- Salem, M.E.; Andre, T.; El-Refai, S.M.; Kopetz, S.; Tabernero, J.; Sinicrope, F.A.; Tie, J.; George, T.J.; VanCutsem, E.; Mauer, E.; et al. Impact of RAS Mutations on Immunologic Characteristics of the Tumor Microenvironment (TME) in Patients with Microsatellite Instability-High (MSI-H) or Mismatch-Repair–Deficient (DMMR) Colorectal Cancer (CRC). J. Clin. Oncol. 2022, 40, 3067. [Google Scholar] [CrossRef]
- Sun, L.; Huang, S.; Li, D.; Mao, Y.; Wang, Y.; Wu, J. Efficacy and Safety of Fruquintinib Plus PD-1 Inhibitors Versus Regorafenib Plus PD-1 Inhibitors in Refractory Microsatellite Stable Metastatic Colorectal Cancer. Front. Oncol. 2021, 11, 754881. [Google Scholar] [CrossRef]
- Nie, C.; Lv, H.; Chen, B.; Xu, W.; Wang, J.; Liu, Y.; Wang, S.; Zhao, J.; He, Y.; Chen, X. Microsatellite Stable Metastatic Colorectal Cancer without Liver Metastasis May Be Preferred Population for Regorafenib or Fruquintinib plus Sintilimab as Third-Line or above Therapy:A Real-World Study. Front. Oncol. 2022, 12, 917353. [Google Scholar] [CrossRef]
- Hutchison Medipharma Limited. An Open-Label, Phase 1b/2 Study to Evaluate the Safety and Efficacy of Fruquintinib in Combination With Tislelizumab in Patients With Advanced Solid Tumors. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04577963 (accessed on 21 November 2022).
- Zhang, J. A Single-Arm, Multicenter Phase II Clinical Study to Evaluate the Efficacy and Safety of Tyrosine Kinase Inhibitor (TKI) in Combination with Anti-PD-1 Antibody in TKI-Responded Microsatellite Stability/Proficient Mismatch Repair (MSS/PMMR) Metastatic Colorectal Adenocarcinoma. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04483219 (accessed on 21 November 2022).
- Tolba, M.F. Revolutionizing the Landscape of Colorectal Cancer Treatment: The Potential Role of Immune Checkpoint Inhibitors. Int. J. Cancer 2020, 147, 2996–3006. [Google Scholar] [CrossRef]
- Coelho, M.A.; de Carné Trécesson, S.; Rana, S.; Zecchin, D.; Moore, C.; Molina-Arcas, M.; East, P.; Spencer-Dene, B.; Nye, E.; Barnouin, K.; et al. Oncogenic RAS Signaling Promotes Tumor Immunoresistance by Stabilizing PD-L1 MRNA. Immunity 2017, 47, 1083–1099.e6. [Google Scholar] [CrossRef]
- Mimura, K.; Shiraishi, K.; Mueller, A.; Izawa, S.; Kua, L.-F.; So, J.; Yong, W.-P.; Fujii, H.; Seliger, B.; Kiessling, R.; et al. The MAPK Pathway Is a Predominant Regulator of HLA-A Expression in Esophageal and Gastric Cancer. J. Immunol. 2013, 191, 6261–6272. [Google Scholar] [CrossRef]
- Ebert, P.J.R.; Cheung, J.; Yang, Y.; McNamara, E.; Hong, R.; Moskalenko, M.; Gould, S.E.; Maecker, H.; Irving, B.A.; Kim, J.M.; et al. MAP Kinase Inhibition Promotes T Cell and Anti-Tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016, 44, 609–621. [Google Scholar] [CrossRef]
- Liu, L.; Mayes, P.A.; Eastman, S.; Shi, H.; Yadavilli, S.; Zhang, T.; Yang, J.; Seestaller-Wehr, L.; Zhang, S.-Y.; Hopson, C.; et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin. Cancer Res. 2015, 21, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Kim, T.W.; Bendell, J.; Argilés, G.; Tebbutt, N.C.; Di Bartolomeo, M.; Falcone, A.; Fakih, M.; Kozloff, M.; Segal, N.H.; et al. Atezolizumab with or without Cobimetinib versus Regorafenib in Previously Treated Metastatic Colorectal Cancer (IMblaze370): A Multicentre, Open-Label, Phase 3, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 849–861. [Google Scholar] [CrossRef] [PubMed]
