The Quality of Life in Surgically Treated Head and Neck Basal Cell Carcinoma Patients: A Comprehensive Review
Abstract
Simple Summary
Abstract
1. Introduction
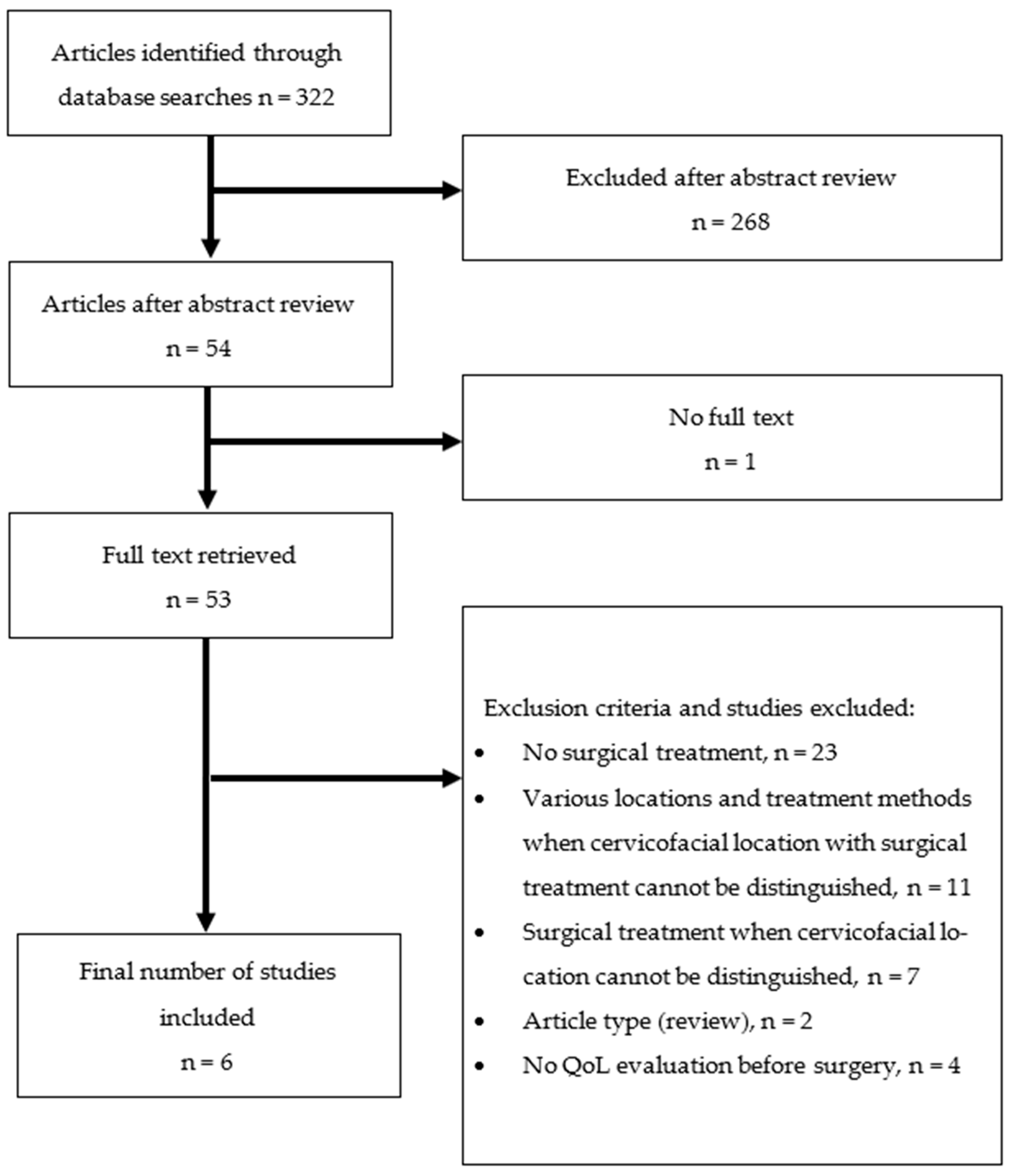
2. Materials and Methods
2.1. Search Strategies
2.2. Study Selection Criteria
- Age: the study included patients over 18 years old
- Location of tumor: craniofacial BCC
- Intervention: surgical treatment of the face and neck BCC
- Outcome: QoL was assessed before and after the surgical treatment
- Article types: prospective studies were included
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kim, D.P.; Kus, K.J.B.; Ruiz, E. Basal Cell Carcinoma Review. Hematol. Oncol. Clin. N. Am. 2019, 33, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Marzuka, A.G.; Book, S.E. Basal cell carcinoma: Pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J. Biol. Med. 2015, 88, 167–179. [Google Scholar] [PubMed]
- Cameron, M.C.; Lee, E.; Hibler, B.P.; Barker, C.A.; Mori, S.; Cordova, M.; Nehal, K.S.; Rossi, A.M. Basal cell carcinoma: Epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J. Am. Acad. Dermatol. 2019, 80, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00140. [Google Scholar] [CrossRef] [PubMed]
- Lai, V.; Cranwell, W.; Sinclair, R. Epidemiology of skin cancer in the mature patient. Clin. Dermatol. 2018, 36, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.J. Incidence of Basal Cell and Squamous Cell Carcinomas in a Population Younger Than 40 Years. JAMA 2005, 294, 681. [Google Scholar] [CrossRef] [PubMed]
- Kauvar, A.N.B.; Cronin, T.; Roenigk, R.; Hruza, G.; Bennett, R. Consensus for Nonmelanoma Skin Cancer Treatment: Basal Cell Carcinoma, Including a Cost Analysis of Treatment Methods. Dermatol. Surg. 2015, 41, 550–571. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; McDonald, J.A.; Greendberg, E.R.; Stukel, T.A.; Weiss, J.E.; Baron, J.A.; Stevens, M.M.; For the Skin Cancer Prevention Study Group. Risk of Basal Cell and Squamous Cell Skin Cancers After Ionizing Radiation Therapy. JNCI J. Natl. Cancer Inst. 1996, 88, 1848–1853. [Google Scholar] [CrossRef]
