Combined Hyperthermia and Re-Irradiation in Non-Breast Cancer Patients: A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- -
- Duplicates;
- -
- Articles that were not clinical studies;
- -
- Studies on breast cancer;
- -
- Articles that were updated in a later publication by the same author(s);
- -
- Studies involving fewer than 10 patients with one tumor type treated with re-irradiation and hyperthermia;
- -
- Case reports, conference abstracts or presentations.
3. Results
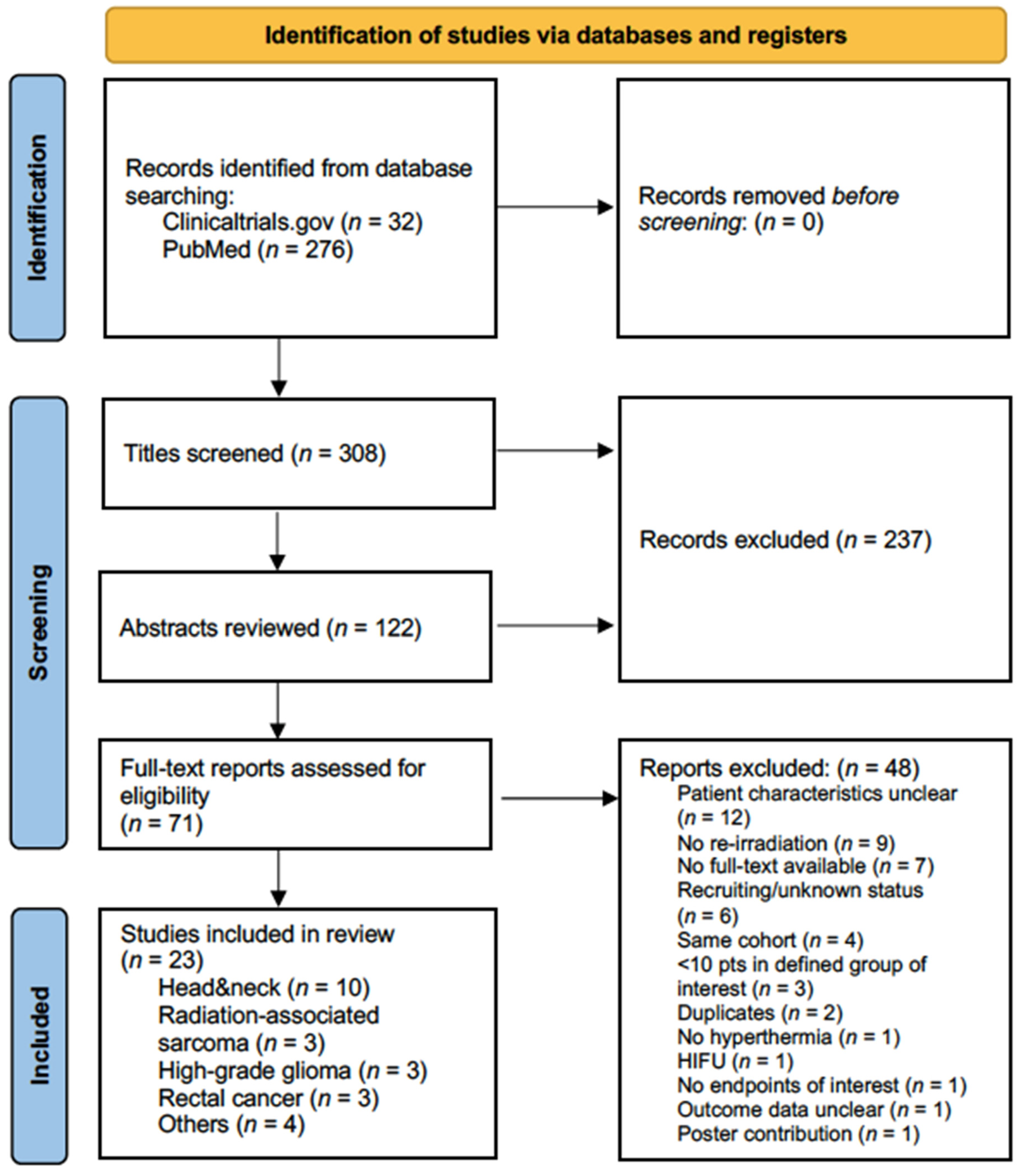
3.1. Study Selection, Patient and Treatment Characteristics
3.2. Bias Assessment
3.3. Head and Neck Cancer
3.4. Glioma
3.5. Radiation-Associated Sarcoma
3.6. Rectal Cancer
3.7. Other Cancer Types
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van der Zee, J.; Gonzalez Gonzalez, D.; van Rhoon, G.C.; van Dijk, J.D.; van Putten, W.L.; Hart, A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Vernon, C.C.; Hand, J.W.; Field, S.B.; Machin, D.; Whaley, J.B.; van der Zee, J.; van Putten, W.L.; van Rhoon, G.C.; van Dijk, J.D.; Gonzalez Gonzalez, D.; et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. International Collaborative Hyperthermia Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Overgaard, J.; Gonzalez Gonzalez, D.; Hulshof, M.C.; Arcangeli, G.; Dahl, O.; Mella, O.; Bentzen, S.M. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 1995, 345, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef]
- Chi, M.S.; Yang, K.L.; Chang, Y.C.; Ko, H.L.; Lin, Y.H.; Huang, S.C.; Huang, Y.Y.; Liao, K.W.; Kondo, M.; Chi, K.H. Comparing the Effectiveness of Combined External Beam Radiation and Hyperthermia Versus External Beam Radiation Alone in Treating Patients With Painful Bony Metastases: A Phase 3 Prospective, Randomized, Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 78–87. [Google Scholar] [CrossRef]
- Elming, P.B.; Sorensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef]
- Horsman, M.R.; Overgaard, J. Hyperthermia: A potent enhancer of radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2007, 19, 418–426. [Google Scholar] [CrossRef]
- Dewey, W.C.; Thrall, D.E.; Gillette, E.L. Hyperthermia and radiation--a selective thermal effect on chronically hypoxic tumor cells in vivo. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 99–103. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Issels, R.; Kampmann, E.; Kanaar, R.; Lindner, L.H. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperth. 2016, 32, 89–95. [Google Scholar] [CrossRef]
- Oldenborg, S.; Rasch, C.R.N.; van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Heymans, M.W.; Zum Vorde Sive Vording, P.J.; Crezee, H.; van Tienhoven, G. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther. Onkol. 2018, 194, 206–214. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grull, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Paulides, M.M.; Dobsicek Trefna, H.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency- andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 3–18. [Google Scholar] [CrossRef]
