Childhood Brain Tumors: A Review of Strategies to Translate CNS Drug Delivery to Clinical Trials
Abstract
Simple Summary
Abstract
1. Introduction
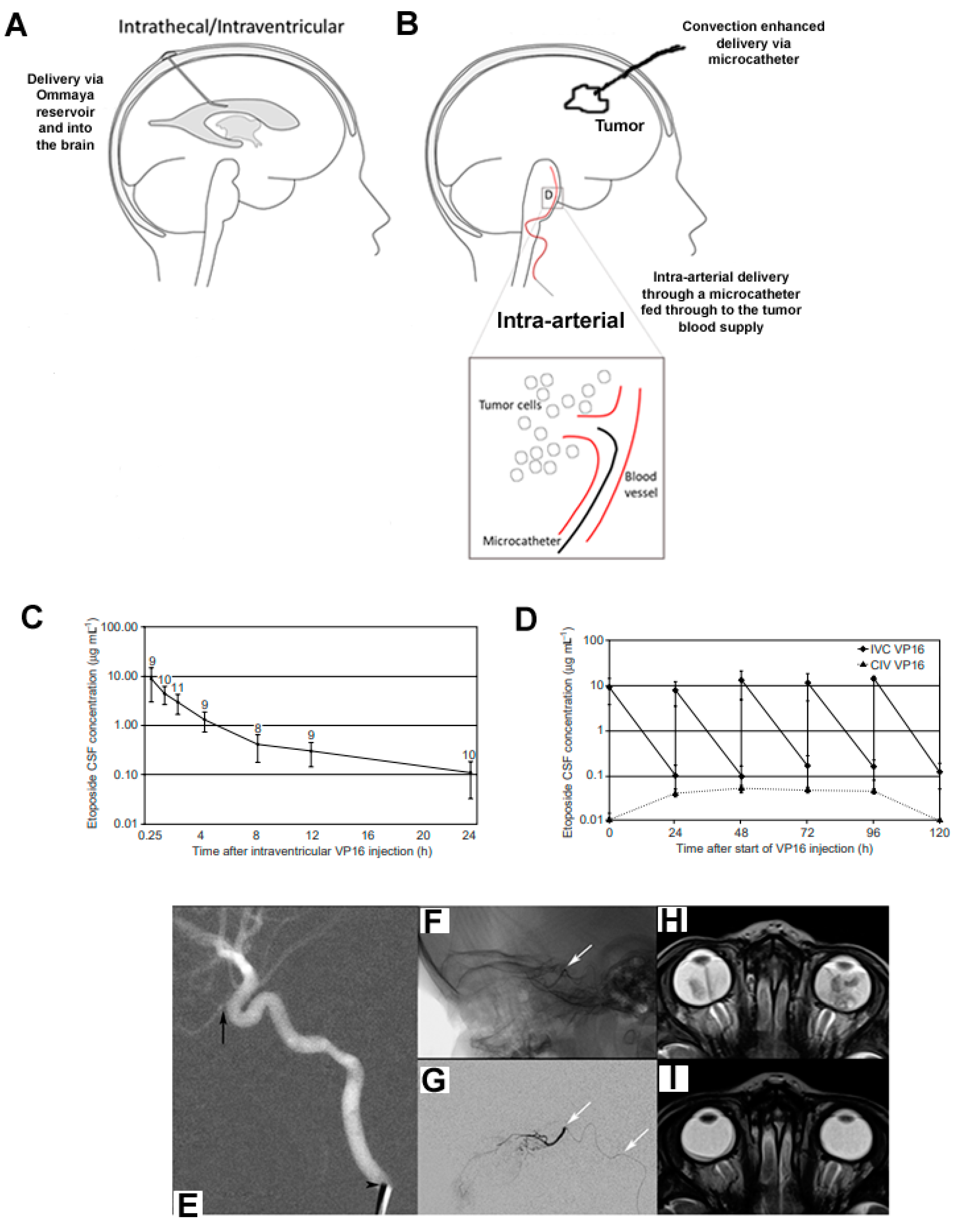
2. Overview of CNS Drug Delivery Methods
3. Augmenting Drug Passage through the BBB
3.1. Pharmacological Modulation of the BBB
3.2. Ultrasound-Induced BBB Disruption
3.3. Intra-Arterial Chemotherapy
4. Bypassing the BBB
4.1. Polymer Therapeutics for Local Delivery
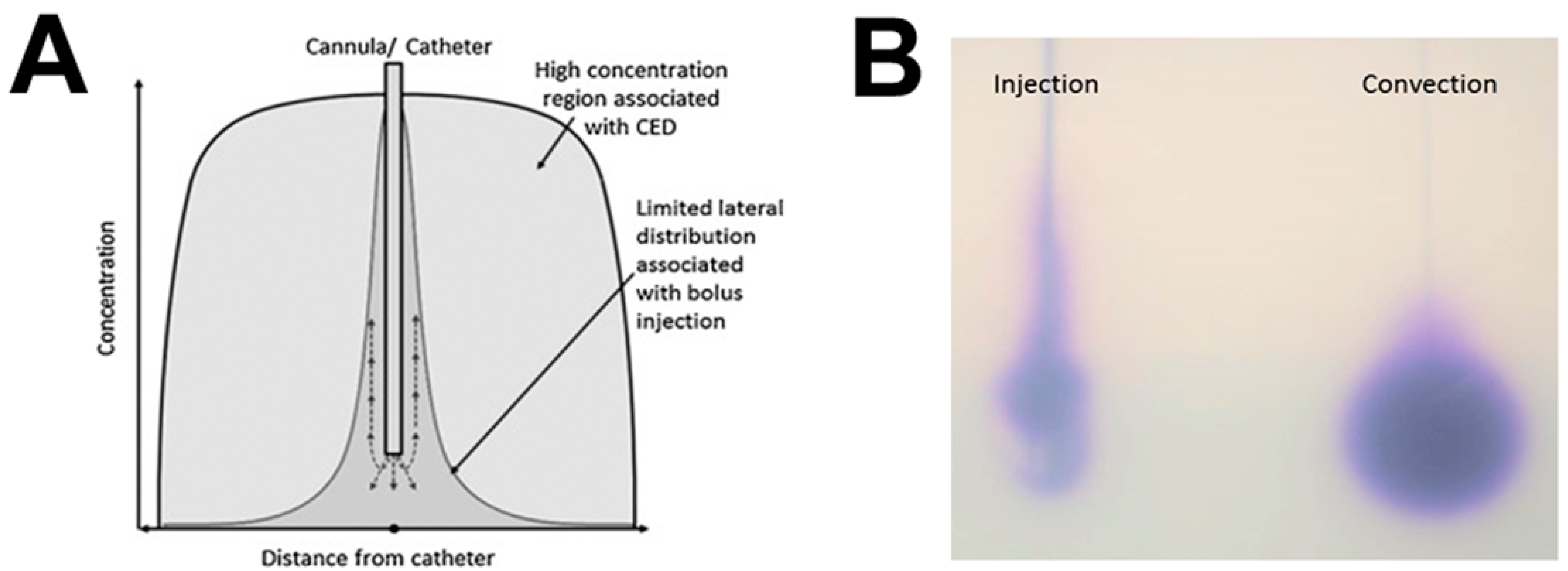
4.2. Convection-Enhanced Delivery
4.2.1. Translation of the Technique from Bench to Bedside
4.2.2. Catheter Design
4.2.3. Convection-Enhanced Delivery Infusion Regimes
4.2.4. Intermittent Convection-Enhanced Delivery
4.2.5. Drug Properties Required for Convection-Enhanced Delivery
4.3. Intra-CSF or Interstitial Administration
5. Repurposing Drugs in Pediatric Neuro-Oncology
Anti-Helminthic/Psychotic/Seizure Drugs
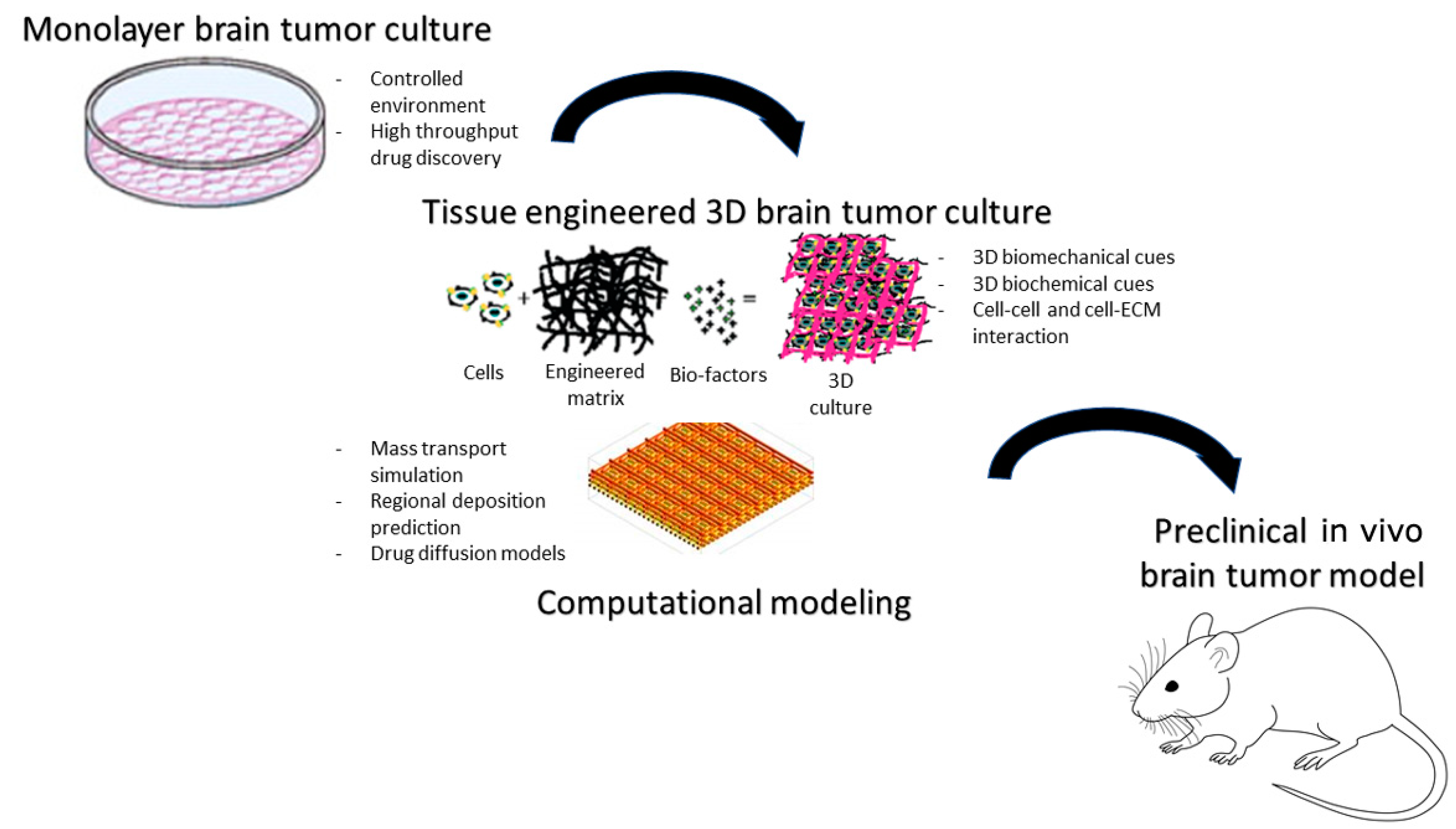
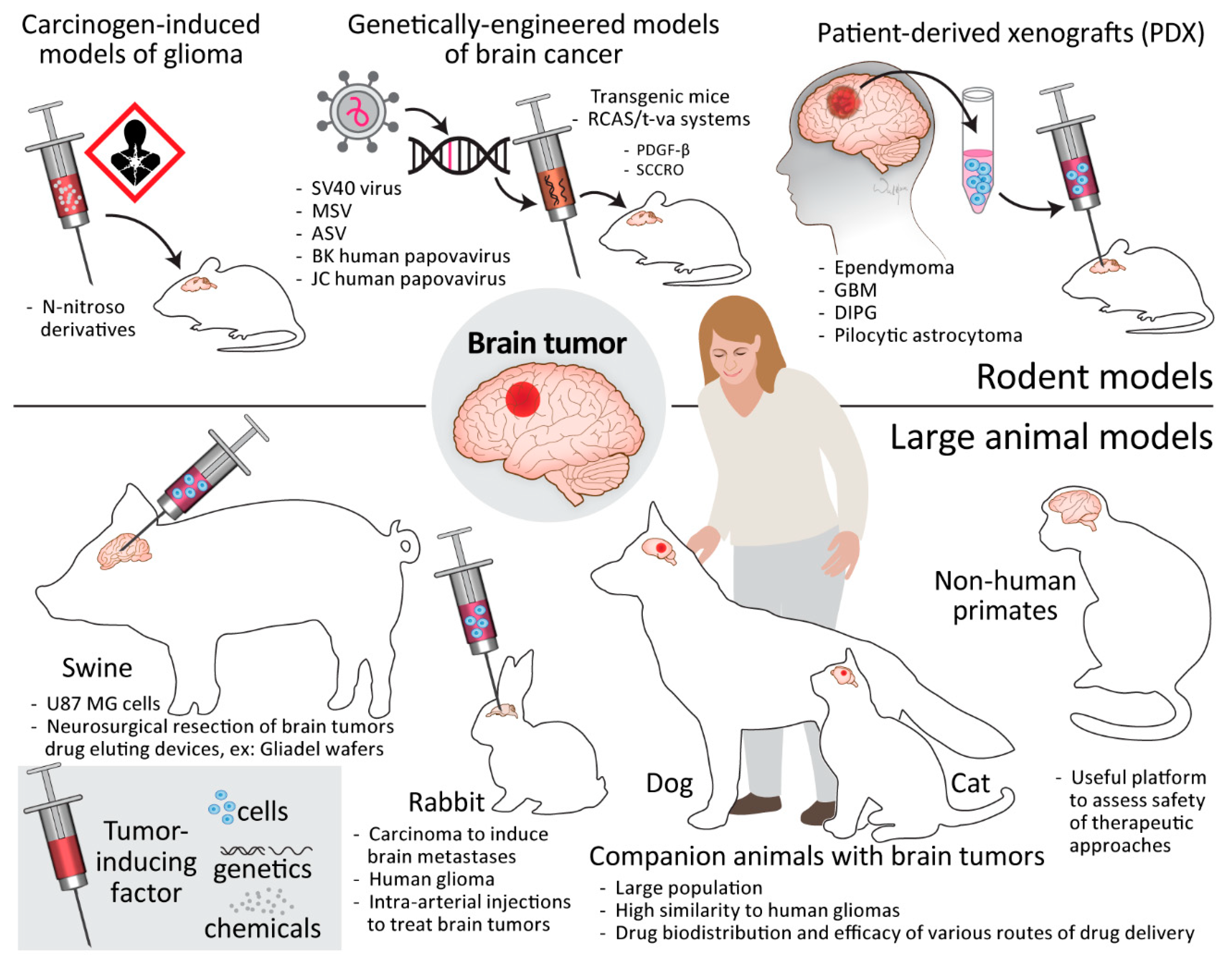
6. Brain Tumor Models
6.1. Human-Specific In Vitro Brain Tumor Models
6.2. Rodent Models
6.2.1. Carcinogen-Induced Models of Glioma
6.2.2. Genetically Engineered Models of Brain Cancer
6.2.3. Patient-Derived Xenografts
6.3. Large Animal Models
6.3.1. Swine
6.3.2. Rabbit
6.3.3. Companion Animals
6.3.4. Non-Human Primates
7. Discussion
7.1. Choose the Tumor Type and Clinical Setting for Targeted Treatments
- Overcome primarily drug-resistant types by ensuring predictable tumor tissue levels of existing or repurposed drugs.
- Target leptomeningeal metastasis with intra-CSF delivery using existing or repurposed drugs to defer or avoid extended-field radiotherapy.
- Enhance the efficacy and reduce systemic toxicity of molecularly targeted treatments that are shown to be effective in early trials.
7.2. Identify the Preclinical Research and Development Needed for Trial Design