- Bichakjian, C.K.; Olencki, T.; Aasi, S.Z.; Alam, M.; Andersen, J.S.; Berg, D.; Bowen, G.M.; Cheney, R.T.; Daniels, G.A.; Glass, L.F.; et al. Basal Cell Skin Cancer, Version 1.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2016, 14, 574–597. [Google Scholar] [CrossRef]
- Didona, D.; Paolino, G.; Bottoni, U.; Cantisani, C. Non Melanoma Skin Cancer Pathogenesis Overview. Biomedicines 2018, 6, 6. [Google Scholar] [CrossRef]
- Rhee, J.S.; Matthews, B.A.; Neuburg, M.; Logan, B.R.; Burzynski, M.; Nattinger, A.B. Validation of a Quality-of-Life Instrument for Patients With Nonmelanoma Skin Cancer. Arch. Facial Plast. Surg. 2006, 8, 314–318. [Google Scholar] [CrossRef]
- Sanchez, N.; Griggs, J.; Nanda, S.; Fayne, R.; Castillo, D.; De Bedout, V.; Meirson, D.; Nichols, A. The Skin Cancer Index: Quality-of-life outcomes of treatments for nonmelanoma skin cancer. J. Dermatol. Treat. 2020, 31, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Gaulin, C.; Sebaratnam, D.F.; Fernández-Peñas, P. Quality of life in non-melanoma skin cancer. Australas. J. Dermatol. 2015, 56, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.S.; Matthews, B.A.; Neuburg, M.; Smith, T.L.; Burzynski, M.; Nattinger, A.B. Quality of Life and Sun-Protective Behavior in Patients With Skin Cancer. Arch. Otolaryngol. Head Neck Surg. 2004, 130, 141–146. [Google Scholar] [CrossRef]
- Maciel, P.C.; Veiga-Filho, J.; Carvalho MP de Fonseca, F.E.M.; Ferreira, L.M.; Veiga, D.F. Quality of life and self-esteem in patients submitted to surgical treatment of skin carcinomas: Long-term results. An. Bras. Dermatol. 