Simple Summary
Acquired melanocytic nevi are classified as common or dysplastic. Cutaneous melanoma may develop in a pre-existing acquired nevus (nevus-associated melanoma, NAM). We conducted a systematic review and meta-analysis of 22 published articles to investigate whether NAM occurs more frequently within a dysplastic or common nevus. Although our meta-analysis showed a similar proportion of 51% dysplastic nevus (compared with common nevus) in NAM, when including those studies with larger patient numbers, there was a higher proportion of 65% NAM developing in a dysplastic nevus. A separate meta-analysis of invasive and in situ NAMs showed that the proportion of dysplastic nevus were 56% and 71%, respectively. The larger proportion of dysplastic nevus in in situ NAMs should be interpreted with caution due to the low numbers and possible misclassification bias of in situ NAM developing in dysplastic nevus that may be challenging to differentiate from a dysplastic nevus. Our meta-analysis had considerable uncertainty and high heterogeneity, highlighting the need for future well-designed studies with uniform histopathological definitions for dysplastic nevus remnants which report the type of nevus in NAM separately for invasive and in situ melanomas, thin tumors, and by histological subtype.
Abstract
Background: Cutaneous melanoma has an adjacent nevus remnant upon histological examination in 30% of cases (nevus-associated melanoma, NAM), while it appears de novo for 70% of tumors. Regarding NAM arising in acquired melanocytic nevus, currently there is no evidence on whether NAM more frequently develops in association with a dysplastic or common melanocytic nevus. Objectives: To conduct a systematic review and meta-analysis to investigate the proportion of dysplastic or common melanocytic nevus in NAM associated with acquired nevus. Methods: A systematic literature search is conducted using PubMed, Scopus, and the Cochrane Library. The PRISMA checklist is used. Studies reporting patients diagnosed with NAM arising in an acquired common or dysplastic melanocytic nevus are included. A meta-analysis of proportions is performed using the random-effects model. The magnitude of heterogeneity is assessed with the I2 statistic. Results: A total of 22 studies with 2174 NAMs with an acquired nevus (dysplastic or common) are included. The proportion of dysplastic nevus in NAM varies considerably in the included studies, ranging from 0% to 100%. In the meta-analysis, the overall estimate of the proportion of having a dysplastic nevus in NAM is 51% (95% CI: 39–63%) with high heterogeneity at I2: 95.8% (p < 0.01). A sensitivity meta-analysis of 12 studies that included 30 or more acquired nevus-NAMs (2023 cases) shows that 65% of the NAMs developed in a dysplastic nevus (95% CI: 51–77%). In a meta-analysis of 4 studies reporting invasive-only acquired nevus-NAMs (764 cases), the proportion of dysplastic nevus is 56% (95% CI: 36–75%). Only 2 studies are found reporting in situ NAMs with an acquired nevus, and the pooled estimated proportion of dysplastic nevus is 71% (95% CI: 63–78%). Conclusions: The results of this meta-analysis suggest a higher proportion of dysplastic nevus in acquired nevus-NAM; however, there is considerable uncertainty and high heterogeneity, highlighting the need for future well-designed studies with uniform histopathological definitions for dysplastic nevus remnants which report the type of nevus in NAM separately for invasive melanomas, thin tumors, and by histological subtype.
1. Introduction
The relation between melanocytic nevi and melanoma is multifaceted. The number of acquired melanocytic nevi on the body, including common and dysplastic nevi, is an established marker of the risk of the development of cutaneous melanoma [1]. Moreover, the possibility of melanocytic nevi to act as precursors for the development of melanoma is more complex and intriguing. The presence of nevi in histological association with melanoma (nevus-associated melanoma, NAM) has fueled the debate on the malignant transformation of nevi to nevus-associated melanoma. Recent research has provided accumulating evidence on the distinct epidemiological, histological, dermoscopic, and genetic characteristics of NAM compared with melanoma developing de novo [2,3,4,5] even in thin melanomas [3], thus supporting a distinct route of stepwise development in NAM [6].
It is not known whether NAM is more likely to develop in a common or dysplastic acquired nevus. In their stepwise model of melanoma progression, Clark et al. proposed that if melanoma is to develop via a precursor lesion, the nevus with melanocytic dysplasia is that precursor [7]. A dysplastic nevus was described as a nevus with atypical melanocytic hyperplasia, melanocytic cytologic atypia, mesenchymal changes in the papillary dermis, and a lymphocytic infiltrate [8,9]. The WHO 2018 histological diagnosis of dysplastic nevi is based on the diagnostic criteria by the International Melanoma Pathology Study Group: (1) width > 4 mm; (2) architecture with irregular/dyscohesive nests of intraepidermal melanocytes and the increased density of non-nested junctional melanocytes; and (3) cytology with atypical melanocytes [10]. A modeling study by Tsao et al. that used population-based total melanocytic nevus counts estimated the malignant transformation rate of an individual nevus to be low and range from 0.00005% to 0.003% per year. However, it was noted that the estimation of the transformation rate of dysplastic nevi into melanoma was not possible due to the absence of the accurate documentation of dysplastic nevi age-specific density in a given individual [11].
We performed a systematic review and meta-analysis of the studies reporting the types of acquired nevus in association with NAM with the aim of answering the question of whether NAM is more likely to develop in association with a dysplastic or common acquired nevus. Such findings are useful to the monitoring follow-up and management of individuals with types of nevi more likely to evolve into melanoma as well as adding evidence regarding the previously proposed model of stepwise progression for melanoma development.
2. Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used to guide the project (Supplementary Table S1) [12]. The systematic review was prospectively registered with PROSPERO (registration number: CRD42022377596).