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organisation. CureAll Framework: WHO Global Initiative for Childhood Cancer: Increasing Access, Advancing Quality, Saving Lives; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Atun, R. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019, 20, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International Classification of Childhood Cancer, third edition. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Steliarova-Foucher, E.; Colombet, M.; Ries, L.A.G.; Moreno, F.; Dolya, A.; Bray, F.; Hesseling, P.; Shin, H.Y.; Stiller, C.A.; The IICC-3 Contributors. International incidence of childhood cancer, 2001–2010: A population-based registry study. Lancet Oncol. 2017, 18, 719–731. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 2 January 2023).
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol. 2022, 18, 515–529. [Google Scholar] [CrossRef]
- Halfpenny, A.M.; Wood, M.D. Review of the Recent Changes in the WHO Classification for Pediatric Brain and Spinal Cord Tumors. Pediatr. Neurosurg. 2023; ahead of print. [Google Scholar]
- Walker, D.A.; Meijer, L.; Coyle, B.; Halsey, C. Leptomeningeal malignancy of childhood: Sharing learning between childhood leukaemia and brain tumour trials. Lancet Child Adolesc. Health 2020, 4, 242–250. [Google Scholar] [CrossRef]
- Blakeley, J. Drug delivery to brain tumors. Curr. Neurol. Neurosci. Rep. 2008, 8, 235–241. [Google Scholar] [CrossRef]
- Rodriguez, A.; Tatter, S.B.; Debinski, W. Neurosurgical Techniques for Disruption of the Blood-Brain Barrier for Glioblastoma Treatment. Pharmaceutics 2015, 7, 175–187. [Google Scholar] [CrossRef]
- Al Ahmad, A.; Taboada, C.B.; Gassmann, M.; Ogunshola, O.O. Astrocytes and pericytes differentially modulate blood-brain barrier characteristics during development and hypoxic insult. J. Cereb. Blood Flow Metab. 2011, 31, 693–705. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Sprowls, S.A.; Lathia, J.D. Breaking down the barrier to medulloblastoma treatment: Piezo2 knockout disrupts the BTB and increases vascular permeability. Neuron 2023, 111, 3–5. [Google Scholar] [CrossRef]