2014, 89, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Çetinarslan, T.; Evrenos, M.K.; Bilaç, C.; Özyurt, B.; Türel Ermertcan, A. Evaluation of the effect of surgical treatment on quality of life with the Dermatology Life Quality Index in patients with facial nonmelanoma skin cancer. Dermatol. Ther. 2020, 33, e14094. [Google Scholar] [CrossRef]
- García-Montero, P.; de Gálvez-Aranda, M.V.; Blázquez-Sánchez, N.; Rivas-Ruíz, F.; Millán-Cayetano, J.F.; Harana, C.G.; de Troya Martín, M. Factors related to the evolution of quality of life in patients with cervicofacial non-melanoma skin cancer. Support Care Cancer 2021, 29, 5187–5195. [Google Scholar] [CrossRef] [PubMed]
- Kinde, B.; Idowu, O.O.; Ashraf, D.C.; Chen, R.M.; Hirabayashi, K.E.; Grob, S.R.; Winn, B.J.; Kersten, R.C.; Vagefi, M.R. Quality-of-Life Outcomes for Excision and Reconstruction of Periocular Nonmelanoma Skin Cancer. Facial Plast. Surg. Aesthet. Med. 2021. ahead of print. [Google Scholar] [CrossRef]
- Sanz Aranda, E.; Bernal Martínez, Á.J.; Reola Ramírez, E.; Perales Enguita, A.; Martí Ayats, J.M. Quality of Life in Patients of Advanced Age With Basal Cell Carcinoma: Analysis and Implications for Approach to Treatment. Actas Dermo-Sifiliogr. 2022, 113, 536–539. [Google Scholar] [CrossRef]
- Constitution of the World Health Organization. Am. J. Public Health Nation’s Health 1946, 36, 1245–1364. [CrossRef]
- Brazier, J.E.; Harper, R.; Jones, N.M.; O’Cathain, A.; Thomas, K.J.; Usherwood, T.; Westlake, L. Validating the SF-36 health survey questionnaire: New outcome measure for primary care. BMJ 1992, 305, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.A.; Donegan, D.; Albert, T. The 36-item short form. J. Am. Acad. Orthop. Surg. 2007, 15, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Lins, L.; Carvalho, F.M. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med. 2016, 4, 205031211667172. [Google Scholar] [CrossRef] [PubMed]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Finlay, A.Y.; Khan, G.K. Dermatology Life Quality Index (DLQI)-a simple practical measure for routine clinical use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef]