2.1. Literature Search and Study Selection
A systematic review of original articles investigating types of acquired melanocytic nevus in NAM was performed by searching in PubMed/MEDLINE, Scopus, and the Cochrane Library from 1 January 1980 through to 20 November 2022. The search strategy included the following keywords in various combinations: “melanoma”, “nevus”, “naevus”, ”nevi”, “naevi”, “acquired”, “dysplastic”, “nevus-associated”, “naevus-associated”. The full list of search terms is detailed in Supplementary Table S2. Inclusion criteria for eligible studies included: original studies reporting the histological association of cutaneous melanoma with the type of acquired nevus (common or dysplastic) in studies with at least 10 patients with NAM. Exclusion criteria were: NAM associated with congenital melanocytic nevus and studies not reporting the type of acquired melanocytic nevus remnants in NAM. We decided not to study congenital nevi and to instead focus on the research question of acquired nevus remnants (common or dysplastic) in NAM, considering the following: (1) The progression of melanoma from congenital nevus is not a debated issue, and the pathway of melanoma arising in congenital nevus is described in the current WHO classification of skin tumors [13]; (2) the risk of progression of congenital nevi to melanoma has been previously studied in individual studies and in systematic reviews [14,15]; (3) however, the development of melanoma within common or dysplastic nevus has not been previously studied in a systematic review and meta-analysis.
All languages were included in the search results. Non-English results were removed during the review process. Furthermore, the references of review articles on the topic were reviewed, and we carried out secondary referencing by manually reviewing reference lists of assessed articles. The authors of studies with missing results were contacted via email.
Two independent reviewers (C.D. and A.B.) selected the potential studies based on the inclusion and exclusion criteria for further assessment in full text and then screened the full texts, identified studies for inclusion, and recorded reasons for exclusion. Any disagreements were resolved by consensus or by consulting a third review author (A.J.S.). Duplicates were identified and removed. We completed a PRISMA flow diagram and a table with characteristics of excluded studies.
2.2. Data Extraction
Data were extracted and recorded using a standardized Excel sheet by two independent reviewers (C.D., A.B.). For each study, the following study characteristics were extracted: author, journal, year, study design, number of melanomas, number of nevus-associated melanomas (NAMs), number of NAMs associated with acquired melanocytic nevus, number of NAMs associated with common acquired nevus, number of NAMs associated with dysplastic nevus, age, sex, Breslow thickness of NAM, in situ or invasive NAM, and type of common nevus (junctional, dermal, or combined) in NAM. Any disagreements were resolved by consensus or by consulting a third review author (A.S.).
2.3. Quality Assessment
The quality of observational studies was assessed by two reviewers (C.D., A.S.) by assigning a risk of bias (high, moderate, or low) based on the limitations in the designs of included studies such as inconsistency and imprecision. Inconsistency was present in studies reporting NAM in non-relevant subgroups: invasive and in situ melanomas, various histological subtypes, or thin and thick melanomas. Imprecision was assessed based on the small number of participants of included studies [16]. The use of the Newcastle–Ottawa quality assessment scale (NOS) was not applicable as this concerns case–control or cohort studies [17].
2.4. Data Analysis
The primary outcome of interest was the proportion of NAM associated with a dysplastic nevus (compared with NAM associated with a common nevus). Meta-analysis of proportions was performed for the synthesis of single group data. The aim was to investigate the proportion of dysplastic or common melanocytic nevus in NAM associated with acquired nevus. Additional pre-planned analyses included a sensitivity meta-analysis excluding studies with a higher risk of bias, a meta-analysis including studies reporting the type of acquired melanocytic nevus, and a separate meta-analysis for invasive NAM. Post-hoc analyses included a meta-analysis stratified by geographical regions and stratified by year of publication (before and after 2000). Meta-analysis was performed with a random-effects model using the Metaprop command in Stata 12. Freeman and Tukey double arcsine transformation was applied [18]. Forest plots were constructed. The percentage of variation attributable to heterogeneity was assessed with the I2 statistic [19]. All statistical tests were two-sided and p-values less than 0.05 were considered statistically significant. The p-values reporting statistical significance are shown for the I2 statistic. The p-values for the estimated proportion in meta-analyses of proportions were not reported as they do not have any clinical significance; the null hypothesis test is the equality of the estimate with zero. The results were evaluated by effect size estimation in the point estimate and its confidence intervals. Analysis was conducted using STATA, version 12.0 (StataCorp LP, College Station, TX, USA).
3. Results
3.1. Included Studies
A PRISMA flow diagram of study identification, screening, and inclusion is shown in Figure 1. The initial search identified a total of 2756 articles, including 2753 records from PubMed, Scopus, and Cochrane as well as a further 3 potentially eligible articles identified through secondary referencing. After excluding 257 reviews from automated processes in PubMed and 122 duplicates, 2377 records were screened for eligibility based on the titles and abstracts. A further 2280 non-eligible articles and non-English language articles from the titles and abstracts were excluded, and 97 potentially eligible articles were assessed from the full text. From these 97 articles, 75 articles were excluded (detailed in Supplementary Table S3 with reasons for exclusion). We contacted the authors of studies with missing results via email but did not receive any replies. As a result, a total of 22 studies were included [7,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40]. Eight studies were assessed to be at low risk of bias, six studies were at moderate risk of bias, and eight studies were at high risk of bias (Supplementary Table S4).
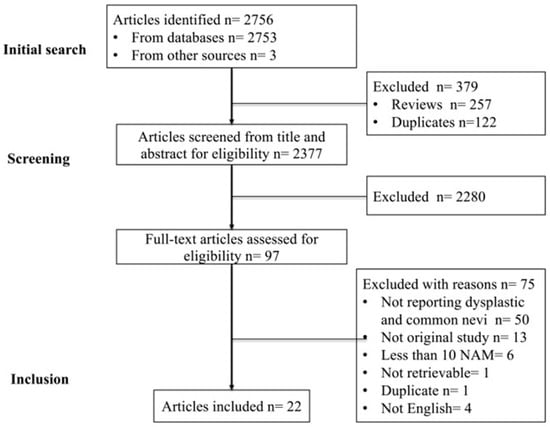
Figure 1.
PRISMA flow diagram of studies identified, screened, and included in the meta-analysis.