- Pollack, I.F.; Stewart, C.F.; Kocak, M.; Poussaint, T.Y.; Broniscer, A.; Banerjee, A.; Douglas, J.G.; Kun, L.E.; Boyett, J.M.; Geyer, J.R. A phase II study of gefitinib and irradiation in children with newly diagnosed brainstem gliomas: A report from the Pediatric Brain Tumor Consortium. Neuro Oncol. 2011, 13, 290–297. [Google Scholar] [CrossRef]
- Pardridge, W.M. Molecular Trojan horses for blood-brain barrier drug delivery. Discov. Med. 2006, 6, 139–143. [Google Scholar] [CrossRef]
- Conroy, S.; Garnett, M.; Vloeberghs, M.; Grundy, R.; Craven, I.; Walker, D. Medulloblastoma in childhood: Revisiting intrathecal therapy in infants and children. Cancer Chemother. Pharmacol. 2010, 65, 1173–1189. [Google Scholar] [CrossRef]
- Atun, R.; Bhakta, N.; Denburg, A.; Frazier, A.L.; Friedrich, P.; Gupta, S.; Lam, C.G.; Ward, Z.J.; Yeh, J.M.; Allemani, C.; et al. Sustainable care for children with cancer: A Lancet Oncology Commission. Lancet Oncol. 2020, 21, e185–e224. [Google Scholar] [CrossRef]
- Wyse, E.; Handa, J.T.; Friedman, A.D.; Pearl, M.S. A review of the literature for intra-arterial chemotherapy used to treat retinoblastoma. Pediatr. Radiol. 2016, 46, 1223–1233. [Google Scholar] [CrossRef]
- Cancer Research UK. Lomustine (CCNU) [Cancer Drugs Information]. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/lomustine-ccnu (accessed on 2 January 2023).
- Estlin, E.J.; Lashford, L.; Ablett, S.; Price, L.; Gowing, R.; Gholkar, A.; Kohler, J.; Lewis, I.; Morland, B.; Pinkerton, C.; et al. Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children’s Cancer Study Group. Br. J. Cancer 1998, 78, 652–661. [Google Scholar] [CrossRef]
- Perry, J.; Chambers, A.; Spithoff, K.; Laperriere, N. Gliadel wafers in the treatment of malignant glioma: a systematic review. Curr. Oncol. 2007, 14, 189–194. [Google Scholar] [CrossRef]
- Cancer Research UK CR. Everolimus [Cancer Drugs Information]. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/everolimus (accessed on 2 January 2023).