- Datta, N.R.; Kok, H.P.; Crezee, H.; Gaipl, U.S.; Bodis, S. Integrating Loco-Regional Hyperthermia Into the Current Oncology Practice: SWOT and TOWS Analyses. Front. Oncol. 2020, 10, 819. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Vujaskovic, Z.; Jones, E.; Thrall, D. Re-setting the biologic rationale for thermal therapy. Int. J. Hyperth. 2005, 21, 779–790. [Google Scholar] [CrossRef]
- Song, C.W. Effect of local hyperthermia on blood flow and microenvironment: A review. Cancer Res. 1984, 44, 4721s–4730s. [Google Scholar]
- Dewey, W.C. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperth. 1994, 10, 457–483. [Google Scholar] [CrossRef]
- van den Tempel, N.; Laffeber, C.; Odijk, H.; van Cappellen, W.A.; van Rhoon, G.C.; Franckena, M.; Kanaar, R. The effect of thermal dose on hyperthermia-mediated inhibition of DNA repair through homologous recombination. Oncotarget 2017, 8, 44593–44604. [Google Scholar] [CrossRef]
- Evans, S.S.; Repasky, E.A.; Fisher, D.T. Fever and the thermal regulation of immunity: The immune system feels the heat. Nat. Rev. Immunol. 2015, 15, 335–349. [Google Scholar] [CrossRef]
- Mantel, F.; Frey, B.; Haslinger, S.; Schildkopf, P.; Sieber, R.; Ott, O.J.; Lodermann, B.; Rodel, F.; Sauer, R.; Fietkau, R.; et al. Combination of ionising irradiation and hyperthermia activates programmed apoptotic and necrotic cell death pathways in human colorectal carcinoma cells. Strahlenther. Onkol. 2010, 186, 587–599. [Google Scholar] [CrossRef]
- Schildkopf, P.; Holmer, R.; Sieber, R.; Ott, O.J.; Janko, C.; Mantel, F.; Frey, B.; Fietkau, R.; Gaipl, U.S. Hyperthermia in combination with X-irradiation induces inflammatory forms of cell death. Autoimmunity 2009, 42, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Schildkopf, P.; Frey, B.; Ott, O.J.; Rubner, Y.; Multhoff, G.; Sauer, R.; Fietkau, R.; Gaipl, U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011, 101, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Werthmoller, N.; Frey, B.; Ruckert, M.; Lotter, M.; Fietkau, R.; Gaipl, U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int. J. Hyperth. 2016, 32, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ostberg, J.R.; Dayanc, B.E.; Yuan, M.; Oflazoglu, E.; Repasky, E.A. Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells. J. Leukoc. Biol. 2007, 82, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Finkel, P.; Frey, B.; Mayer, F.; Bosl, K.; Werthmoller, N.; Mackensen, A.; Gaipl, U.S.; Ullrich, E. The dual role of NK cells in antitumor reactions triggered by ionizing radiation in combination with hyperthermia. Oncoimmunology 2016, 5, e1101206. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Oleson, J.R.; Kirkpatrick, J.; Secomb, T.W. Accurate Three-Dimensional Thermal Dosimetry and Assessment of Physiologic Response Are Essential for Optimizing Thermoradiotherapy. Cancers 2022, 14, 1701. [Google Scholar] [CrossRef]