- Chernyshov, P.V.; Lallas, A.; Tomas-Aragones, L.; Arenbergerova, M.; Samimi, M.; Manolache, L.; Svensson, A.; Marron, S.E.; Sampogna, F.; Spillekom-vanKoulil, S.; et al. Quality of life measurement in skin cancer patients: Literature review and position paper of the European Academy of Dermatology and Venereology Task Forces on Quality of Life and Patient Oriented Outcomes, Melanoma and Non-Melanoma Skin Cancer. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 816–827. [Google Scholar] [CrossRef]

| Author | Study Type | Study Description | Sample | Sample Description | QoL Outcome Measures | Main Conclusions |
|---|---|---|---|---|---|---|
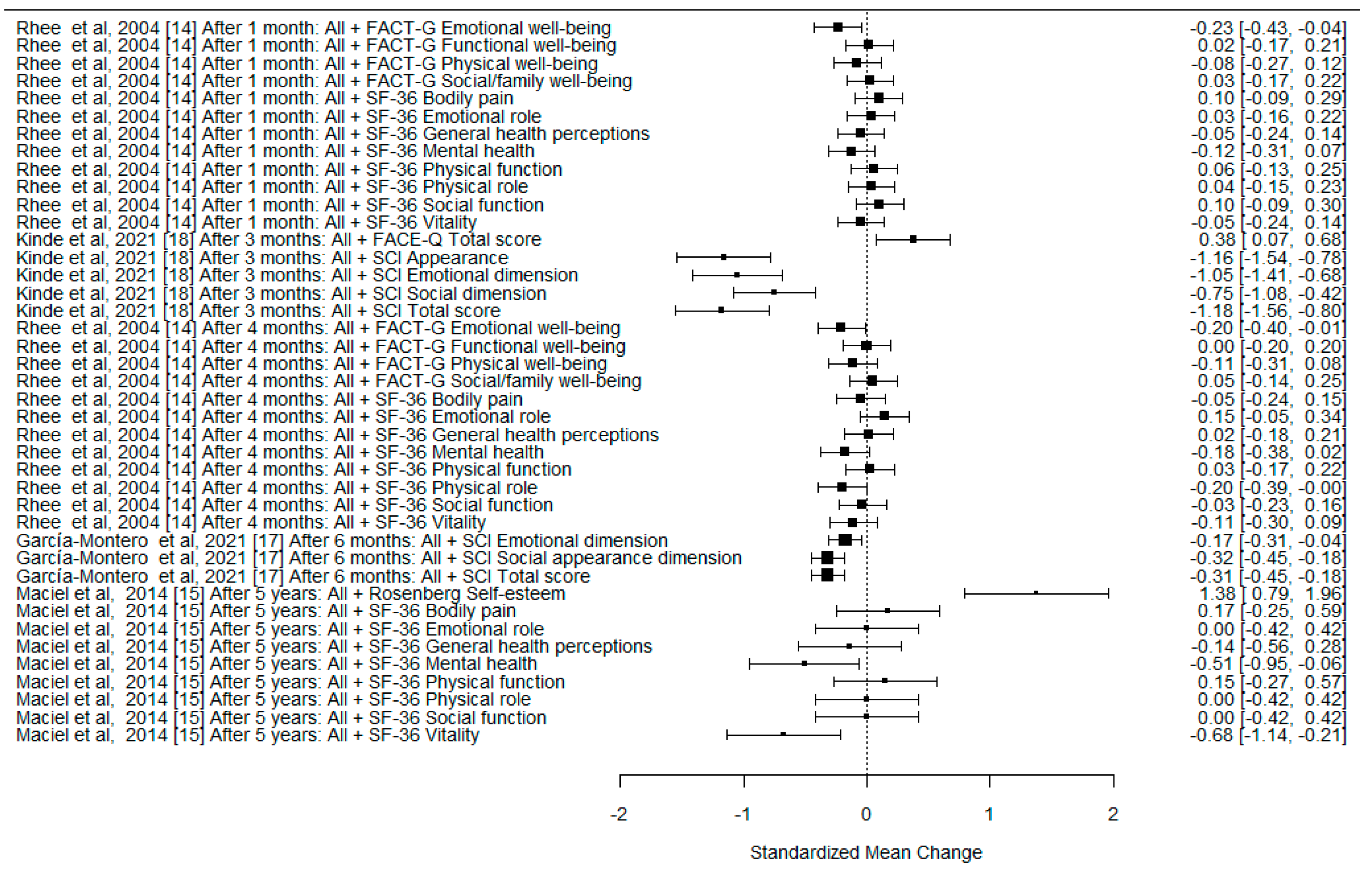
| Rhee et al., 2004 [14] | Longitudinal prospective research | QoL assessment of cervicofacial skin cancer treated with Mohs surgery. QoL data collected at baseline, 1 month and 4 months | n = 121—initial visit n = 105—first follow up n = 102—second follow up | Biopsy-proved NMSC cervicofacial skin cancer: n = 103—BCC, n = 16—SCC, n = 2—other | SF-36, 10-cm visual analog scale (VAS), Functional Assessment of Cancer Therapy-General (FACT-G) | Little change of QoL was noticed following the treatment of NMSC; the improvements in emotional, and mental health following treatment of NMSC were established (specifically <65 years and employed patients). |
| Maciel et al., 2014 [15] | Prospective, analytical clinical study | Assessment of late impact of surgical treatment of cervicofacial skin carcinomas on QoL and self-esteem. QoL data collected at baseline and 5 years after surgery | n = 55—initial visit n = 22—5-years after surgery follow up | Biopsy-proved NMSC cervicofacial skin cancer larger than 1 cm: n = 19—BCC, n = 3—SCC | SF-36, the Rosenberg Self-Esteem Scale/UNIFESP-EPM | Improvement in mental health and self-esteem was observed in the late postoperative period after surgical treatment of NMSC. |