- Franz, D.N.; Agricola, K.; Mays, M.; Tudor, C.; Care, M.M.; Holland-Bouley, K.; Berkowitz, N.; Miao, S.; Peyrard, S.; Krueger, D.A. Everolimus for subependymal giant cell astrocytoma: 5-year final analysis. Ann. Neurol. 2015, 78, 929–938. [Google Scholar] [CrossRef]
- England) SCTN. Clinical Commissioning Policy: Everolimus for Subependymal Giant Cell Astrocytoma (SEGA) Associated with Tuberous Sclerosis Complex: NHS England; 2016 [Clinical Commissioning Policy]. Available online: https://www.england.nhs.uk/publication/clinical-commissioning-policy-everolimus-for-subependymal-giant-cell-astrocytoma-sega-associated-with-tuberous-sclerosis-complex/ (accessed on 2 January 2023).
- Marini, B.L.; Benitez, L.L.; Zureick, A.H.; Salloum, R.; Gauthier, A.C.; Brown, J.; Wu, Y.M.; Robinson, D.R.; Kumar, C.; Lonigro, R.; et al. Blood-brain barrier-adapted precision medicine therapy for pediatric brain tumors. Transl. Res. 2017, 188, 27.E1–27.E14. [Google Scholar] [CrossRef]
- Pardridge, W. The blood-brain barrier: Bottleneck in brain drug delivery. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro Oncol. 2016, 18, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Elliott, P.J.; Hayward, N.J.; Dean, R.L.; Blunt, D.G.; Bartus, R.T. Intravenous RMP-7 selectively increases uptake of carboplatin into rat brain tumors. Cancer Res. 1996, 56, 3998–4005. [Google Scholar] [PubMed]
- Prados, M.D.; Schold, S.C., Jr.; Fine, H.A.; Jaeckle, K.; Hochberg, F.; Mechtler, L.; Fetell, M.R.; Phuphanich, S.; Feun, L.; Janus, T.J.; et al. A randomized, double-blind, placebo-controlled, phase 2 study of RMP-7 in combination with carboplatin administered intravenously for the treatment of recurrent malignant glioma. Neuro Oncol. 2003, 5, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.; Widemann, B.C.; Pastakia, D.; Chen, C.C.; Yang, S.X.; Cole, D.; Balis, F.M. Pharmacokinetic and pharmacodynamic study of tariquidar (XR9576), a P-glycoprotein inhibitor, in combination with doxorubicin, vinorelbine, or docetaxel in children and adolescents with refractory solid tumors. Cancer Chemother. Pharmacol. 2015, 76, 1273–1283. [Google Scholar] [CrossRef]
- Bakay, L.; Ballantine, H.T., Jr.; Hueter, T.F.; Sosa, D. Ultrasonically produced changes in the blood-brain barrier. AMA Arch. Neurol. Psychiatry 1956, 76, 457–467. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.; Hynynen, K. Cellular mechanisms of the blood-brain barrier opening induced by ultrasound in presence of microbubbles. Ultrasound Med. Biol. 2004, 30, 979–989. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Goldwirt, L.; Fernandez, C.; Piquet, J.; Perier, M.C.; Lafon, C.; Chapelon, J.Y.; Carpentier, A. Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan delivery: An experimental study in rabbits. J. Neurosurg. 2016, 124, 1602–1610. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood-brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Ganguly, M.; Francia, G.; Kerbel, R.; Wels, W.S.; Hynynen, K. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013, 73, 1892–1899. [Google Scholar] [CrossRef]
- Fan, C.H.; Ting, C.Y.; Chang, Y.C.; Wei, K.C.; Liu, H.L.; Yeh, C.K. Drug-loaded bubbles with matched focused ultrasound excitation for concurrent blood-brain barrier opening and brain-tumor drug delivery. Acta Biomater. 2015, 15, 89–101. [Google Scholar] [CrossRef]
- Ting, C.Y.; Fan, C.H.; Liu, H.L.; Huang, C.Y.; Hsieh, H.Y.; Yen, T.C.; Wei, K.C.; Yeh, C.K. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials 2012, 33, 704–712. [Google Scholar] [CrossRef]
- Treat, L.H.; McDannold, N.; Zhang, Y.; Vykhodtseva, N.; Hynynen, K. Improved anti-tumor effect of liposomal doxorubicin after targeted blood-brain barrier disruption by MRI-guided focused ultrasound in rat glioma. Ultrasound Med. Biol. 2012, 38, 1716–1725. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Hynynen, K. Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology 2012, 263, 96–106. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging- guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332; discussion 332. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Goldwirt, L.; Fernandez, C.; Adam, C.; Piquet, J.; Autret, G.; Clement, O.; Lafon, C.; Chapelon, J.Y.; et al. Opening of the blood-brain barrier with an unfocused ultrasound device in rabbits. J. Neurosurg. 2013, 119, 887–898. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A.; et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Mainprize, T.; Lipsman, N.; Huang, Y.; Meng, Y.; Bethune, A.; Ironside, S.; Heyn, C.; Alkins, R.; Trudeau, M.; Sahgal, A.; et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci. Rep. 2019, 9, 321. [Google Scholar] [CrossRef]
- Ostapowicz, G. Behavior of pressure of the cerebrospinal fluid in intraarterial infusion in carotid artery in animal experiments. Z. Gesamte Inn. Med. 1952, 7, 933–948. [Google Scholar]
- French, J.D.; West, P.M.; Von Amerongen, F.K.; Magoun, H.W. Effects of intracarotid administration of nitrogen mustard on normal brain and brain tumors. J. Neurosurg. 1952, 9, 378–389. [Google Scholar] [CrossRef]
- Gobin, Y.P.; Cloughesy, T.F.; Chow, K.L.; Duckwiler, G.R.; Sayre, J.W.; Milanese, K.; Vinuela, F. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology 2001, 218, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Filippi, C.G.; Burkhardt, J.K.; Fralin, S.; Ray, A.; Wong, T.; Ortiz, R.; Langer, D.J.; Boockvar, J.A. Durability of single dose intra-arterial bevacizumab after blood/brain barrier disruption for recurrent glioblastoma. J. Exp. Ther. Oncol. 2016, 11, 261–267. [Google Scholar] [PubMed]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Du, Y.; Pomper, M.G.; Walczak, P.; Janowski, M.P. A Distinct Advantage to Intraarterial Delivery of 89Zr-Bevacizumab in PET Imaging of Mice With and Without Osmotic Opening of the Blood-Brain Barrier. J. Nucl. Med. 2019, 60, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Janowski, M.; Walczak, P.; Pearl, M.S. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J. Cereb. Blood Flow Metab. 2016, 36, 569–575. [Google Scholar] [CrossRef]