- Datta, N.R.; Puric, E.; Klingbiel, D.; Gomez, S.; Bodis, S. Hyperthermia and Radiation Therapy in Locoregional Recurrent Breast Cancers: A Systematic Review and Meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 1073–1087. [Google Scholar] [CrossRef]
- Kaidar-Person, O.; Oldenborg, S.; Poortmans, P. Re-irradiation and Hyperthermia in Breast Cancer. Clin. Oncol. (R. Coll. Radiol.) 2018, 30, 73–84. [Google Scholar] [CrossRef]
- Bakker, A.; van der Zee, J.; van Tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar] [CrossRef]
- Oldenborg, S.; Griesdoorn, V.; van Os, R.; Kusumanto, Y.H.; Oei, B.S.; Venselaar, J.L.; Zum Vorde Sive Vording, P.J.; Heymans, M.W.; Kolff, M.W.; Rasch, C.R.; et al. Reirradiation and hyperthermia for irresectable locoregional recurrent breast cancer in previously irradiated area: Size matters. Radiother. Oncol. 2015, 117, 223–228. [Google Scholar] [CrossRef]
- Notter, M.; Thomsen, A.R.; Nitsche, M.; Hermann, R.M.; Wolff, H.A.; Habl, G.; Munch, K.; Grosu, A.L.; Vaupel, P. Combined wIRA-Hyperthermia and Hypofractionated Re-Irradiation in the Treatment of Locally Recurrent Breast Cancer: Evaluation of Therapeutic Outcome Based on a Novel Size Classification. Cancers 2020, 12, 606. [Google Scholar] [CrossRef]
- Diagnosis and Treatment of Patients with Early and Advanced Breast Cancer—Loco-Regional Recurrence, Guidelines Breast Version 2022.1E. AGO e.V. 2022. Available online: https://www.ago-online.de/leitlinien-empfehlungen/leitlinien-empfehlungen/kommission-mamma (accessed on 29 September 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Petrovich, Z.; Lam, K.; Langholz, B.; Astrahan, M.; Luxton, G.; Rice, D. Interstitial thermoradiotherapy for recurrent head and neck cancer. Am. J. Otolaryngol. 1989, 10, 257–260. [Google Scholar] [CrossRef]
- Emami, B.; Scott, C.; Perez, C.A.; Asbell, S.; Swift, P.; Grigsby, P.; Montesano, A.; Rubin, P.; Curran, W.; Delrowe, J.; et al. Phase III study of interstitial thermoradiotherapy compared with interstitial radiotherapy alone in the treatment of recurrent or persistent human tumors. A prospectively controlled randomized study by the Radiation Therapy Group. Int. J. Radiat. Oncol. Biol. Phys. 1996, 34, 1097–1104. [Google Scholar] [CrossRef]
- Feyerabend, T.; Steeves, R.; Jager, B.; Wiedemann, G.; Sommer, K.; Richter, E.; Katschinski, D.; Robins, H. Local hyperthermia, hyperfractionated radiation, and cisplatin in preirradiated recurrent lymph node metastases of recurrent head and neck cancer. Int. J. Oncol. 1997, 10, 591–595. [Google Scholar] [CrossRef]
- Puthawala, A.; Nisar Syed, A.M.; Gamie, S.; Chen, Y.J.; Londrc, A.; Nixon, V. Interstitial low-dose-rate brachytherapy as a salvage treatment for recurrent head-and-neck cancers: Long-term results. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 354–362. [Google Scholar] [CrossRef]