3.2. Characteristics of NAM with an Acquired Nevus in Included Studies
The details of the 22 included studies are shown in Table 1. These studies included 3088 NAMs, of which 2174 were NAMs with an acquired nevus (common or dysplastic). All the studies were of a retrospective design. Regarding the inclusion of invasive or in situ melanomas, 8 studies included only invasive melanomas [21,22,24,27,29,30,32,33], 4 studies did not report whether the melanomas were invasive or in situ [7,23,25,26], and 10 studies included invasive and in situ melanomas [20,28,31,34,35,36,37,38,39,40]. Of the 10 studies that included invasive as well as in situ melanomas, 4 studies included both invasive and in situ melanomas without reporting the case numbers [34,35,36,39], 4 studies reported the number of invasive and in situ melanomas without specifying the nevus remnant type [20,28,37,38], and 2 studies reported the number of invasive and in situ melanomas and specified the nevus remnant type in in situ NAMs [31,40] (Table 1).

Table 1.
Included studies reporting the type of acquired melanocytic nevus in histological association with cutaneous melanoma (n = 22).
Of the 22 studies, 12 studies included 30 or more NAMs with an acquired nevus. The mean or median Breslow thickness of the NAMs were reported in only 9 studies (mean Breslow range: 0.51–1.38 mm and median Breslow range: 0.35–2.3 mm, Table 1).
The histologic subtypes of NAM were reported in 15 studies, of which one study had all preselected SSM and one study had all preselected NM. In the remaining 13 studies, the most common subtypes were SSM and NM, and the percentage of SSM ranged from 50% to 100%, while the percentage of NM ranged from 0 to 13% (Table 1).
The type of common nevus in NAM was reported in eight studies, of which three studies used the histological criteria described by Ackerman [41,42], classifying acquired nevi as Clark’s nevi, Miescher’s nevi, and Unna’s nevi. Miescher’s nevi present as dome- or wedge-shaped lesions with mostly endophytic distributed melanocytes, and Unna’s nevi are exophytic, papillomatous lesions confined to a thickened papillary dermis. Unna and Miescher are classified as intradermal [23] (Table 1).
The histopathological criteria for dysplastic nevus remnants in NAM were variably defined in the included studies and are shown in Table 2.

Table 2.
Histopathological criteria for dysplastic nevus remnants in nevus-associated melanoma as defined in included individual studies.
3.3. Meta-Analysis of the Proportion of Dysplastic or Common Acquired Nevus in NAM Studies (n = 22)
There were 22 studies reporting NAM associated with a dysplastic or common acquired nevus [7,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40] and the overall estimate of the proportion of a dysplastic nevus in NAM was 51% (95% CI: 39–63%) as shown in the forest plot in Figure 2. The estimate of the proportion of a dysplastic nevus in NAM was stratified according to the risk of bias (low, moderate, high) (Supplementary Figure S1). The proportion of a dysplastic nevus in NAM was higher in studies with a low risk of bias (60%, 95% CI: 45–73%) compared with studies with a high risk of bias (38%, 95% CI: 13–66%).
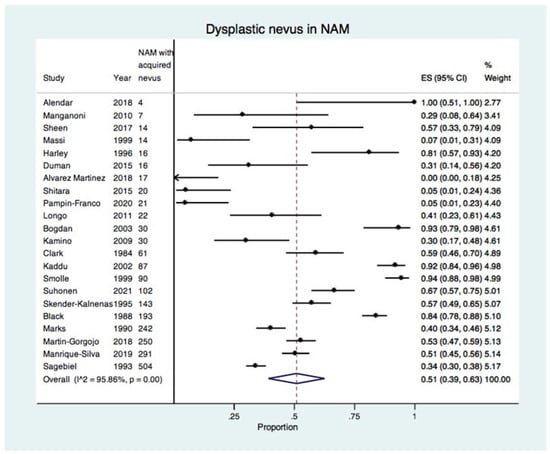
Figure 2.
Forest plot showing the proportion of dysplastic nevus in all included studies reporting NAM developing in an acquired nevus (22 studies, 2174 NAMs with an acquired nevus) [7,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
Overall, there was high heterogeneity with I2 of 95.86%, which was statistically significant (p < 0.01). This was due to the wide variation in the proportion of dysplastic nevus in NAM in the included studies, ranging from 0% to 100% (Table 1). In order to increase precision and to explore heterogeneity, a sensitivity meta-analysis was performed for studies reporting 30 or more NAMs with an acquired nevus.
3.4. Sensitivity Meta-Analysis of Studies Including 30 or More NAMs with an Acquired Nevus (n = 12)
There were 12 studies reporting 30 or more NAMs with an acquired nevus, including a total of 2023 cases [7,22,23,26,27,30,31,32,35,38,39,40] (Table 1). The meta-analysis of these studies showed the presence of dysplastic nevus in 65% of acquired nevus-NAM (95% CI: 51–77%) (Figure 3). There was high heterogeneity with I2: 97.16% (p < 0.01), which was due to the variability of the proportion of dysplastic nevus in the included studies, ranging from 30% to 94.4% (Table 1).
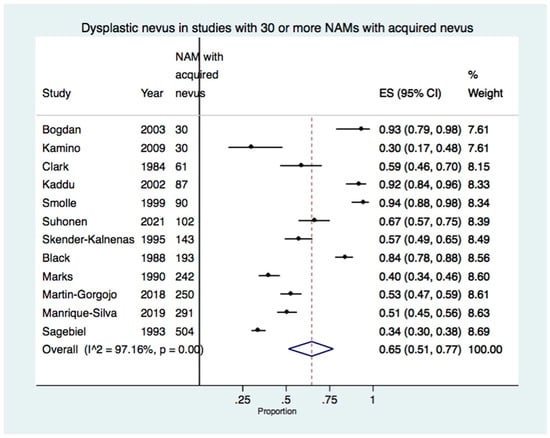
Figure 3.
Forest plot showing the proportion of dysplastic nevus in studies with 30 or more NAMs developing in an acquired nevus (12 studies, 2023 NAMs with an acquired nevus) [7,22,23,26,27,30,31,32,35,38,39,40].
When the study of Suhonen et al. which had a large number of missing values in the presence or absence of nevus in melanoma was additionally excluded from the meta-analysis, the results remained similar, with dysplastic nevus present in 65% of NAM (95% CI: 50–78%).