- Groothuis, D.R. The blood-brain and blood-tumor barriers: A review of strategies for increasing drug delivery. Neuro Oncol. 2000, 2, 45–59. [Google Scholar] [CrossRef]
- Chew, S.A.; Danti, S. Biomaterial-Based Implantable Devices for Cancer Therapy. Adv. Healthc. Mater. 2017, 6, 1600766. [Google Scholar] [CrossRef]
- Brem, H.; Piantadosi, S.; Burger, P.C.; Walker, M.; Selker, R.; Vick, N.A.; Black, K.; Sisti, M.; Brem, S.; Mohr, G.; et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet 1995, 345, 1008–1012. [Google Scholar] [CrossRef]
- Li, K.W.; Dang, W.; Tyler, B.M.; Troiano, G.; Tihan, T.; Brem, H.; Walter, K.A. Polilactofate microspheres for Paclitaxel delivery to central nervous system malignancies. Clin. Cancer Res. 2003, 9, 3441–3447. [Google Scholar]
- Tyler, B.; Fowers, K.D.; Li, K.W.; Recinos, V.R.; Caplan, J.M.; Hdeib, A.; Grossman, R.; Basaldella, L.; Bekelis, K.; Pradilla, G.; et al. A thermal gel depot for local delivery of paclitaxel to treat experimental brain tumors in rats. J. Neurosurg. 2010, 113, 210–217. [Google Scholar] [CrossRef]
- Mastorakos, P.; Zhang, C.; Song, E.; Kim, Y.E.; Park, H.W.; Berry, S.; Choi, W.K.; Hanes, J.; Suk, J.S. Biodegradable brain-penetrating DNA nanocomplexes and their use to treat malignant brain tumors. J. Control. Release 2017, 262, 37–46. [Google Scholar] [CrossRef]
- Tuma, P.; Hubbard, A.L. Transcytosis: Crossing cellular barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, Q.; Chow, P.K.; Wang, D.; Wang, C.H. Transferrin-conjugated magnetic silica PLGA nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials 2013, 34, 8511–8520. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.F.; Bryant, J.; Charles, A.; Boado, R.J.; Pardridge, W.M. Intravenous RNA interference gene therapy targeting the human epidermal growth factor receptor prolongs survival in intracranial brain cancer. Clin. Cancer Res. 2004, 10, 3667–3677. [Google Scholar] [CrossRef]
- Ren, J.; Shen, S.; Wang, D.; Xi, Z.; Guo, L.; Pang, Z.; Qian, Y.; Sun, X.; Jiang, X. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 2012, 33, 3324–3333. [Google Scholar] [CrossRef]
- Huttunen, J.; Gynther, M.; Huttunen, K.M. Targeted efflux transporter inhibitors—A solution to improve poor cellular accumulation of anti-cancer agents. Int. J. Pharm. 2018, 550, 278–289. [Google Scholar] [CrossRef]
- Bhowmik, A.; Chakravarti, S.; Ghosh, A.; Shaw, R.; Bhandary, S.; Bhattacharyya, S.; Sen, P.C.; Ghosh, M.K. Anti-SSTR2 peptide based targeted delivery of potent PLGA encapsulated 3,3’-diindolylmethane nanoparticles through blood brain barrier prevents glioma progression. Oncotarget 2017, 8, 65339–65358. [Google Scholar] [CrossRef]
- Barua, N.U.; Gill, S.S.; Love, S. Convection-enhanced drug delivery to the brain: Therapeutic potential and neuropathological considerations. Brain Pathol. 2014, 24, 117–127. [Google Scholar] [CrossRef]
- Morrison, P.F.; Laske, D.W.; Bobo, H.; Oldfield, E.H.; Dedrick, R.L. High-flow microinfusion: Tissue penetration and pharmacodynamics. Am. J. Physiol. 1994, 266 Pt 2, R292–R305. [Google Scholar] [CrossRef]
- Lidar, Z.; Mardor, Y.; Jonas, T.; Pfeffer, R.; Faibel, M.; Nass, D.; Hadani, M.; Ram, Z. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: A phase I/II clinical study. J. Neurosurg. 2004, 100, 472–479. [Google Scholar] [CrossRef]
- Gill, T.; Barua, N.U.; Woolley, M.; Bienemann, A.S.; Johnson, D.E.; Sullivan, S.O.; Murray, G.; Fennelly, C.; Lewis, O.; Irving, C.; et al. In vitro and in vivo testing of a novel recessed-step catheter for reflux-free convection-enhanced drug delivery to the brain. J. Neurosci. Methods 2013, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Souweidane, M.M.; Kramer, K.; Pandit-Taskar, N.; Zhou, Z.; Haque, S.; Zanzonico, P.; Carrasquillo, J.A.; Lyashchenko, S.K.; Thakur, S.B.; Donzelli, M.; et al. Convection-enhanced delivery for diffuse intrinsic pontine glioma: A single-centre, dose-escalation, phase 1 trial. Lancet Oncol. 2018, 19, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Sonabend, A.M.; Stuart, R.M.; Yun, J.; Yanagihara, T.; Mohajed, H.; Dashnaw, S.; Bruce, S.S.; Brown, T.; Romanov, A.; Sebastian, M.; et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol. 2011, 13, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Barua, N.U.; Hopkins, K.; Woolley, M.; O’Sullivan, S.; Harrison, R.; Edwards, R.J.; Bienemann, A.S.; Wyatt, M.J.; Arshad, A.; Gill, S.S. A novel implantable catheter system with transcutaneous port for intermittent convection-enhanced delivery of carboplatin for recurrent glioblastoma. Drug Deliv. 2016, 23, 167–173. [Google Scholar] [CrossRef]
- Barua, N.U.; Miners, J.S.; Bienemann, A.S.; Wyatt, M.J.; Welser, K.; Tabor, A.B.; Hailes, H.C.; Love, S.; Gill, S.S. Convection-enhanced delivery of neprilysin: A novel amyloid-beta-degrading therapeutic strategy. J. Alzheimers Dis. 2012, 32, 43–56. [Google Scholar] [CrossRef]
- Singleton, W.G.; Collins, A.M.; Bienemann, A.S.; Killick-Cole, C.L.; Haynes, H.R.; Asby, D.J.; Butts, C.P.; Wyatt, M.J.; Barua, N.U.; Gill, S.S. Convection enhanced delivery of panobinostat (LBH589)-loaded pluronic nano-micelles prolongs survival in the F98 rat glioma model. Int. J. Nanomed. 2017, 12, 1385–1399. [Google Scholar] [CrossRef]