- Geiger, M.; Strnad, V.; Lotter, M.; Sauer, R. Pulsed-dose rate brachytherapy with concomitant chemotherapy and interstitial hyperthermia in patients with recurrent head-and-neck cancer. Brachytherapy 2002, 1, 149–153. [Google Scholar] [CrossRef]
- Gabriele, P.; Ferrara, T.; Baiotto, B.; Garibaldi, E.; Marini, P.G.; Penduzzu, G.; Giovannini, V.; Bardati, F.; Guiot, C. Radio hyperthermia for re-treatment of superficial tumours. Int. J. Hyperth. 2009, 25, 189–198. [Google Scholar] [CrossRef]
- Bartochowska, A.; Wierzbicka, M.; Skowronek, J.; Leszczyńska, M.; Szyfter, W. High-dose-rate and pulsed-dose-rate brachytherapy in palliative treatment of head and neck cancers. Brachytherapy 2012, 11, 137–143. [Google Scholar] [CrossRef]
- Verduijn, G.M.; de Wee, E.M.; Rijnen, Z.; Togni, P.; Hardillo, J.A.U.; Ten Hove, I.; Franckena, M.; van Rhoon, G.C.; Paulides, M.M. Deep hyperthermia with the HYPERcollar system combined with irradiation for advanced head and neck carcinoma—A feasibility study. Int. J. Hyperth. 2018, 34, 994–1001. [Google Scholar] [CrossRef]
- Kroesen, M.; van Holthe, N.; Sumser, K.; Chitu, D.; Vernhout, R.; Verduijn, G.; Franckena, M.; Hardillo, J.; van Rhoon, G.; Paulides, M. Feasibility, SAR Distribution, and Clinical Outcome upon Reirradiation and Deep Hyperthermia Using the Hypercollar3D in Head and Neck Cancer Patients. Cancers 2021, 13, 6149. [Google Scholar] [CrossRef] [PubMed]
- Zschaeck, S.; Weingärtner, J.; Ghadjar, P.; Wust, P.; Mehrhof, F.; Kalinauskaite, G.; Ehrhardt, V.H.; Hartmann, V.; Tinhofer, I.; Heiland, M.; et al. Fever range whole body hyperthermia for re-irradiation of head and neck squamous cell carcinomas: Final results of a prospective study. Oral Oncol. 2021, 116, 105240. [Google Scholar] [CrossRef] [PubMed]
- Juffermans, J.H.; Hanssens, P.E.; van Putten, W.L.; van Rhoon, G.C.; van Der Zee, J. Reirradiation and hyperthermia in rectal carcinoma: A retrospective study on palliative effect. Cancer 2003, 98, 1759–1766. [Google Scholar] [CrossRef] [PubMed]
- Milani, V.; Pazos, M.; Issels, R.D.; Buecklein, V.; Rahman, S.; Tschoep, K.; Schaffer, P.; Wilkowski, R.; Duehmke, E.; Schaffer, M. Radiochemotherapy in combination with regional hyperthermia in preirradiated patients with recurrent rectal cancer. Strahlenther. Onkol. 2008, 184, 163–168. [Google Scholar] [CrossRef]
- Ott, O.J.; Gani, C.; Lindner, L.H.; Schmidt, M.; Lamprecht, U.; Abdel-Rahman, S.; Hinke, A.; Weissmann, T.; Hartmann, A.; Issels, R.D.; et al. Neoadjuvant Chemoradiation Combined with Regional Hyperthermia in Locally Advanced or Recurrent Rectal Cancer. Cancers 2021, 13, 1279. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Rothe, R.; Scholz, R.; Gneveckow, U.; Wust, P.; Thiesen, B.; Feussner, A.; von Deimling, A.; Waldoefner, N.; Felix, R.; et al. Intracranial thermotherapy using magnetic nanoparticles combined with external beam radiotherapy: Results of a feasibility study on patients with glioblastoma multiforme. J. Neurooncol. 2007, 81, 53–60. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Heo, J.; Kim, S.H.; Oh, Y.T.; Chun, M.; Noh, O.K. Concurrent hyperthermia and re-irradiation for recurrent high-grade gliomas. Neoplasma 2017, 64, 803–808. [Google Scholar] [CrossRef]