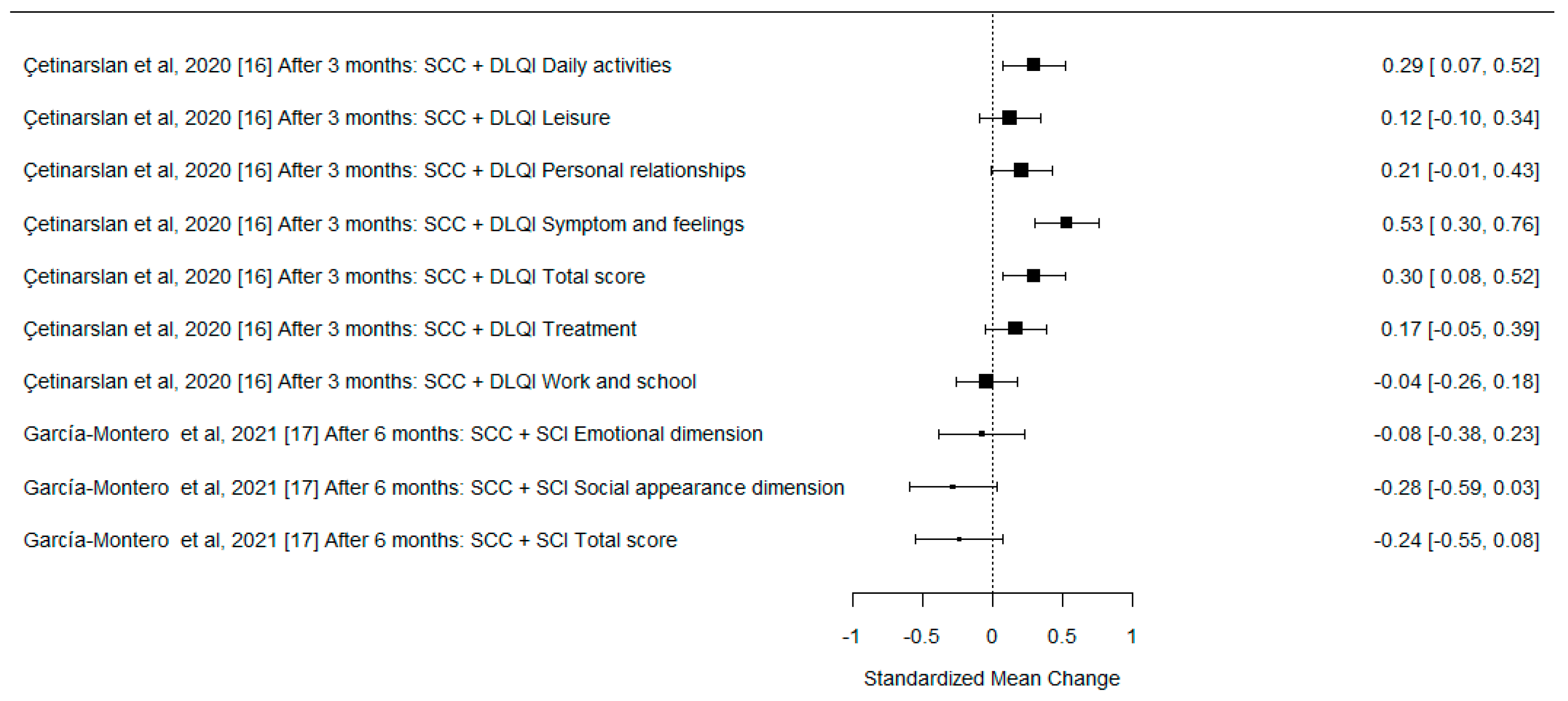
| Çetinarslan et al., 2020 [16] | Prospective study | Determination of the factors affecting QoL and the effect of surgical treatment on QoL of patients with facial NMSC. QoL data collected at baseline and 3 months after surgery | n = 255—initial visit | Histologically or clinically diagnosed facial BCC or SCC: n = 174—BCC, n = 81—SCC | DLQI | The QoL is minimally affected in patients with NMSC using DLQI; the QoL 3 months after surgery showed a significant improvement in patients with facial NMSC. |
| García-Montero et al., 2021 [17] | Prospective cohort study | Identification of the factors related to the favorable evaluation of QoL during follow-up after treatment of cervicofacial NMSC. QoL data collected at the time of diagnosis, 7 days, 1 month and 6 months after treatment | n = 229—initially included n = 220—completed questionnaires | Cervicofacial NMSC, confirmed by skin biopsy: n = 179—BCC, n= 41—SCC Type of treatment: n = 190—surgery n = 19—photodynamic therapy n = 3 imiquimod 5% n = 8 cryotherapy or electrosurgery | SCI, VAS, clinical interview | Scores of the SCI improve after the treatment of cervicofacial NMSC. |
| Kinde et al., 2021 [18] | Prospective study | Measurement of QoL of individuals with surgically treated periocular NMSC. QoL data collected at baseline, 1 week and 3 months after surgery | n = 57—enrolled patients n = 45 completed questionnaires | Patients diagnosed for the first time with periocular NMSC who underwent Mohs micrographic surgery and reconstruction: n= 37—BCC n= 8—SCC | SCI, FACE-Q | Mohs resection of periocular NMSC patients demonstrated reduced QoL as measured by the SCI and FACE-Q surveys; the significant improvement of Qol after this surgery was reported. The highest improvements were in the late postoperative period. |