- McCrorie, P.; Vasey, C.E.; Smith, S.J.; Marlow, M.; Alexander, C.; Rahman, R. Biomedical engineering approaches to enhance therapeutic delivery for malignant glioma. J. Control. Release 2020, 328, 917–931. [Google Scholar] [CrossRef]
- Allard, E.; Passirani, C.; Benoit, J.P. Convection-enhanced delivery of nanocarriers for the treatment of brain tumors. Biomaterials 2009, 30, 2302–2318. [Google Scholar] [CrossRef]
- Tosi, U.; Kommidi, H.; Bellat, V.; Marnell, C.S.; Guo, H.; Adeuyan, O.; Schweitzer, M.E.; Chen, N.; Su, T.; Zhang, G.; et al. Real-Time, in Vivo Correlation of Molecular Structure with Drug Distribution in the Brain Striatum Following Convection Enhanced Delivery. ACS Chem. Neurosci. 2019, 10, 2287–2298. [Google Scholar] [CrossRef]
- Arshad, A.; Yang, B.; Bienemann, A.S.; Barua, N.U.; Wyatt, M.J.; Woolley, M.; Johnson, D.E.; Edler, K.J.; Gill, S.S. Convection-Enhanced Delivery of Carboplatin PLGA Nanoparticles for the Treatment of Glioblastoma. PLoS ONE 2015, 10, e0132266. [Google Scholar] [CrossRef]
- Zhou, J.; Patel, T.R.; Sirianni, R.W.; Strohbehn, G.; Zheng, M.Q.; Duong, N.; Schafbauer, T.; Huttner, A.J.; Huang, Y.; Carson, R.E.; et al. Highly penetrative, drug-loaded nanocarriers improve treatment of glioblastoma. Proc. Natl. Acad. Sci. USA 2013, 110, 11751–11756. [Google Scholar] [CrossRef]
- Sawyer, A.J.; Saucier-Sawyer, J.K.; Booth, C.J.; Liu, J.; Patel, T.; Piepmeier, J.M.; Saltzman, W.M. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv. Transl. Res 2011, 1, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Hadjipanayis, C.G.; Machaidze, R.; Kaluzova, M.; Wang, L.; Schuette, A.J.; Chen, H.; Wu, X.; Mao, H. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010, 70, 6303–6312. [Google Scholar] [CrossRef] [PubMed]
- Bernal, G.M.; LaRiviere, M.J.; Mansour, N.; Pytel, P.; Cahill, K.E.; Voce, D.J.; Kang, S.; Spretz, R.; Welp, U.; Noriega, S.E.; et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine 2014, 10, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Fouladi, M.; Gajjar, A.; Boyett, J.M.; Walter, A.W.; Thompson, S.J.; Merchant, T.E.; Jenkins, J.J.; Langston, J.W.; Liu, A.; Kun, L.E.; et al. Comparison of CSF cytology and spinal magnetic resonance imaging in the detection of leptomeningeal disease in pediatric medulloblastoma or primitive neuroectodermal tumor. J. Clin. Oncol. 1999, 17, 3234–3237. [Google Scholar] [CrossRef]
- Khang, M.; Bindra, R.S.; Mark Saltzman, W. Intrathecal delivery and its applications in leptomeningeal disease. Adv. Drug Deliv. Rev. 2022, 186, 114338. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar]
- Schmidt, C.; Schubert, N.A.; Brabetz, S.; Mack, N.; Schwalm, B.; Chan, J.A.; Selt, F.; Herold-Mende, C.; Witt, O.; Milde, T.; et al. Preclinical drug screen reveals topotecan, actinomycin D, and volasertib as potential new therapeutic candidates for ETMR brain tumor patients. Neuro-Oncology 2017, 19, 1607–1617. [Google Scholar] [CrossRef]
- Spence, T.; Perotti, C.; Sin-Chan, P.; Picard, D.; Wu, W.; Singh, A.; Anderson, C.; Blough, M.D.; Cairncross, J.G.; Lafay-Cousin, L.; et al. A novel C19MC amplified cell line links Lin28/let-7 to mTOR signaling in embryonal tumor with multilayered rosettes. Neuro-Oncology 2014, 16, 62–71. [Google Scholar] [CrossRef]
- Pei, Y.; Liu, K.W.; Wang, J.; Garancher, A.; Tao, R.; Esparza, L.A.; Maier, D.L.; Udaka, Y.T.; Murad, N.; Morrissy, S.; et al. HDAC and PI3K Antagonists Cooperate to Inhibit Growth of MYC-Driven Medulloblastoma. Cancer Cell 2016, 29, 311–323. [Google Scholar] [CrossRef]
- Veringa, S.J.; Biesmans, D.; van Vuurden, D.G.; Jansen, M.H.; Wedekind, L.E.; Horsman, I.; Wesseling, P.; Vandertop, W.P.; Noske, D.P.; Kaspers, G.J.L.; et al. In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. PLoS ONE 2013, 8, e61512. [Google Scholar] [CrossRef]
- Halvorson, K.G.; Barton, K.L.; Schroeder, K.; Misuraca, K.L.; Hoeman, C.; Chung, A.; Crabtree, N.M.; Cordero, F.J.; Singh, R.; Spasojevic, I.; et al. A high-throughput in vitro drug screen in a genetically engineered mouse model of diffuse intrinsic pontine glioma identifies BMS-754807 as a promising therapeutic agent. PLoS ONE 2015, 10, e0118926. [Google Scholar] [CrossRef]
- Grasso, C.S.; Tang, Y.; Truffaux, N.; Berlow, N.E.; Liu, L.; Debily, M.A.; Quist, M.J.; Davis, L.E.; Huang, E.C.; Woo, P.J.; et al. Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Nat. Med. 2015, 21, 827. [Google Scholar] [CrossRef]
- De Witt, M.; Gamble, A.; Hanson, D.; Markowitz, D.; Powell, C.; Al Dimassi, S.; Atlas, M.; Boockvar, J.; Ruggieri, R.; Symons, M. Repurposing Mebendazole as a Replacement for Vincristine for the Treatment of Brain Tumors. Mol. Med. 2017, 23, 50–56. [Google Scholar] [CrossRef]
- Ajaz, M.; Jefferies, S.; Brazil, L.; Watts, C.; Chalmers, A. Current and investigational drug strategies for glioblastoma. Clin. Oncol. 2014, 26, 419–430. [Google Scholar] [CrossRef]
- Boyle, F.M.; Eller, S.L.; Grossman, S.A. Penetration of intra-arterially administered vincristine in experimental brain tumor. Neuro Oncol. 2004, 6, 300–305. [Google Scholar] [CrossRef]
- Phoenix, T.N.; Patmore, D.M.; Boop, S.; Boulos, N.; Jacus, M.O.; Patel, Y.T.; Roussel, M.F.; Finkelstein, D.; Goumnerova, L.; Perreault, S.; et al. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell 2016, 29, 508–522. [Google Scholar] [CrossRef]
- Moore, A.; Pinkerton, R. Vincristine: Can its therapeutic index be enhanced? Pediatr. Blood Cancer 2009, 53, 1180–1187. [Google Scholar] [CrossRef]
- Bai, R.Y.; Staedtke, V.; Aprhys, C.M.; Gallia, G.L.; Riggins, G.J. Antiparasitic mebendazole shows survival benefit in 2 preclinical models of glioblastoma multiforme. Neuro Oncol. 2011, 13, 974–982. [Google Scholar] [CrossRef]