3.5. Sensitivity Meta-Analysis of Studies Including Invasive-Only NAM
There were eight studies including invasive-only acquired nevus-NAMs [21,22,24,27,29,30,32,33] (Table 1). In the meta-analysis, the estimated proportion of dysplastic nevus in invasive acquired nevus-NAM was 36% (95% CI: 19–55%) (Supplementary Figure S2). However, there were studies with a very small number of acquired nevus-NAMs, ranging from 7 NAMs in a study by Manganoni et al. to 17 NAMs in a study by Alvarez Martinez et al. Moreover, the study by Alvarez Martinez et al. reported 0 cases of dysplastic nevus NAM, while a study by Massi et al. reported 7% of 14 NAMs developed in a dysplastic nevus.
When excluding studies with less than 30 acquired nevus-NAMs, 4 studies with a total of 764 invasive acquired nevus-NAMs were included in the meta-analysis [22,27,30,32]. The estimated proportion of dysplastic nevus in invasive NAM was 56% (95% CI: 36–75%). There was a high degree of heterogeneity at 96.4% (p < 0.01) (Figure 4).
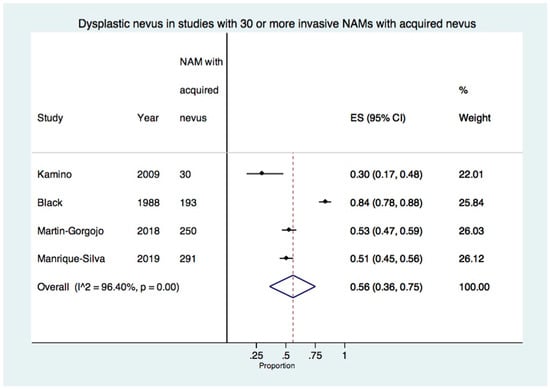
Figure 4.
Forest plot of the proportion of dysplastic nevus in studies with 30 or more invasive-only acquired nevus NAMs (4 studies, 764 invasive-only acquired nevus NAMs) [22,27,30,32].
3.6. Sensitivity Meta-Analysis of Studies Including In Situ NAM
Regarding in situ acquired nevus-NAMs, there were only 2 studies reporting acquired nevus remnant type [31,40]; Marks et al. reported 50/85 in situ dysplastic nevus NAM (59%) [31], and Suhonen et al. reported 46/53 in situ dysplastic nevus NAM (87%) [40]. In a meta-analysis of these 2 studies, there was a pooled proportion of 71% (95% CI: 63–78%) dysplastic nevus in in situ NAMs.
3.7. Meta-Analysis Stratified by Region
There were six studies including patients from Southern Europe [28,29,30,32,33,34]; six studies from Northern/Western Europe [20,21,23,26,39,40]; five studies from North America [7,22,25,27,35]; two studies from Australia [31,38]; one study from Eastern Asia [36]; one study from Western Asia [24]; and one study from Brazil and Austria [37] that could not be classified into one region.
In a meta-analysis stratified by region, the proportion of dysplastic nevus NAM was 46% (95% CI: 41–51%) for Australia, 58% (95% CI: 30–84%) for North America, 76% (95% CI: 49–96%) for Northern/Western Europe, and 32% (95% CI: 19–47%) for Southern Europe (Supplementary Figure S3).
In a meta-analysis of studies including 30 or more acquired nevus-NAMs stratified by region, the proportion of dysplastic nevus NAM remained the same for Australia, 53% (95% CI: 23–82%) for North America, 88% (95% CI: 73–98%) for Northern/Western Europe, and 52% (95% CI: 47–56%) for Southern Europe (Figure 5). The studies from Northern/Western Europe all reported a higher proportion of dysplastic nevi in NAM. By contrast, the studies from the USA showed discrepant results with the proportion of dysplastic nevus in NAM ranging from 30% to 84%. Only four studies reported the number of invasive acquired nevus NAMs (Figure 5).
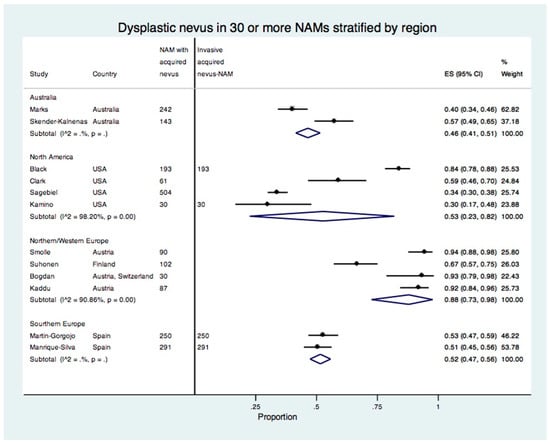
Figure 5.
Forest plot of the proportion of dysplastic nevi in studies with 30 or more acquired nevus NAMs stratified by region.
4. Discussion
It is unclear whether individual dysplastic nevi progress to melanoma at higher rates than banal common nevi. Prospective studies are hindered by the need for long follow-ups and by the overall low rate of nevi transformation. Published studies have indirectly addressed this question by comparing the frequency of NAMs developing in remnants of dysplastic nevi versus the remnants of common nevi with variable and conflicting results. In order to investigate whether NAM occurs more frequently with a dysplastic or a common nevus, we performed a systematic review and meta-analysis of studies reporting NAM in acquired nevi. Although our meta-analysis of 22 studies (n = 2174) showed a similar proportion of 51% of a dysplastic nevus (compared with common nevus) in NAM, when including the 12 studies with 30 or more NAMs with an acquired nevus (n = 2023), there was a higher proportion of NAM developing in a dysplastic nevus at 65%. The separate meta-analysis of invasive and in situ NAMs showed that the proportions of dysplastic nevus were 56% and 71%, respectively.