- McGregor, M.; Price, T.J. IMblaze 370: Lessons Learned and Future Strategies in Colorectal Cancer Treatment. Ann. Transl. Med. 2019, 7, 602. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Kim, T.-W.; Lee, C.B.; Goh, B.-C.; Miller, W.H.; Oh, D.-Y.; Jamal, R.; Chee, C.-E.; Chow, L.Q.M.; Gainor, J.F.; et al. Phase Ib Study of Atezolizumab Combined with Cobimetinib in Patients with Solid Tumors. Ann. Oncol. 2019, 30, 1134–1142. [Google Scholar] [CrossRef]
- Friedrich, T.; Blatchford, P.J.; Lentz, R.W.; Davis, S.L.; Kim, S.S.; Leal, A.D.; Voorde, Z.V.D.; Lee, M.R.; Waring, M.; Cull, T.; et al. A Phase II Study of Pembrolizumab, Binimetinib, and Bevacizumab in Patients with Microsatellite-Stable, Refractory, Metastatic Colorectal Cancer (MCRC). J. Clin. Oncol. 2022. [Google Scholar] [CrossRef]
- Lee, J.J.; Chu, E. Recent Advances in the Clinical Development of Immune Checkpoint Blockade Therapy for Mismatch Repair Proficient (PMMR)/Non-MSI-H Metastatic Colorectal Cancer. Clin. Color. Cancer 2018, 17, 258–273. [Google Scholar] [CrossRef]
- A Study to Assess the Safety, Tolerability and Anti-Tumour Activity of Ascending Doses of Selumetinib in Combination with MEDI4736 and Selumetinib in Combination with MEDI4736 and Tremelimumab in Patients with Advanced Solid Tumours. Available online: https://clinicaltrials.gov/ct2/show/NCT02586987 (accessed on 12 November 2022).
- Goel, A.; Nagasaka, T.; Arnold, C.N.; Inoue, T.; Hamilton, C.; Niedzwiecki, D.; Compton, C.; Mayer, R.J.; Goldberg, R.; Bertagnolli, M.M.; et al. The CpG Island Methylator Phenotype and Chromosomal Instability Are Inversely Correlated in Sporadic Colorectal Cancer. Gastroenterology 2007, 132, 127–138. [Google Scholar] [CrossRef]
- Molina-Cerrillo, J.; San Román, M.; Pozas, J.; Alonso-Gordoa, T.; Pozas, M.; Conde, E.; Rosas, M.; Grande, E.; García-Bermejo, M.L.; Carrato, A. BRAF Mutated Colorectal Cancer: New Treatment Approaches. Cancers 2020, 12, 1571. [Google Scholar] [CrossRef]
- Corcoran, R.; Giannakis, M.; Allen, J.; Chen, J.; Pelka, K.; Chao, S.; Meyerhardt, J.; Enzinger, A.; Enzinger, P.; McCleary, N.; et al. SO-26 Clinical Efficacy of Combined BRAF, MEK, and PD-1 Inhibition in BRAFV600E Colorectal Cancer Patients. Ann. Oncol. 2020, 31, S226–S227. [Google Scholar] [CrossRef]
- Ros, J.; Baraibar, I.; Sardo, E.; Mulet, N.; Salvà, F.; Argilés, G.; Martini, G.; Ciardiello, D.; Cuadra, J.L.; Tabernero, J.; et al. BRAF, MEK and EGFR Inhibition as Treatment Strategies in BRAF V600E Metastatic Colorectal Cancer. Adv. Med. Oncol 2021, 13, 175883592199297. [Google Scholar] [CrossRef]
- A Study of Select Drug Combinations in Adult Patients with Advanced/Metastatic BRAF V600 Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT04294160 (accessed on 13 November 2022).
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRAS G12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Fakih, M.G.; Kopetz, S.; Kuboki, Y.; Kim, T.W.; Munster, P.N.; Krauss, J.C.; Falchook, G.S.; Han, S.-W.; Heinemann, V.; Muro, K.; et al. Sotorasib for Previously Treated Colorectal Cancers with KRASG12C Mutation (CodeBreaK100): A Prespecified Analysis of a Single-Arm, Phase 2 Trial. Lancet Oncol. 2022, 23, 115–124. [Google Scholar] [CrossRef]
- A Phase 1/2, Study Evaluating the Safety, Tolerability, PK, and Efficacy of Sotorasib (AMG 510) in Subjects with Solid Tumors with a Specific KRAS Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT03600883 (accessed on 13 November 2022).
- Study of JDQ443 in Patients with Advanced Solid Tumors Harboring the KRAS G12C Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT04699188 (accessed on 13 November 2022).