- de Jong, M.A.; Oldenborg, S.; Bing Oei, S.; Griesdoorn, V.; Kolff, M.W.; Koning, C.C.; van Tienhoven, G. Reirradiation and hyperthermia for radiation-associated sarcoma. Cancer 2012, 118, 180–187. [Google Scholar] [CrossRef]
- Linthorst, M.; van Geel, A.N.; Baartman, E.A.; Oei, S.B.; Ghidey, W.; van Rhoon, G.C.; van der Zee, J. Effect of a combined surgery, re-irradiation and hyperthermia therapy on local control rate in radio-induced angiosarcoma of the chest wall. Strahlenther. Onkol. 2013, 189, 387–393. [Google Scholar] [CrossRef]
- Notter, M.; Stutz, E.; Thomsen, A.R.; Vaupel, P. Radiation-Associated Angiosarcoma of the Breast and Chest Wall Treated with Thermography-Controlled, Contactless wIRA-Hyperthermia and Hypofractionated Re-Irradiation. Cancers 2021, 13, 3911. [Google Scholar] [CrossRef]
- Surwit, E.A.; Manning, M.R.; Aristizabal, S.A.; Oleson, J.R.; Cetas, T.C. Interstitial thermoradiotherapy in recurrent gynecologic malignancies. Gynecol. Oncol. 1983, 15, 95–102. [Google Scholar] [CrossRef]
- Gupta, A.K.; Vicini, F.A.; Frazier, A.J.; Barth-Jones, D.C.; Edmundson, G.K.; Mele, E.; Gustafson, G.S.; Martinez, A.A. Iridium-192 transperineal interstitial brachytherapy for locally advanced or recurrent gynecological malignancies. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 1055–1060. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Ohguri, T.; Imada, H.; Yahara, K.; Moon, S.D.; Higure, A.; Yamaguchi, K.; Yoshikawa, I.; Harada, M.; Korogi, Y. Multimodal approaches including three-dimensional conformal re-irradiation for recurrent or persistent esophageal cancer: Preliminary results. J. Radiat. Res. 2011, 52, 812–820. [Google Scholar] [CrossRef]
- Ohguri, T.; Imada, H.; Yahara, K.; Moon, S.D.; Yamaguchi, S.; Yatera, K.; Mukae, H.; Hanagiri, T.; Tanaka, F.; Korogi, Y. Re-irradiation plus regional hyperthermia for recurrent non-small cell lung cancer: A potential modality for inducing long-term survival in selected patients. Lung Cancer 2012, 77, 140–145. [Google Scholar] [CrossRef]
- Emami, B.; Perez, C.A.; Leybovich, L.; Straube, W.; Vongerichten, D. Interstitial thermoradiotherapy in treatment of malignant tumours. Int. J. Hyperth. 1987, 3, 107–118. [Google Scholar] [CrossRef]
- Lam, K.; Astrahan, M.; Langholz, B.; Jepson, J.; Cohen, D.; Luxton, G.; Petrovich, Z. Interstitial thermoradiotherapy for recurrent or persistent tumours. Int. J. Hyperth. 1988, 4, 259–266. [Google Scholar] [CrossRef]
- Strnad, V.; Lotter, M.; Kreppner, S.; Fietkau, R. Reirradiation for recurrent head and neck cancer with salvage interstitial pulsed-dose-rate brachytherapy: Long-term results. Strahlenther. Onkol. 2015, 191, 495–500. [Google Scholar] [CrossRef]