- Bai, R.Y.; Staedtke, V.; Rudin, C.M.; Bunz, F.; Riggins, G.J. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol. 2015, 17, 545–554. [Google Scholar] [CrossRef]
- Pantziarka, P.; Bouche, G.; Meheus, L.; Sukhatme, V.; Sukhatme, V.P. Repurposing Drugs in Oncology (ReDO)-mebendazole as an anti-cancer agent. Ecancermedicalscience 2014, 8, 443. [Google Scholar] [CrossRef]
- Huang, X.; He, Y.; Dubuc, A.M.; Hashizume, R.; Zhang, W.; Reimand, J.; Yang, H.; Wang, T.A.; Stehbens, S.J.; Younger, S.; et al. EAG2 potassium channel with evolutionarily conserved function as a brain tumor target. Nat. Neurosci. 2015, 18, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Patties, I.; Kortmann, R.D.; Menzel, F.; Glasow, A. Enhanced inhibition of clonogenic survival of human medulloblastoma cells by multimodal treatment with ionizing irradiation, epigenetic modifiers, and differentiation-inducing drugs. J. Exp. Clin. Cancer Res. 2016, 35, 94. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.G. Comparison of valproate concentrations in human plasma, CSF and brain tissue after administration of different formulations of valproate or valpromide. Epilepsy Res. 1991, 9, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Masoudi, A.; Elopre, M.; Amini, E.; Nagel, M.E.; Ater, J.L.; Gopalakrishnan, V.; Wolff, J.E. Influence of valproic acid on outcome of high-grade gliomas in children. Anticancer Res. 2008, 28, 2437–2442. [Google Scholar] [PubMed]
- Hollingworth, M.; Hurter, C.; Wooley, M.; Lewis, O.; Gill, S.; Zacharoulis, S. DIPG-65. Preliminary Experience of chronic intermittent convection enhanced delivery of carboplatin and valproic acid for the treatment of diffuse intrinsic pontine glioma following radiation therapy. Neuro-Oncology 2018, 20 (Suppl_2), i62. [Google Scholar] [CrossRef]
- Ananthanarayanan, B.; Kim, Y.; Kumar, S. Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials 2011, 32, 7913–7923. [Google Scholar] [CrossRef]
- Heffernan, J.M.; Overstreet, D.J.; Le, L.D.; Vernon, B.L.; Sirianni, R.W. Bioengineered Scaffolds for 3D Analysis of Glioblastoma Proliferation and Invasion. Ann. Biomed. Eng. 2015, 43, 1965–1977. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Parker, T.L.; Walker, D.A.; Alexander, C.; Ashford, M.B.; Gellert, P.R.; Garnett, M.C. In vitro co-culture model of medulloblastoma and human neural stem cells for drug delivery assessment. J. Biotechnol. 2015, 205, 3–13. [Google Scholar] [CrossRef]
- Meng, W.N.; Garnett, M.C.; Walker, D.A.; Parker, T.L. Penetration and intracellular uptake of poly(glycerol-adipate) nanoparticles into three-dimensional brain tumour cell culture models. Exp. Biol. Med. 2016, 241, 466–477. [Google Scholar] [CrossRef]
- Torres, A.J.; Zhu, C.; Shuler, M.L.; Pannullo, S. Paclitaxel delivery to brain tumors from hydrogels: A computational study. Biotechnol. Prog. 2011, 27, 1478–1487. [Google Scholar] [CrossRef]
- Cooke, J.N.; Ellis, J.A.; Hossain, S.; Nguyen, J.; Bruce, J.N.; Joshi, S. Computational pharmacokinetic rationale for intra-arterial delivery to the brain. Drug Deliv. Transl. Res. 2016, 6, 622–629. [Google Scholar] [CrossRef]
- Gutin, P.H.; Hilton, J.; Walker, M.D. Experimental brain tumor chemotherapy: DNA damage in the rat gliosarcoma 9L treated with CCNU. Clin. Neurosurg. 1977, 24, 653–664. [Google Scholar] [CrossRef]
- Wechlser, W.; Kleihues, P.; Zulch, K.L.; Ivankovic, S.; Preussmann, R.; Druckrey, H. Pathology of experimental neurogenic tumors chemically induced during prenatal and postnatal life. Ann. N. Y. Acad. Sci. 1969, 159, 360–408. [Google Scholar]
- Benda, P.; Someda, K.; Messer, J.; Sweet, W.H. Morphological and immunochemical studies of rat glial tumors and clonal strains propagated in culture. J. Neurosurg. 1971, 34, 310–323. [Google Scholar] [CrossRef]
- Barker, M.; Hoshino, T.; Gurcay, O.; Wilson, C.B.; Nielsen, S.L.; Downie, R.; Eliason, J. Development of an animal brain tumor model and its response to therapy with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1973, 33, 976–986. [Google Scholar]
- Gutin, P.H.; Hilton, J.; Fein, V.J.; Allan, A.E.; Rottman, A.; Walker, M.D. DNA damage in the intracerebral rat gliosarcoma 9L treated with 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea. Cancer Res. 1977, 37, 3761–3765. [Google Scholar]
- Owens, G.C.; Orr, E.A.; DeMasters, B.K.; Muschel, R.J.; Berens, M.E.; Kruse, C.A. Overexpression of a transmembrane isoform of neural cell adhesion molecule alters the invasiveness of rat CNS-1 glioma. Cancer Res. 1998, 58, 2020–2028. [Google Scholar]
- Rabson, A.S.; O’Conor, G.T.; Kirschstein, R.L.; Branigan, W.J. Papillary ependymomas produced in Rattus (Mastomys) natalensis inoculated with vacuolating virus (SV40). J. Natl. Cancer Inst. 1962, 29, 765–787. [Google Scholar]
- Rabotti, G.F.; Raine, W.A.; Sellers, R.L. Brain Tumors (Gliomas) Induced in Hamsters by Bryan’s Strain of Rous Sarcoma Virus. Science 1965, 147, 504–506. [Google Scholar] [CrossRef]
- Masui, K.; Suzuki, S.O.; Torisu, R.; Goldman, J.E.; Canoll, P.; Iwaki, T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. GLIA 2010, 58, 1050–1065. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Parada, L.F.; Holland, E.C.; Charest, A. Genetic modeling of gliomas in mice: New tools to tackle old problems. GLIA 2011, 59, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Shackleford, G.M.; Shi, X.H.; Swanson, K.S.; Mahdi, M.Y.; Gonzalez-Gomez, I.; Asgharzadeh, S.; D’Apuzzo, M.; Erdreich-Epstein, A.; Moats, R.A. BarTeL, a Genetically Versatile, Bioluminescent and Granule Neuron Precursor-Targeted Mouse Model for Medulloblastoma. PLoS ONE 2016, 11, e0156907. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, T.; Olow, A.K.; Yang, X.; Hashizume, R.; Nicolaides, T.P.; Tom, M.; Aoki, Y.; Berger, M.S.; Weiss, W.A.; Stalpers, L.J.; et al. Survival advantage combining a BRAF inhibitor and radiation in BRAF V600E-mutant glioma. J. Neuro-Oncol. 2016, 126, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.N. Factors Influencing the Use and Interpretation of Animal Models in the Development of Parenteral Drug Delivery Systems. AAPS J. 2011, 13, 632–649. [Google Scholar] [CrossRef]