- Chen, X.; Zou, F.; Hu, Z.; Du, G.; Yu, P.; Wang, W.; Wang, H.; Ye, L.; Tian, J. PCC0208023, a Potent SHP2 Allosteric Inhibitor, Imparts an Antitumor Effect against KRAS Mutant Colorectal Cancer. Toxicol. Appl. Pharmacol. 2020, 398, 115019. [Google Scholar] [CrossRef]
- Baraibar, I.; Mirallas, O.; Saoudi, N.; Ros, J.; Salvà, F.; Tabernero, J.; Élez, E. Combined Treatment with Immunotherapy-Based Strategies for MSS Metastatic Colorectal Cancer. Cancers 2021, 13, 6311. [Google Scholar] [CrossRef]
- Morschhauser, F.; Machiels, J.-P.; Salles, G.; Rottey, S.; Rule, S.A.J.; Cunningham, D.; Peyrade, F.; Fruchart, C.; Arkenau, H.-T.; Genvresse, I.; et al. On-Target Pharmacodynamic Activity of the PI3K Inhibitor Copanlisib in Paired Biopsies from Patients with Malignant Lymphoma and Advanced Solid Tumors. Mol. Cancer Ther. 2020, 19, 468–478. [Google Scholar] [CrossRef]
- Jakubowski, C.; Collins, N.B.; Sugar, E.A.; Berg, M.; Cao, H.; Giannakis, M.; Jaffee, E.M.; Azad, N.S. A Phase I/II Study of PI3Kinase Inhibition with Copanlisib Combined with the Anti-PD-1 Antibody Nivolumab in Relapsed/Refractory Solid Tumors with Expansions in MSS Colorectal Cancer. J. Clin. Oncol. 2020, 38, TPS4114. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I Study of Single-Agent Anti–Programmed Death-1 (MDX-1106) in Refractory Solid Tumors: Safety, Clinical Activity, Pharmacodynamics, and Immunologic Correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. CheckMate 067: 6.5-Year Outcomes in Patients (Pts) with Advanced Melanoma. J. Clin. Oncol. 2021, 39, 9506. [Google Scholar] [CrossRef]
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. Effect of Combined Immune Checkpoint Inhibition vs. Best Supportive Care Alone in Patients with Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol. 2020, 6, 831. [Google Scholar] [CrossRef]
- Vaccaro, G.M.; Rothe, M.; Mangat, P.K.; Garrett-Mayer, E.; Hwang, J.J.; Alese, O.B.; Khalil, M.F.; Hameed, M.K.; Duvivier, H.L.; Cannon, T.L.; et al. Nivolumab plus Ipilimumab (N+I) in Patients (Pts) with Colorectal Cancer (CRC) with High Tumor Mutational Burden (HTMB): Results from the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. J. Clin. Oncol. 2022, 40, 107. [Google Scholar] [CrossRef]
- Bullock, A.; Grossman, J.; Fakih, M.; Lenz, H.; Gordon, M.; Margolin, K.; Wilky, B.; Mahadevan, D.; Trent, J.; Bockorny, B.; et al. LBA O-9 Botensilimab, a Novel Innate/Adaptive Immune Activator, plus Balstilimab (Anti-PD-1) for Metastatic Heavily Pretreated Microsatellite Stable Colorectal Cancer. Ann. Oncol. 2022, 33, S376. [Google Scholar] [CrossRef]
- Fc-Engineered Anti-CTLA-4 Monoclonal Antibody in Advanced Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03860272 (accessed on 17 November 2022).