- Paulides, M.M.; Verduijn, G.M.; Van Holthe, N. Status quo and directions in deep head and neck hyperthermia. Radiat. Oncol. 2016, 11, 21. [Google Scholar] [CrossRef]
- Ward, M.C.; Riaz, N.; Caudell, J.J.; Dunlap, N.E.; Isrow, D.; Zakem, S.J.; Dault, J.; Awan, M.J.; Vargo, J.A.; Heron, D.E.; et al. Refining Patient Selection for Reirradiation of Head and Neck Squamous Carcinoma in the IMRT Era: A Multi-institution Cohort Study by the MIRI Collaborative. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 586–594. [Google Scholar] [CrossRef]
- Datta, N.R.; Rogers, S.; Ordonez, S.G.; Puric, E.; Bodis, S. Hyperthermia and radiotherapy in the management of head and neck cancers: A systematic review and meta-analysis. Int. J. Hyperth. 2016, 32, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.L.; Chi, M.S.; Ko, H.L.; Huang, Y.Y.; Wu, R.H.; Hao, S.P.; Chi, K.H. Adding Hyperthermia To Salvage Concurrent Chemoradiotherapy For Previously Irradiated Unresectable Recurrent Head And Neck Cancer: A Phase II Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e790. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Wong, E.T.; Hess, K.R.; Gleason, M.J.; Jaeckle, K.A.; Kyritsis, A.P.; Prados, M.D.; Levin, V.A.; Yung, W.K. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J. Clin. Oncol. 1999, 17, 2572–2578. [Google Scholar] [CrossRef] [PubMed]
- Kaul, D.; Pudlitz, V.; Böhmer, D.; Wust, P.; Budach, V.; Grün, A. Reirradiation of High-Grade Gliomas: A Retrospective Analysis of 198 Patients Based on the Charité Data Set. Adv. Radiat. Oncol. 2020, 5, 959–964. [Google Scholar] [CrossRef]
- Fogh, S.E.; Andrews, D.W.; Glass, J.; Curran, W.; Glass, C.; Champ, C.; Evans, J.J.; Hyslop, T.; Pequignot, E.; Downes, B.; et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J. Clin. Oncol. 2010, 28, 3048–3053. [Google Scholar] [CrossRef]
- Combs, S.E.; Gutwein, S.; Thilmann, C.; Huber, P.; Debus, J.; Schulz-Ertner, D. Stereotactically guided fractionated re-irradiation in recurrent glioblastoma multiforme. J. Neurooncol. 2005, 74, 167–171. [Google Scholar] [CrossRef]
- Combs, S.E.; Bischof, M.; Welzel, T.; Hof, H.; Oertel, S.; Debus, J.; Schulz-Ertner, D. Radiochemotherapy with temozolomide as re-irradiation using high precision fractionated stereotactic radiotherapy (FSRT) in patients with recurrent gliomas. J. Neurooncol. 2008, 89, 205–210. [Google Scholar] [CrossRef]
- Minniti, G.; Armosini, V.; Salvati, M.; Lanzetta, G.; Caporello, P.; Mei, M.; Osti, M.F.; Maurizi, R.E. Fractionated stereotactic reirradiation and concurrent temozolomide in patients with recurrent glioblastoma. J. Neurooncol. 2011, 103, 683–691. [Google Scholar] [CrossRef]
- Rombouts, A.J.M.; Huising, J.; Hugen, N.; Siesling, S.; Poortmans, P.M.; Nagtegaal, I.D.; de Wilt, J.H.W. Assessment of Radiotherapy-Associated Angiosarcoma After Breast Cancer Treatment in a Dutch Population-Based Study. JAMA Oncol. 2019, 5, 267–269. [Google Scholar] [CrossRef]