The numbers of in situ and invasive NAMs included in the individual studies may have influenced the proportion of the detected dysplastic nevus remnant component. In our meta-analysis, in 4 studies including 30 or more invasive-only acquired nevus-NAMs (764 cases), the proportion of dysplastic nevus was 56% (95% CI: 36–75%). However, in 2 available studies that reported in situ NAMs, (138 cases), the proportion of dysplastic nevus was 71% (95% CI: 63–78%). These results should be interpreted with caution due to the small numbers and possible misclassification bias of in situ NAMs. The larger proportion of dysplastic nevus in in situ melanomas may indicate some misclassification arising from upgrading the histologic diagnosis of dysplastic nevus to melanomas in situ [50]. This “diagnostic drift” may arise from the pressure of medical liability and the variability in and challenges to the histopathological diagnosis of dysplastic nevi versus melanoma in situ [51,52]. The addition of dermoscopy to contribute to the diagnosis of melanoma in situ may aid in decreasing the misclassification of these lesions [53], while reflectance confocal microscopy may further aid correct diagnosis [34]. In addition, the dermoscopic characteristics of NAM compared with de novo melanoma have been described. Reiter et al. showed that NAMs were 2.5 times more likely to show a negative pigment network compared with de novo melanomas, even though the nevus component of NAM could not be identified dermoscopically [4]. Regarding the nevus component of NAM, Zalaudek et al. reported dermoscopic characteristics of congenital versus noncongenital NAMs, and in their series, the nevus component was mostly evident and characterized by regular dots/clods and structureless brown areas [54].
In our meta-analysis, there was high heterogeneity due to the variability of the proportion of dysplastic nevus in individual studies. This variability could be attributed mostly to differences in the included melanomas regarding the invasive or in situ type as well as the variable criteria used for the histological definition of dysplastic nevus remnants. It could also possibly be attributed to differences in the Breslow thickness and histological subtypes. It has been shown that the proportion of NAM is higher in thinner melanomas while thicker tumors may obliterate the nevus remnants [3,30], and NAM is more frequent in SSM compared with other subtypes [3,6,30,32]; however, it is not known whether the development of NAM with a common or dysplastic nevus could be different depending on the thickness of the melanoma or the histological subtype. In our systematic review, only the study by Martin-Gorgojo et al. analyzed dysplastic nevus NAM versus common nevus NAM and reported no significant differences in Breslow thickness or subtype [32]. Future studies should report the nevus remnant type in NAM separately for thin melanomas and if possible by histological subtype so that more clear evidence may accumulate.
Our meta-analysis stratified by region showed a higher proportion of dysplastic nevi in NAM in studies from Northern/Western Europe and discrepant estimates in individual studies from North America. These results may be explained by the differences in the number of invasive or in situ melanomas in individual studies. Regarding environmental and genetic factors, NAMs are more likely than de novo melanomas to be located on non-chronically sun-damaged skin, such as on the trunk and extremities [38,55], which in turn is more frequently associated with BRAF mutations [55,56]. In their landmark genetic study of 37 primary melanomas and their adjacent nonmalignant melanocytic neoplasms, Bastian and colleagues investigated the genetic evolution of melanomas from nevi, the sequence of mutations in melanocytic neoplasms, and a stepwise model of mutations [5]. The burden of point mutations escalated with each histologic stage, from benign nevi to intermediate lesions, melanoma in situ, and to invasive melanoma [5,57]. The study by Martin-Gorgojo et al. of 250 invasive NAMs reported no significant differences between common nevus NAM and dysplastic nevus NAM for age, sex, phototype, hair color, location, total body nevus counts, BRAF, NRAS mutations, or MC1R RHC gene variants [32]. Compared with dysplastic nevus NAM, common nevus NAM was more likely to have mitoses present [32]. Future studies may further investigate whether genetic and environmental factors differentially modulate the risk of NAMs developing in a dysplastic or common nevus.
The limitations of our meta-analysis pertain to the limitations of the included studies. Inconsistency underscores the need for studies in relevant patient/melanoma subgroups and for investigating separately in situ and invasive melanomas. Imprecision underscores the need for more studies that report the type of associated nevus in NAM. Our systematic review and meta-analysis examined the frequency of dysplastic nevus versus common nevus in NAM to help improve our understanding of the existing evidence on the potential of nevus transformation to melanoma. However, most existing studies do not distinguish between severe dysplasia and mild or moderate dysplasia, and it is not known if it would be correct to assume a uniform rate of the transformation of dysplastic nevi to melanoma [58]. An additional limitation was that eight studies had a low risk of bias while six had a moderate risk and eight studies had a high risk of bias. Moreover, the very small sample size of some of the included studies may have influenced the accuracy of the estimated proportion. The estimation is likely to be more precise in the meta-analysis including studies with 30 or more NAMs, which supports a pooled proportion of 65% dysplastic nevus in NAM, taking into consideration the fact that the reporting of in situ and invasive NAMs together may have contributed to these results.
The clinical implications of the possibility of nevi progressing to NAM are not clear. Congenital melanocytic nevi are recognized precursors of melanoma arising in congenital nevus, that is a melanoma subtype in the current WHO classification associated with a distinct pathway of development [10,14,15]. By contrast, acquired nevi are frequent, growth-arrested, clonal neoplasms of melanocytes [59]. Even though NAM represents 30% of melanomas [60,61], an acquired nevus rarely progresses to melanoma. The decision regarding when to follow up versus excise clinically atypical nevi incorporates findings with dermoscopy (dermatoscopy, epiluminescence microscopy, or skin surface microscopy) and, in high-risk patients, total-body photography [9]. Investigating the genetics of dermoscopic nevus patterns may shed further light on dysplastic nevi more likely to evolve into melanoma [62].
5. Conclusions
In conclusion, this systematic review synthesized what is known on the frequency of a dysplastic or common nevus associated with NAM, addressing the question of the development of NAM within an acquired nevus. The results of our meta-analysis suggest that a higher proportion of acquired nevus-NAMs develop within a dysplastic nevus (65%); however, there was considerable uncertainty (95% CI: 51–77%) and high heterogeneity, highlighting the need for future well-designed studies with uniform histopathological definitions for dysplastic nevus remnants which report the type of nevus in NAM separately for invasive and in situ melanomas, thin tumors, and by histological subtype.