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver Metastasis Restrains Immunotherapy Efficacy via Macrophage-Mediated T Cell Elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Garralda, E.; Sukari, A.; Lakhani, N.J.; Patnaik, A.; Lou, Y.; Im, S.-A.; Golan, T.; Geva, R.; Wermke, M.; De Miguel, M.; et al. A Phase 1 First-in-Human Study of the Anti-LAG-3 Antibody MK4280 (Favezelimab) plus Pembrolizumab in Previously Treated, Advanced Microsatellite Stable Colorectal Cancer. J. Clin. Oncol. 2021, 39, 3584. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Demaria, S.; Rodriguez-Ruiz, M.E.; Zarour, H.M.; Melero, I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 1845–1855. [Google Scholar] [CrossRef]
- Segal, N.H.; Kemeny, N.E.; Cercek, A.; Reidy, D.L.; Raasch, P.J.; Warren, P.; Hrabovsky, A.E.; Campbell, N.; Shia, J.; Goodman, K.A.; et al. Non-Randomized Phase II Study to Assess the Efficacy of Pembrolizumab (Pem) plus Radiotherapy (RT) or Ablation in Mismatch Repair Proficient (PMMR) Metastatic Colorectal Cancer (MCRC) Patients. J. Clin. Oncol. 2016, 34, 3539. [Google Scholar] [CrossRef]
- Parikh, A.R.; Clark, J.W.; Wo, J.Y.-L.; Yeap, B.Y.; Allen, J.N.; Blaszkowsky, L.S.; Ryan, D.P.; Giantonio, B.J.; Weekes, C.D.; Zhu, A.X.; et al. A Phase II Study of Ipilimumab and Nivolumab with Radiation in Microsatellite Stable (MSS) Metastatic Colorectal Adenocarcinoma (MCRC). JCO 2019, 37, 3514. [Google Scholar] [CrossRef]
- Segal, N.H.; Cercek, A.; Ku, G.; Wu, A.J.; Rimner, A.; Khalil, D.N.; Reidy-Lagunes, D.; Cuaron, J.; Yang, T.J.; Weiser, M.R.; et al. Phase II Single-Arm Study of Durvalumab and Tremelimumab with Concurrent Radiotherapy in Patients with Mismatch Repair–Proficient Metastatic Colorectal Cancer. Clin. Cancer Res. 2021, 27, 2200–2208. [Google Scholar] [CrossRef]
- Zheng, W.; Skowron, K.B.; Namm, J.P.; Burnette, B.; Fernandez, C.; Arina, A.; Liang, H.; Spiotto, M.T.; Posner, M.C.; Fu, Y.-X.; et al. Combination of Radiotherapy and Vaccination Overcomes Checkpoint Blockade Resistance. Oncotarget 2016, 7, 43039–43051. [Google Scholar] [CrossRef]
- Atezolizumab with Stereotactic Ablative Radiotherapy in Patients with Metastatic Tumours. Available online: https://clinicaltrials.gov/ct2/show/NCT02992912 (accessed on 12 November 2022).
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Cañellas, A.; Hernando-Momblona, X.; et al. TGFβ Drives Immune Evasion in Genetically Reconstituted Colon Cancer Metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, K.W.; Ahn, J.B.; Lee, J.; Ryu, J.; Oh, B.; Ock, C.-Y.; Hwang, S.; Hahm, K.B.; Kim, S.-J.; et al. Efficacy and Safety of Vactosertib and Pembrolizumab Combination in Patients with Previously Treated Microsatellite Stable Metastatic Colorectal Cancer. J. Clin. Oncol. 2021, 39, 3573. [Google Scholar] [CrossRef]
- Morris, V.K.; Overman, M.J.; Lam, M.; Parseghian, C.M.; Johnson, B.; Dasari, A.; Raghav, K.; Kee, B.K.; Huey, R.; Wolff, R.A.; et al. Bintrafusp Alfa, an Anti-PD-L1:TGFβ Trap Fusion Protein, in Patients with CtDNA-Positive, Liver-Limited Metastatic Colorectal Cancer. Cancer Res. Commun. 2022, 2, 979–986. [Google Scholar] [CrossRef]
- Dodagatta-Marri, E.; Meyer, D.S.; Reeves, M.Q.; Paniagua, R.; To, M.D.; Binnewies, M.; Broz, M.L.; Mori, H.; Wu, D.; Adoumie, M.; et al. α-PD-1 Therapy Elevates Treg/Th Balance and Increases Tumor Cell PSmad3 That Are Both Targeted by α-TGFβ Antibody to Promote Durable Rejection and Immunity in Squamous Cell Carcinomas. J. Immunother. Cancer 2019, 7, 62. [Google Scholar] [CrossRef]
- Novartis Pharmaceuticals. A Phase I/Ib, Open-Label, Multi-Center Dose Escalation Study of NIS793 in Combination with PDR001 in Adult Patients with Advanced Malignancies. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT02947165 (accessed on 27 December 2022).
- Seshagiri, S.; Stawiski, E.W.; Durinck, S.; Modrusan, Z.; Storm, E.E.; Conboy, C.B.; Chaudhuri, S.; Guan, Y.; Janakiraman, V.; Jaiswal, B.S.; et al. Recurrent R-Spondin Fusions in Colon Cancer. Nature 2012, 488, 660–664. [Google Scholar] [CrossRef]
- Eisai Inc. An Open-Label, Multicenter, Phase 1b/2 Study of E7386 in Combination with Pembrolizumab in Previously Treated Subjects With Selected Solid Tumors. 2023. Available online: https://clinicaltrials.gov/ct2/show/NCT05091346 (accessed on 27 December 2022).