- Taffurelli, M.; Pellegrini, A.; Meattini, I.; Orzalesi, L.; Tinterri, C.; Roncella, M.; Terribile, D.; Caruso, F.; Tazzioli, G.; Pollini, G.; et al. Secondary breast angiosarcoma: A multicentre retrospective survey by the national Italian association of Breast Surgeons (ANISC). Breast 2019, 45, 56–60. [Google Scholar] [CrossRef]
- Salminen, S.H.; Wiklund, T.; Sampo, M.M.; Tarkkanen, M.; Pulliainen, L.; Bohling, T.O.; Tukiainen, E.; Hukkinen, K.; Blomqvist, C.P. Treatment and Prognosis of Radiation-Associated Breast Angiosarcoma in a Nationwide Population. Ann. Surg. Oncol. 2020, 27, 1002–1010. [Google Scholar] [CrossRef]
- Lae, M.; Lebel, A.; Hamel-Viard, F.; Asselain, B.; Trassard, M.; Sastre, X.; Kirova, Y.M. Can c-myc amplification reliably discriminate postradiation from primary angiosarcoma of the breast? Cancer Radiother. 2015, 19, 168–174. [Google Scholar] [CrossRef]
- Mito, J.K.; Mitra, D.; Barysauskas, C.M.; Marino-Enriquez, A.; Morgan, E.A.; Fletcher, C.D.M.; Raut, C.P.; Baldini, E.H.; Doyle, L.A. A Comparison of Outcomes and Prognostic Features for Radiation-Associated Angiosarcoma of the Breast and Other Radiation-Associated Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 425–435. [Google Scholar] [CrossRef]
- Torres, K.E.; Ravi, V.; Kin, K.; Yi, M.; Guadagnolo, B.A.; May, C.D.; Arun, B.K.; Hunt, K.K.; Lam, R.; Lahat, G.; et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann. Surg. Oncol. 2013, 20, 1267–1274. [Google Scholar] [CrossRef]
- Palta, M.; Morris, C.G.; Grobmyer, S.R.; Copeland, E.M., 3rd; Mendenhall, N.P. Angiosarcoma after breast-conserving therapy: Long-term outcomes with hyperfractionated radiotherapy. Cancer 2010, 116, 1872–1878. [Google Scholar] [CrossRef]
- Scott, M.T.; Portnow, L.H.; Morris, C.G.; Marcus, R.B., Jr.; Mendenhall, N.P.; Mendenhall, W.M.; Indelicato, D.J. Radiation therapy for angiosarcoma: The 35-year University of Florida experience. Am J Clin Oncol 2013, 36, 174–180. [Google Scholar] [CrossRef]
- Bahadoer, R.R.; Dijkstra, E.A.; van Etten, B.; Marijnen, C.A.M.; Putter, H.; Kranenbarg, E.M.; Roodvoets, A.G.H.; Nagtegaal, I.D.; Beets-Tan, R.G.H.; Blomqvist, L.K.; et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 29–42. [Google Scholar] [CrossRef]
- De Haas-Kock, D.F.; Buijsen, J.; Pijls-Johannesma, M.; Lutgens, L.; Lammering, G.; van Mastrigt, G.A.; De Ruysscher, D.K.; Lambin, P.; van der Zee, J. Concomitant hyperthermia and radiation therapy for treating locally advanced rectal cancer. Cochrane Database Syst. Rev. 2009, 3, CD006269. [Google Scholar] [CrossRef]
- Harima, Y.; Ohguri, T.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int. J. Hyperth. 2016, 32, 801–808. [Google Scholar] [CrossRef]