Supplementary Materials
The following supporting information is available at https://www.mdpi.com/article/10.3390/cancers15030856/s1: Supplementary Table S1. PRISMA 2020 Checklist; Supplementary Table S2. Search terms in PubMed; Supplementary Table S3. Reasons for exclusion of fully assessed articles; Supplementary Table S4. Assessment of risk of bias; Supplementary Figure S1. Forest plot of the proportion of dysplastic nevus in NAM stratified by the risk of bias of individual studies; Supplementary Figure S2. Forest plot of the proportion of dysplastic nevus in studies with invasive-only acquired nevus-NAM; Supplementary Figure S3. Forest plot of the proportion of dysplastic nevus in acquired nevus-NAM stratified by region.
Author Contributions
Conceptualization, C.D. and A.J.S.; methodology, C.D. and A.J.S.; data collection, C.D. and A.B.; data analysis, C.D. and A.J.S.; software, C.D.; writing—original draft preparation, C.D., A.B. and A.J.S.; writing—review and editing, A.J.S., C.D. and A.B.; supervision, A.J.S., C.D., A.B. and A.J.S. had access to all the raw datasets. C.D., A.B. and A.J.S. verified the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
Alexander J. Stratigos: Advisory Board: Regeneron, Novartis. Honoraria: LeoPharma, Novartis, MSD. Research Support: Roche, Genesis Pharma, Janssen Cilag, Abbvie, unrelated to the content of this work; Clio Dessinioti: no conflict of interest; Aggeliki Befon: no conflict of interest.
References
- Gandini, S.; Sera, F.; Cattaruzza, M.S.; Pasquini, P.; Abeni, D.; Boyle, P.; Melchi, C.F. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur. J. Cancer 2005, 41, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Amate, X.; Podlipnik, S.; Riquelme-Mc Loughlin, C.; Carrera, C.; Barreiro-Capurro, A.; Garcia-Herrera, A.; Alos, L.; Malvehy, J.; Puig, S. Clinicopathological, Genetic and Survival Advantages of Naevus-associated Melanomas: A Cohort Study. Acta Derm. Venereol. 2021, 101, adv00425. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Geller, A.C.; Stergiopoulou, A.; Dimou, N.; Lo, S.; Keim, U.; Gershenwald, J.E.; Haydu, L.E.; Dummer, R.; Mangana, J.; et al. A multicentre study of naevus-associated melanoma vs. de novo melanoma, tumour thickness and body site differences. Br. J. Derm. 2021, 185, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Reiter, O.; Kurtansky, N.; Nanda, J.K.; Busam, K.J.; Scope, A.; Musthaq, S.; Marghoob, A.A. The differences in clinical and dermoscopic features between in situ and invasive nevus-associated melanomas and de novo melanomas. J. Eur. Acad. Derm. Venereol. 2021, 35, 1111–1118. [Google Scholar] [CrossRef]
- Shain, A.H.; Yeh, I.; Kovalyshyn, I.; Sriharan, A.; Talevich, E.; Gagnon, A.; Dummer, R.; North, J.; Pincus, L.; Ruben, B.; et al. The Genetic Evolution of Melanoma from Precursor Lesions. N. Engl. J. Med. 2015, 373, 1926–1936. [Google Scholar] [CrossRef]
- Dessinioti, C.; Geller, A.C.; Stratigos, A.J. A review of nevus-associated melanoma: What is the evidence? J. Eur. Acad. Derm. Venereol. 2022, 36, 1927–1936. [Google Scholar] [CrossRef]
- Clark, W.H., Jr.; Elder, D.E.; Guerry, D.t.; Epstein, M.N.; Greene, M.H.; Van Horn, M. A study of tumor progression: The precursor lesions of superficial spreading and nodular melanoma. Hum. Pathol. 1984, 15, 1147–1165. [Google Scholar] [CrossRef]
- Clark, W.H., Jr.; Reimer, R.R.; Greene, M.; Ainsworth, A.M.; Mastrangelo, M.J. Origin of familial malignant melanomas from heritable melanocytic lesions. ‘The B-K mole syndrome’. Arch. Derm. 1978, 114, 732–738. [Google Scholar] [CrossRef]
- Drozdowski, R.; Spaccarelli, N.; Peters, M.S.; Grant-Kels, J.M. Dysplastic nevus part I: Historical perspective, classification, and epidemiology. J. Am. Acad. Derm. 2023, 88, 1–10. [Google Scholar] [CrossRef]
- Elder, D.; Barnhill, R.L.; Bastian, B.C.; Duncan, L.M.; Massi, D.; Mihm, M.C.J.; Piepkorn, M.; Rabkin, M.; Scolyer, R.A. Dysplastic naevus. In WHO Classification of Skin Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2018; Volume 11, pp. 82–86. [Google Scholar]
- Tsao, H.; Bevona, C.; Goggins, W.; Quinn, T. The transformation rate of moles (melanocytic nevi) into cutaneous melanoma: A population-based estimate. Arch. Derm. 2003, 139, 282–288. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.; Barnhill, R.L.; Bastian, B.C.; Cook, M.G.; de la Fouchardiere, A.; Gerami, P.; Lazar, A.J.; Massi, D.; Mihm, M.C.J.; Nagore, E.; et al. Melanocytic tumour classification and the pathway concept of melanoma pathogenesis. In WHO Classification of Skin Tumours, 4th ed.; Elder, D.E., Massi, D., Scolyer, R.A., Willemze, R., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2018; Volume 11, pp. 66–71. [Google Scholar]
- Krengel, S.; Hauschild, A.; Schafer, T. Melanoma risk in congenital melanocytic naevi: A systematic review. Br. J. Derm. 2006, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Scard, C.; Aubert, H.; Wargny, M.; Martin, L.; Barbarot, S. Risk of melanoma in congenital melanocytic nevi of all sizes: A systematic review. J. Eur. Acad. Derm. Venereol. 2023, 37, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Schumermann, H.J.; Higgins, J.P.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Cochrane Handbook for Systematic Reviews of Interventions. Chapter 14: Completing ‘Summary of Findings’ Tables and Grading the Certainty of Evidence. Available online: https://training.cochrane.org/handbook/current/chapter-14#section-14-2-2 (accessed on 18 December 2022).