- Yamada, K.; Hori, Y.; Inoue, S.; Yamamoto, Y.; Iso, K.; Kamiyama, H.; Yamaguchi, A.; Kimura, T.; Uesugi, M.; Ito, J.; et al. E7386, a Selective Inhibitor of the Interaction between β-Catenin and CBP, Exerts Antitumor Activity in Tumor Models with Activated Canonical Wnt Signaling. Cancer Res. 2021, 81, 1052–1062. [Google Scholar] [CrossRef]
- M.D. Anderson Cancer Center. A Phase Ib/II Trial of M7824 in Solid Tumors with Microsatellite Instability with Consensus Molecular Subtype 4 Metastatic Colorectal Cancer in Combination with Radiation, or in Colorectal Cancer Patients With Detectable Circulating Tumor DNA Following Definitive Therapy. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03436563 (accessed on 27 December 2022).
- Greco, R.; Qu, H.; Qu, H.; Theilhaber, J.; Shapiro, G.; Gregory, R.; Winter, C.; Malkova, N.; Sun, F.; Jaworski, J.; et al. Pan-TGFβ Inhibition by SAR439459 Relieves Immunosuppression and Improves Antitumor Efficacy of PD-1 Blockade. OncoImmunology 2020, 9, 1811605. [Google Scholar] [CrossRef]
- Eli Lilly and Company. A Phase 1b/2 Dose Escalation and Cohort Expansion Study of the Safety, Tolerability and Efficacy of a Novel Transforming Growth Factor-Beta Receptor I Kinase Inhibitor (Galunisertib) Administered in Combination with Anti-PD-1 (Nivolumab) in Advanced Refractory Solid Tumors (Phase 1b) and in Recurrent or Refractory Non-Small Cell Lung Cancer or Hepatocellular Carcinoma (Phase 2). 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT02734160 (accessed on 27 December 2022).
- Jones, P.A.; Ohtani, H.; Chakravarthy, A.; De Carvalho, D.D. Epigenetic Therapy in Immune-Oncology. Nat. Rev. Cancer 2019, 19, 151–161. [Google Scholar] [CrossRef]
- Kuang, C.; Park, Y.; Augustin, R.C.; Lin, Y.; Hartman, D.J.; Seigh, L.; Pai, R.K.; Sun, W.; Bahary, N.; Ohr, J.; et al. Pembrolizumab plus Azacitidine in Patients with Chemotherapy Refractory Metastatic Colorectal Cancer: A Single-Arm Phase 2 Trial and Correlative Biomarker Analysis. Clin. Epigenet. 2022, 14, 3. [Google Scholar] [CrossRef]
- Celleron Therapeutics Reports 3-Year Survival Data from Phase II Clinical Trial in MSS Colorectal Cancer Patients Treated with Zabadinostat and Nivolumab Combination—Celleron Therapeutics. Available online: https://cellerontherapeutics.com/ (accessed on 29 November 2022).
- A Trial of CXD101 in Combination with Nivolumab in Patients with Metastatic Microsatellite-Stable Colorectal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03993626 (accessed on 19 November 2022).
- Azad, N.S.; Shirai, K.; McRee, A.J.; Opyrchal, M.; Johnson, D.B.; Ordentlich, P.; Brouwer, S.; Sankoh, S.; Schmidt, E.V.; Meyers, M.L.; et al. ENCORE 601: A Phase 2 Study of Entinostat in Combination with Pembrolizumab in Patients with Microsatellite Stable Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 3557. [Google Scholar] [CrossRef]
- Wang, H.; Liu, G.; Jin, X.; Song, S.; Chen, S.; Zhou, P.; Li, H.; Liang, J.; Li, B.; Zhang, C.; et al. BET Inhibitor JQ1 Enhances Anti-Tumor Immunity and Synergizes with PD-1 Blockade in CRC. J. Cancer 2022, 13, 2126–2137. [Google Scholar] [CrossRef]
- Tabernero, J.; Melero, I.; Ros, W.; Argiles, G.; Marabelle, A.; Rodriguez-Ruiz, M.E.; Albanell, J.; Calvo, E.; Moreno, V.; Cleary, J.M.; et al. Phase Ia and Ib Studies of the Novel Carcinoembryonic Antigen (CEA) T-Cell Bispecific (CEA CD3 TCB) Antibody as a Single Agent and in Combination with Atezolizumab: Preliminary Efficacy and Safety in Patients with Metastatic Colorectal Cancer (MCRC). J. Clin. Oncol. 2017, 35, 3002. [Google Scholar] [CrossRef]