- Coldwell, D.M.; Huff, J.; Marcellus, H.; Coldwell, S.G. Experimental intra-arterial administration of SR-2508 in the rabbit V2 carcinoma model. Br. J. Radiol. 1989, 62, 234–236. [Google Scholar] [CrossRef]
- Qin, H.; Janowski, M.; Pearl, M.S.; Malysz-Cymborska, I.; Li, S.; Eberhart, C.G.; Walczak, P. Rabbit Model of Human Gliomas: Implications for Intra-Arterial Drug Delivery. PLoS ONE 2017, 12, e0169656. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.; Brown, D.E.; Koehler, J.W.; Miller, A.D.; Miller, C.R.; Bentley, R.T.; Packer, R.A.; Breen, M.; Boudreau, C.E.; et al. Creation of an NCI comparative brain tumor consortium: Informing the translation of new knowledge from canine to human brain tumor patients. Neuro Oncol. 2016, 18, 1209–1218. [Google Scholar] [CrossRef]
- Heidner, G.L.; Kornegay, J.N.; Page, R.L.; Dodge, R.K.; Thrall, D.E. Analysis of survival in a retrospective study of 86 dogs with brain tumors. J. Vet. Intern. Med. 1991, 5, 219–226. [Google Scholar] [CrossRef]
- MacDiarmid, J.A.; Langova, V.; Bailey, D.; Pattison, S.T.; Pattison, S.L.; Christensen, N.; Armstrong, L.R.; Brahmbhatt, V.N.; Smolarczyk, K.; Harrison, M.T.; et al. Targeted Doxorubicin Delivery to Brain Tumors via Minicells: Proof of Principle Using Dogs with Spontaneously Occurring Tumors as a Model. PLoS ONE 2016, 11, e0151832. [Google Scholar] [CrossRef]
- Joshi, A.D.; Botham, R.C.; Schlein, L.J.; Roth, H.S.; Mangraviti, A.; Borodovsky, A.; Tyler, B.; Joslyn, S.; Looper, J.S.; Podell, M.; et al. Synergistic and targeted therapy with a procaspase-3 activator and temozolomide extends survival in glioma rodent models and is feasible for the treatment of canine malignant glioma patients. Oncotarget 2017, 8, 80124–80138. [Google Scholar] [CrossRef]
- Rossmeisl, J.H., Jr.; Garcia, P.A.; Pancotto, T.E.; Robertson, J.L.; Henao-Guerrero, N.; Neal, R.E., II; Ellis, T.L.; Davalos, R.V. Safety and feasibility of the NanoKnife system for irreversible electroporation ablative treatment of canine spontaneous intracranial gliomas. J. Neurosurg. 2015, 123, 1008–1025. [Google Scholar] [CrossRef]
- Raimondi, F.; Shihab, N.; Gutierrez-Quintana, R.; Smith, A.; Trevail, R.; Sanchez-Masian, D.; Smith, P.M. Magnetic resonance imaging findings in epileptic cats with a normal interictal neurological examination: 188 cases. Vet. Rec. 2017, 180, 610. [Google Scholar] [CrossRef]
- Cusick, P.K.; Parker, A.J. Brain stem gliomas in cats. Vet. Pathol. 1975, 12, 460–461. [Google Scholar] [CrossRef]
- Dickinson, P.J.; LeCouteur, R.A.; Higgins, R.J.; Bringas, J.R.; Larson, R.F.; Yamashita, Y.; Krauze, M.T.; Forsayeth, J.; Noble, C.O.; Drummond, D.C.; et al. Canine spontaneous glioma: A translational model system for convection-enhanced delivery. Neuro Oncol. 2010, 12, 928–940. [Google Scholar] [CrossRef]
- Walczak, P.; Wojtkiewicz, J.; Nowakowski, A.; Habich, A.; Holak, P.; Xu, J.; Adamiak, Z.; Chehade, M.; Pearl, M.S.; Gailloud, P.; et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J. Cereb. Blood Flow Metab. 2017, 37, 2346–2358. [Google Scholar] [CrossRef]
- Rissi, D.R.; Miller, A.D. Feline glioma: A retrospective study and review of the literature. J. Feline Med. Surg. 2017, 19, 1307–1314. [Google Scholar] [CrossRef]
- Tabuchi, K.; Nishimoto, A.; Matsumoto, K.; Satoh, T.; Nakasone, S.; Fujiwara, T.; Ogura, H. Establishment of a brain-tumor model in adult monkeys. J. Neurosurg. 1985, 63, 912–916. [Google Scholar] [CrossRef]
- London, W.T.; Houff, S.A.; McKeever, P.E.; Wallen, W.C.; Sever, J.L.; Padgett, B.L.; Walker, D.L. Viral-induced astrocytomas in squirrel monkeys. Prog. Clin. Biol. Res. 1983, 105, 227–237. [Google Scholar]
- Houff, S.A.; London, W.T.; Zu Rhein, G.M.; Padgett, B.L.; Walker, D.L.; Sever, J.L. New world primates as a model of viral-induced astrocytomas. Prog. Clin. Biol. Res. 1983, 105, 223–226. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, R.; Janowski, M.; Killick-Cole, C.L.; Singleton, W.G.B.; Campbell, E.; Walczak, P.; Khatua, S.; Faltings, L.; Symons, M.; Schneider, J.R.; et al. Childhood Brain Tumors: A Review of Strategies to Translate CNS Drug Delivery to Clinical Trials. Cancers 2023, 15, 857. https://doi.org/10.3390/cancers15030857
Rahman R, Janowski M, Killick-Cole CL, Singleton WGB, Campbell E, Walczak P, Khatua S, Faltings L, Symons M, Schneider JR, et al. Childhood Brain Tumors: A Review of Strategies to Translate CNS Drug Delivery to Clinical Trials. Cancers. 2023; 15(3):857. https://doi.org/10.3390/cancers15030857
Chicago/Turabian StyleRahman, Ruman, Miroslaw Janowski, Clare L. Killick-Cole, William G. B. Singleton, Emma Campbell, Piotr Walczak, Soumen Khatua, Lukas Faltings, Marc Symons, Julia R. Schneider, and et al. 2023. "Childhood Brain Tumors: A Review of Strategies to Translate CNS Drug Delivery to Clinical Trials" Cancers 15, no. 3: 857. https://doi.org/10.3390/cancers15030857
APA StyleRahman, R., Janowski, M., Killick-Cole, C. L., Singleton, W. G. B., Campbell, E., Walczak, P., Khatua, S., Faltings, L., Symons, M., Schneider, J. R., Kwan, K., Boockvar, J. A., Gill, S. S., Oliveira, J. M., Beccaria, K., Carpentier, A., Canney, M., Pearl, M., Veal, G. J., ... Walker, D. A. (2023). Childhood Brain Tumors: A Review of Strategies to Translate CNS Drug Delivery to Clinical Trials. Cancers, 15(3), 857. https://doi.org/10.3390/cancers15030857