- Wells, G.A.; Shea, B.; O’’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 4 May 2021).
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 39. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef]
- Alendar, T.; Kittler, H. Morphologic characteristics of nevi associated with melanoma: A clinical, dermatoscopic and histopathologic analysis. Derm. Pract. Concept. 2018, 8, 104–108. [Google Scholar] [CrossRef]
- Alvarez Martinez, D.; Boehncke, W.H.; Kaya, G.; Merat, R. Recognition of early melanoma: A monocentric dermoscopy follow-up study comparing de novo melanoma with nevus-associated melanoma. Int. J. Dermatol. 2018, 57, 692–702. [Google Scholar] [CrossRef]
- Black, W.C. Residual dysplastic and other nevi in superficial spreading melanoma. Clinical correlations and association with sun damage. Cancer 1988, 62, 163–173. [Google Scholar] [CrossRef]
- Bogdan, I.; Smolle, J.; Kerl, H.; Burg, G.; Boni, R. Melanoma ex naevo: A study of the associated naevus. Melanoma Res. 2003, 13, 213–217. [Google Scholar] [CrossRef]
- Duman, N.; Erkin, G.; Gokoz, O.; Karahan, S.; Kayikcioglu, A.U.; Celik, I. Nevus-Associated versus de novo Melanoma: Do They Have Different Characteristics and Prognoses? Dermatopathology 2015, 2, 46–51. [Google Scholar] [CrossRef]
- Harley, S.; Walsh, N. A new look at nevus-associated melanomas. Am. J. Derm. 1996, 18, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Kaddu, S.; Smolle, J.; Zenahlik, P.; Hofmann-Wellenhof, R.; Kerl, H. Melanoma with benign melanocytic naevus components: Reappraisal of clinicopathological features and prognosis. Melanoma Res. 2002, 12, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Kamino, H.; Tam, S.; Tapia, B.; Toussaint, S. The use of elastin immunostain improves the evaluation of melanomas associated with nevi. J. Cutan. Pathol. 2009, 36, 845–852. [Google Scholar] [CrossRef]
- Longo, C.; Rito, C.; Beretti, F.; Cesinaro, A.M.; Pineiro-Maceira, J.; Seidenari, S.; Pellacani, G. De novo melanoma and melanoma arising from pre-existing nevus: In vivo morphologic differences as evaluated by confocal microscopy. J. Am. Acad. Derm. 2011, 65, 604–614. [Google Scholar] [CrossRef]
- Manganoni, A.M.; Farisoglio, C.; Gavazzoni, F.; Facchetti, F.; Zanotti, F.; Calzavara-Pinton, P. Nodular melanomas associated with nevi. J. Am. Acad. Derm. 2010, 63, e97. [Google Scholar] [CrossRef] [PubMed]
- Manrique-Silva, E.; Reyes-Garcia, D.; Folgado, B.; Martin-Gorgojo, A.; Traves, V.; Requena, C.; Nagore, E. The proportion of nevus-associated invasive melanoma differs with Breslow thickness: A cross-sectional study of 1087 cutaneous melanomas. J. Am. Acad. Derm. 2019, 81, 852–854. [Google Scholar] [CrossRef]
- Marks, R.; Dorevitch, A.P.; Mason, G. Do all melanomas come from “moles”? A study of the histological association between melanocytic naevi and melanoma. Australas. J. Dermatol. 1990, 31, 77–80. [Google Scholar] [CrossRef]
- Martin-Gorgojo, A.; Requena, C.; Garcia-Casado, Z.; Traves, V.; Kumar, R.; Nagore, E. Dysplastic vs. Common Naevus-associated vs. De novo Melanomas: An Observational Retrospective Study of 1,021 Patients. Acta Derm. Venereol. 2018, 98, 556–562. [Google Scholar] [CrossRef]
- Massi, D.; Carli, P.; Franchi, A.; Santucci, M. Naevus-associated melanomas: Cause or chance? Melanoma Res. 1999, 9, 85–91. [Google Scholar] [CrossRef]
- Pampin-Franco, A.; Gamo-Villegas, R.; Floristan-Muruzabal, U.; Pinedo-Moraleda, F.J.; Perez-Fernandez, E.; Garcia-Zamora, E.; Lopez-Estebaranz, J.L. Nevus-associated melanoma: An observational retrospective study of 22 patients evaluated with dermoscopy and reflectance confocal microscopy. Ski. Res. Technol. 2020, 26, 99–104. [Google Scholar] [CrossRef]
- Sagebiel, R.W. Melanocytic nevi in histologic association with primary cutaneous melanoma of superficial spreading and nodular types: Effect of tumor thickness. J. Invest. Derm. 1993, 100, 322S–325S. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.S.; Liao, Y.H.; Lin, M.H.; Chen, J.S.; Liau, J.Y.; Liang, C.W.; Chang, Y.L.; Chu, C.Y. Clinicopathological features and prognosis of patients with de novo versus nevus-associated melanoma in Taiwan. PLoS ONE 2017, 12, e0177126. [Google Scholar] [CrossRef] [PubMed]
- Shitara, D.; Tell-Marti, G.; Badenas, C.; Enokihara, M.M.; Alos, L.; Larque, A.B.; Michalany, N.; Puig-Butille, J.A.; Carrera, C.; Malvehy, J.; et al. Mutational status of naevus-associated melanomas. Br. J. Derm. 2015, 173, 671–680. [Google Scholar] [CrossRef]
- Skender-Kalnenas, T.M.; English, D.R.; Heenan, P.J. Benign melanocytic lesions: Risk markers or precursors of cutaneous melanoma? J. Am. Acad. Derm. 1995, 33, 1000–1007. [Google Scholar] [CrossRef]
- Smolle, J.; Kaddu, S.; Kerl, H. Non-random spatial association of melanoma and naevi--a morphometric analysis. Melanoma Res. 1999, 9, 407–412. [Google Scholar] [CrossRef]