- Haag, G.M.; Halama, N.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschaebitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; et al. Combined PD-1 Inhibition (Pembrolizumab) and CCR5 Inhibition (Maraviroc) for the Treatment of Refractory Microsatellite Stable (MSS) Metastatic Colorectal Cancer (MCRC): First Results of the PICCASSO Phase I Trial. J. Clin. Oncol. 2020, 38, 3010. [Google Scholar] [CrossRef]
- Haag, G.M.; Springfeld, C.; Grün, B.; Apostolidis, L.; Zschäbitz, S.; Dietrich, M.; Berger, A.-K.; Weber, T.F.; Zoernig, I.; Schaaf, M.; et al. Pembrolizumab and Maraviroc in Refractory Mismatch Repair Proficient/Microsatellite-Stable Metastatic Colorectal Cancer—The PICCASSO Phase I Trial. Eur. J. Cancer 2022, 167, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Tan, E.; Zhou, J.-M.; Schell, M.J.; Martinez, M.; Yu, J.; Carballido, E.; Mehta, R.; Strosberg, J.; Imanirad, I.; et al. A Phase 1/2 Trial of Ibrutinib in Combination with Pembrolizumab in Patients with Mismatch Repair Proficient Metastatic Colorectal Cancer. Br. J. Cancer 2021, 124, 1803–1808. [Google Scholar] [CrossRef]
- AbbVie. A Phase 1 First-in Human, Multi-Center, Open Label Dose-Escalation Study to Determine the Safety, Tolerability, Pharmacokinetics and RP2D of ABBV-151 as a Single Agent and in Combination With ABBV-181 in Subjects with Locally Advanced or Metastatic Solid Tumors. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03821935 (accessed on 15 December 2022).
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.-J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in Patients with Metastatic DNA Mismatch Repair-Deficient or Microsatellite Instability-High Colorectal Cancer (CheckMate 142): An Open-Label, Multicentre, Phase 2 Study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef]
- Shlien, A.; Campbell, B.B.; de Borja, R.; Alexandrov, L.B.; Merico, D.; Wedge, D.; Van Loo, P.; Tarpey, P.S.; Coupland, P.; Behjati, S.; et al. Combined Hereditary and Somatic Mutations of Replication Error Repair Genes Result in Rapid Onset of Ultra-Hypermutated Cancers. Nat. Genet. 2015, 47, 257–262. [Google Scholar] [CrossRef]
- The CORGI Consortium; The WGS500 Consortium; Palles, C.; Cazier, J.-B.; Howarth, K.M.; Domingo, E.; Jones, A.M.; Broderick, P.; Kemp, Z.; Spain, S.L.; et al. Germline Mutations Affecting the Proofreading Domains of POLE and POLD1 Predispose to Colorectal Adenomas and Carcinomas. Nat. Genet. 2013, 45, 136–144. [Google Scholar] [CrossRef]
- Keshinro, A.; Vanderbilt, C.; Kim, J.K.; Firat, C.; Chen, C.-T.; Yaeger, R.; Ganesh, K.; Segal, N.H.; Gonen, M.; Shia, J.; et al. Tumor-Infiltrating Lymphocytes, Tumor Mutational Burden, and Genetic Alterations in Microsatellite Unstable, Microsatellite Stable, or Mutant POLE/POLD1 Colon Cancer. JCO Precis. Oncol. 2021, 5, 817–826. [Google Scholar] [CrossRef]
- Garmezy, B.; Gheeya, J.; Lin, H.Y.; Huang, Y.; Kim, T.; Jiang, X.; Thein, K.Z.; Pilié, P.G.; Zeineddine, F.; Wang, W.; et al. Clinical and Molecular Characterization of POLE Mutations as Predictive Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Cancers. JCO Precis. Oncol. 2022, 6, e2100267. [Google Scholar] [CrossRef]
- High Activity of Nivolumab in Patients with Pathogenic Exonucleasic Domain POLE (EdPOLE) Mutated Mismatch Repair Proficient (MMRp) Advanced Tumours|OncologyPRO. Available online: https://oncologypro.esmo.org/meeting-resources/esmo-virtual-congress-2020/high-activity-of-nivolumab-in-patients-with-pathogenic-exonucleasic-domain-pole-edpole-mutated-mismatch-repair-proficient-mmrp-advanced-tumours (accessed on 22 November 2022).
- Canadian Cancer Trials Group. A Phase II Open Label, Randomized Non-Comparative Trial of Nivolumab Alone or in Combination with Ipilimumab for the Treatment of Patients With Advanced Hypermutated Solid Tumors Detected by a Blood Based Assay. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03461952 (accessed on 15 December 2022).