| Sanz Aranda et al., 2021 [19] | Prospective observational study | QoL in histologically confirmed BCC patients older than 85 years treated with surgery. QoL data collected before and 3 months after surgery | n = 48—presurgery questionnaire n = 25—postoperative survey | Histologically confirmed BCC patients older than 85 years | Spanish SF-36 | A significant improvement of QoL after surgery was not detected; the authors believe that surgery as a first-line treatment for BCC should be discussed with patients and their caregivers or relatives, along with alternative options. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stundys, D.; Ulianskaite, G.; Stundiene, I.; Grigaitiene, J.; Jancoriene, L. The Quality of Life in Surgically Treated Head and Neck Basal Cell Carcinoma Patients: A Comprehensive Review. Cancers 2023, 15, 801. https://doi.org/10.3390/cancers15030801
Stundys D, Ulianskaite G, Stundiene I, Grigaitiene J, Jancoriene L. The Quality of Life in Surgically Treated Head and Neck Basal Cell Carcinoma Patients: A Comprehensive Review. Cancers. 2023; 15(3):801. https://doi.org/10.3390/cancers15030801
Chicago/Turabian StyleStundys, Domantas, Gintare Ulianskaite, Ieva Stundiene, Jurate Grigaitiene, and Ligita Jancoriene. 2023. "The Quality of Life in Surgically Treated Head and Neck Basal Cell Carcinoma Patients: A Comprehensive Review" Cancers 15, no. 3: 801. https://doi.org/10.3390/cancers15030801
APA StyleStundys, D., Ulianskaite, G., Stundiene, I., Grigaitiene, J., & Jancoriene, L. (2023). The Quality of Life in Surgically Treated Head and Neck Basal Cell Carcinoma Patients: A Comprehensive Review. Cancers, 15(3), 801. https://doi.org/10.3390/cancers15030801