- Suhonen, V.; Rummukainen, J.; Siiskonen, H.; Mannermaa, A.; Harvima, I.T. High regional mortality due to malignant melanoma in Eastern Finland may be explained by the increase in aggressive melanoma types. BMC Cancer 2021, 21, 1155. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Magana-Garcia, M. Naming acquired melanocytic nevi. Unna’s, Miescher’s, Spitz’s Clark’s. Am. J. Derm. 1990, 12, 193–209. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Milde, P. Naming acquired melanocytic nevi. Common and dysplastic, normal and atypical, or Unna, Miescher, Spitz, and Clark? Am. J. Derm. 1992, 14, 447–453. [Google Scholar] [CrossRef]
- Stolz, W.; Schmoeckel, C.; Landthaler, M.; Braun-Falco, O. Association of early malignant melanoma with nevocytic nevi. Cancer 1989, 63, 550–555. [Google Scholar] [CrossRef]
- Rhodes, A.R.; Harrist, T.J.; Day, C.L.; Mihm, M.C., Jr.; Fitzpatrick, T.B.; Sober, A.J. Dysplastic melanocytic nevi in histologic association with 234 primary cutaneous melanomas. J. Am. Acad. Derm. 1983, 9, 563–574. [Google Scholar] [CrossRef]
- Cook, M.G.; Robertson, I. Melanocytic dysplasia and melanoma. Histopathology 1985, 9, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, A.R.; Melski, J.W.; Sober, A.J.; Harrist, T.J.; Mihm, M.C., Jr.; Fitzpatrick, T.B. Increased intraepidermal melanocyte frequency and size in dysplastic melanocytic nevi and cutaneous melanoma. A comparative quantitative study of dysplastic melanocytic nevi, superficial spreading melanoma, nevocellular nevi, and solar lentigines. J. Invest. Derm. 1983, 80, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Elder, D.; Green, M.H.; Bondi, E.E. Acquired melanocytic nevi and melanoma; the dysplastic nevus syndrome. In Pathology of Malignant Melanoma; Ackerman, A.B., Ed.; Masson Publishing: New York, NY, USA, 1981; pp. 185–215. [Google Scholar]
- Lever, W.; Schaumburg-Lever, G. Histopathology of the Skin, 6th ed.; JB Lippincott Co.: Philadelphia, PA, USA, 1983. [Google Scholar]
- Duffy, K.; Grossman, D. The dysplastic nevus: From historical perspective to management in the modern era: Part I. Historical, histologic, and clinical aspects. J. Am. Acad. Derm. 2012, 67, 1.e1–1.e16. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Nufer, K.L.; Raphael, A.P.; Soyer, H.P. Dermoscopy and Overdiagnosis of Melanoma In Situ. JAMA Derm. 2018, 154, 398–399. [Google Scholar] [CrossRef]
- Farmer, E.R.; Gonin, R.; Hanna, M.P. Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum. Pathol. 1996, 27, 528–531. [Google Scholar] [CrossRef]
- Lallas, A.; Longo, C.; Manfredini, M.; Benati, E.; Babino, G.; Chinazzo, C.; Apalla, Z.; Papageorgiou, C.; Moscarella, E.; Kyrgidis, A.; et al. Accuracy of Dermoscopic Criteria for the Diagnosis of Melanoma In Situ. JAMA Derm. 2018, 154, 414–419. [Google Scholar] [CrossRef]
- Zalaudek, I.; Conforti, C.; Guarneri, F.; Vezzoni, R.; Deinlein, T.; Hofmann-Wellenhof, R.; Longo, C.; Moscarella, E.; Kittler, H.; Argenziano, G.; et al. Clinical and dermoscopic characteristics of congenital and noncongenital nevus-associated melanomas. J. Am. Acad. Derm. 2020, 83, 1080–1087. [Google Scholar] [CrossRef]
- Pandeya, N.; Kvaskoff, M.; Olsen, C.M.; Green, A.C.; Perry, S.; Baxter, C.; Davis, M.B.; Mortimore, R.; Westacott, L.; Wood, D.; et al. Factors Related to Nevus-Associated Cutaneous Melanoma: A Case-Case Study. J. Invest. Derm. 2018, 138, 1816–1824. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.N.; Hahn, H.J.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Metaanalysis of BRAF mutations and clinicopathologic characteristics in primary melanoma. J. Am. Acad. Derm. 2015, 72, 1036–1046.e1032. [Google Scholar] [CrossRef]
- Shain, A.H.; Joseph, N.M.; Yu, R.; Benhamida, J.; Liu, S.; Prow, T.; Ruben, B.; North, J.; Pincus, L.; Yeh, I.; et al. Genomic and Transcriptomic Analysis Reveals Incremental Disruption of Key Signaling Pathways during Melanoma Evolution. Cancer Cell 2018, 34, 45–55.e44. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.C.; Swetter, S.M.; Curiel-Lewandrowski, C.; Grichnik, J.M.; Grossman, D.; Halpern, A.C.; Kirkwood, J.M.; Leachman, S.A.; Marghoob, A.A.; Ming, M.E.; et al. Addressing the knowledge gap in clinical recommendations for management and complete excision of clinically atypical nevi/dysplastic nevi: Pigmented Lesion Subcommittee consensus statement. JAMA Derm. 2015, 151, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Roh, M.R.; Eliades, P.; Gupta, S.; Tsao, H. Genetics of melanocytic nevi. Pigment. Cell Melanoma Res. 2015, 28, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A meta-analysis of nevus-associated melanoma: Prevalence and practical implications. J. Am. Acad. Derm. 2017, 77, 938–945.e934. [Google Scholar] [CrossRef]
- Lin, W.M.; Luo, S.; Muzikansky, A.; Lobo, A.Z.; Tanabe, K.K.; Sober, A.J.; Cosimi, A.B.; Tsao, H.; Duncan, L.M. Outcome of patients with de novo versus nevus-associated melanoma. J. Am. Acad. Derm. 2015, 72, 54–58. [Google Scholar] [CrossRef]
- Tan, J.M.; Tom, L.N.; Soyer, H.P.; Stark, M.S. Defining the Molecular Genetics of Dermoscopic Naevus Patterns. Dermatology 2019, 235, 19–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).