- Kim, T.W. A Phase II Study of Durvalumab in Patients with Mismatch Repair Deficient or POLE Mutated Metastatic Colorectal Cancer. 2020. Available online: https://clinicaltrials.gov (accessed on 21 November 2022).
- Addeo, A.; Friedlaender, A.; Banna, G.L.; Weiss, G.J. TMB or Not TMB as a Biomarker: That Is the Question. Crit. Rev. Oncol. Hematol. 2021, 163, 103374. [Google Scholar] [CrossRef]
- Fabrizio, D.A.; George, T.J., Jr.; Dunne, R.F.; Frampton, G.; Sun, J.; Gowen, K.; Kennedy, M.; Greenbowe, J.; Schrock, A.B.; Hezel, A.F.; et al. Beyond Microsatellite Testing: Assessment of Tumor Mutational Burden Identifies Subsets of Colorectal Cancer Who May Respond to Immune Checkpoint Inhibition. J. Gastrointest. Oncol. 2018, 9, 610–617. [Google Scholar] [CrossRef]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB Measurement in Clinical Practice: Considerations on Assay Requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Wu, Z.; Zeng, F.; Song, B.; Zhang, Y.; Li, J.; Lui, S.; Wu, M. Tumor Mutational Burden Predicting the Efficacy of Immune Checkpoint Inhibitors in Colorectal Cancer: A Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 751407. [Google Scholar] [CrossRef]
- Research, C. for D.E. and FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors; FDA: Silver Spring, MD, USA, 2020.
- Friedman, C.F.; Hainsworth, J.D.; Kurzrock, R.; Spigel, D.R.; Burris, H.A.; Sweeney, C.J.; Meric-Bernstam, F.; Wang, Y.; Levy, J.; Grindheim, J.; et al. Atezolizumab Treatment of Tumors with High Tumor Mutational Burden from MyPathway, a Multicenter, Open-Label, Phase IIa Multiple Basket Study. Cancer Discov. 2022, 12, 654–669. [Google Scholar] [CrossRef]
- Mlecnik, B.; Bindea, G.; Angell, H.K.; Maby, P.; Angelova, M.; Tougeron, D.; Church, S.E.; Lafontaine, L.; Fischer, M.; Fredriksen, T.; et al. Integrative Analyses of Colorectal Cancer Show Immunoscore Is a Stronger Predictor of Patient Survival Than Microsatellite Instability. Immunity 2016, 44, 698–711. [Google Scholar] [CrossRef]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Bi, K.; Zhang, X.; Chen, W.; Diao, H. MicroRNAs Regulate Intestinal Immunity and Gut Microbiota for Gastrointestinal Health: A Comprehensive Review. Genes 2020, 11, 1075. [Google Scholar] [CrossRef]
- Tarallo, S.; Ferrero, G.; Gallo, G.; Francavilla, A.; Clerico, G.; Realis Luc, A.; Manghi, P.; Thomas, A.M.; Vineis, P.; Segata, N.; et al. Altered Fecal Small RNA Profiles in Colorectal Cancer Reflect Gut Microbiome Composition in Stool Samples. mSystems 2019, 4, e00289-19. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Bhathena, J.; Martoni, C.; Prakash, S. Estimation of the Potential Antitumor Activity of Microencapsulated Lactobacillus Acidophilus Yogurt Formulation in the Attenuation of Tumorigenesis in Apc(Min/+) Mice. Dig. Dis Sci 2009, 54, 264–273. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, W.-C.; Kong, M.-S.; Shi, H.N.; Walker, W.A.; Lin, C.-Y.; Huang, C.-T.; Lin, Y.-C.; Jung, S.-M.; Lin, T.-Y. Oral Inoculation of Probiotics Lactobacillus Acidophilus NCFM Suppresses Tumour Growth Both in Segmental Orthotopic Colon Cancer and Extra-Intestinal Tissue. Br. J. Nutr. 2012, 107, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Khodaii, Z.; Mehrabani Natanzi, M.; Khalighfard, S.; Ghandian Zanjan, M.; Gharghi, M.; Khori, V.; Amiriani, T.; Rahimkhani, M.; Alizadeh, A.M. Novel Targets in Rectal Cancer by Considering LncRNA–MiRNA–MRNA Network in Response to Lactobacillus Acidophilus Consumption: A Randomized Clinical Trial. Sci. Rep. 2022, 12, 9168. [Google Scholar] [CrossRef] [PubMed]
- Goubet, A.-G.; Daillère, R.; Routy, B.; Derosa, L.M.; Roberti, P.; Zitvogel, L. The Impact of the Intestinal Microbiota in Therapeutic Responses against Cancer. Comptes Rendus Biol. 2018, 341, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Man Lei, Y.; Jabri, B.; Alegre, M.-L.; et al. Commensal Bifidobacterium Promotes Antitumor Immunity and Facilitates Anti–PD-L1 Efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.-L.; Luke, J.J.; Gajewski, T.F. The Commensal Microbiome Is Associated with Anti–PD-1 Efficacy in Metastatic Melanoma Patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Sumransub, N.; Vantanasiri, K.; Prakash, A.; Lou, E. Advances and New Frontiers for Immunotherapy in Colorectal Cancer: Setting the Stage for Neoadjuvant Success? Mol. Ther. Oncolytics 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Cremonesi, E.; Governa, V.; Garzon, J.F.G.; Mele, V.; Amicarella, F.; Muraro, M.G.; Trella, E.; Galati-Fournier, V.; Oertli, D.; Däster, S.R.; et al. Gut Microbiota Modulate T Cell Trafficking into Human Colorectal Cancer. Gut 2018, 67, 1984–1994. [Google Scholar] [CrossRef]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal Microbiota Transplant Promotes Response in Immunotherapy-Refractory Melanoma Patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef]
- Lin, S. Investigator-Initiated Trial of Fecal Microbiota Transplant (FMT) Capsule for Improving the Efficacy of Anti-PD-1 in Patients with PD-1 Resistant Digestive System Cancers. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04130763 (accessed on 26 December 2022).
- Park, S.R. Utilization of Microbiome as Biomarkers and Therapeutics in Immuno-Oncology. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04264975 (accessed on 26 December 2022).
- Zhou, A. Phase II, Single-Arm Study of FMT Combined with Immune Checkpoint Inhibitor and TKI in the Treatment of Colorectal Cancer Patients with Advanced Stage. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT05279677 (accessed on 26 December 2022).
- Chen, E.X.; Jonker, D.J.; Loree, J.M.; Kennecke, H.F.; Berry, S.R.; Couture, F.; Ahmad, C.E.; Goffin, J.R.; Kavan, P.; Harb, M.; et al. CCTG CO.26: Updated Analysis and Impact of Plasma-Detected Microsatellite Stability (MSS) and Tumor Mutation Burden (TMB) in a Phase II Trial of Durvalumab (D) plus Tremelimumab (T) and Best Supportive Care (BSC) versus BSC Alone in Patients (Pts) with Refractory Metastatic Colorectal Carcinoma (RmCRC). J. Clin. Oncol. 2019, 37, 3512. [Google Scholar]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic Review of Microsatellite Instability and Colorectal Cancer Prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Fumet, J.-D. Is There a Place for Immunotherapy for Metastatic Microsatellite Stable Colorectal Cancer? Front. Immunol. 2019, 10, 1816. [Google Scholar] [CrossRef]
- Dechant, M.; Weisner, W.; Berger, S.; Peipp, M.; Beyer, T.; Schneider-Merck, T.; Lammerts van Bueren, J.J.; Bleeker, W.K.; Parren, P.W.H.I.; van de Winkel, J.G.J.; et al. Complement-Dependent Tumor Cell Lysis Triggered by Combinations of Epidermal Growth Factor Receptor Antibodies. Cancer Res. 2008, 68, 4998–5003. [Google Scholar] [CrossRef]
- Grimaldi, A.; Cammarata, I.; Martire, C.; Focaccetti, C.; Piconese, S.; Buccilli, M.; Mancone, C.; Buzzacchino, F.; Berrios, J.R.G.; D’Alessandris, N.; et al. Combination of Chemotherapy and PD-1 Blockade Induces T Cell Responses to Tumor Non-Mutated Neoantigens. Commun. Biol. 2020, 3, 85. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Morano, F.; Lonardi, S.; Raimondi, A.; Salvatore, L.; Marmorino, F.; Murgioni, S.; Pella, N.; Antonuzzo, L.; Ritorto, G.; et al. 383O MAYA Trial: Temozolomide (TMZ) Priming Followed by Combination with Low-Dose Ipilimumab and Nivolumab in Patients with Microsatellite Stable (MSS), MGMT Silenced Metastatic Colorectal Cancer (MCRC). Ann. Oncol. 2021, 32, S530–S531. [Google Scholar] [CrossRef]
- Elez, E.; Baraibar, I. Immunotherapy in Colorectal Cancer: An Unmet Need Deserving of Change. Lancet Oncol. 2022, 23, 830–831. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).