- Ohguri, T.; Harima, Y.; Imada, H.; Sakurai, H.; Ohno, T.; Hiraki, Y.; Tuji, K.; Tanaka, M.; Terashima, H. Relationships between thermal dose parameters and the efficacy of definitive chemoradiotherapy plus regional hyperthermia in the treatment of locally advanced cervical cancer: Data from a multicentre randomised clinical trial. Int. J. Hyperth. 2018, 34, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Fukuda, H.; Matsui, K.; Hirashima, T.; Hosono, M.; Takada, Y.; Inoue, Y. Non-small-cell lung cancer: Reirradiation for loco-regional relapse previously treated with radiation therapy. Int. J. Clin. Oncol. 2005, 10, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Murakami, M.; Yoden, E.; Sasaki, R.; Okuno, Y.; Nakajima, T.; Kuroda, Y. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Bruggmoser, G.; Bauchowitz, S.; Canters, R.; Crezee, H.; Ehmann, M.; Gellermann, J.; Lamprecht, U.; Lomax, N.; Messmer, M.B.; Ott, O.; et al. Guideline for the clinical application, documentation and analysis of clinical studies for regional deep hyperthermia: Quality management in regional deep hyperthermia. Strahlenther. Onkol. 2012, 188 (Suppl. 2), 198–211. [Google Scholar] [CrossRef]
- Dobsicek Trefna, H.; Crezee, J.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Nadobny, J.; Hartmann, J.; Lomax, N.; Abdel-Rahman, S.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: II. Technical requirements for heating devices. Strahlenther. Onkol. 2017, 193, 351–366. [Google Scholar] [CrossRef]
- Dobsicek Trefna, H.; Schmidt, M.; van Rhoon, G.C.; Kok, H.P.; Gordeyev, S.S.; Lamprecht, U.; Marder, D.; Nadobny, J.; Ghadjar, P.; Abdel-Rahman, S.; et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019, 36, 277–294. [Google Scholar] [CrossRef]
- Trefna, H.D.; Crezee, H.; Schmidt, M.; Marder, D.; Lamprecht, U.; Ehmann, M.; Hartmann, J.; Nadobny, J.; Gellermann, J.; van Holthe, N.; et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017, 33, 471–482. [Google Scholar] [CrossRef]
- Organ Preservation in Locally Advanced Rectal Cancer by Radiochemotherapy Followed by Consolidation Chemotherapy. Available online: https://clinicaltrials.gov/ct2/show/NCT03561142 (accessed on 19 June 2018).
- Bhandari, V.; Hoey, C.; Liu, L.Y.; Lalonde, E.; Ray, J.; Livingstone, J.; Lesurf, R.; Shiah, Y.J.; Vujcic, T.; Huang, X.; et al. Molecular landmarks of tumor hypoxia across cancer types. Nat. Genet. 2019, 51, 308–318. [Google Scholar] [CrossRef]
- Hyperthermia Enhanced Re-Irradiation of Loco-Regional Recurrent Tumors. Available online: https://clinicaltrials.gov/ct2/show/NCT04889742 (accessed on 17 May 2021).
| Author | Year | Entity Group | Type of Study | N | Treatment | Type of HT | COI | Funding |
|---|---|---|---|---|---|---|---|---|
| Petrovich et al. [34] | 1989 | HN | Single institution, prospective phase I/II, single arm | 20 | Interstitial BT + iHT | Interstitial, MW | n.r. | n.r. |
| Emami et al. [35] | 1996 | HN | Multi-center, prospective, randomized phase III | 40 | Interstitial BT + iHT vs. interstitial BT | Interstitial, MW/RF | n.r. | n.r. |
| Feyerabend et al. [36] | 1997 | HN | Single institution, prospective phase I/II, single arm | 13 | EBRT + HT + CT | Superficial, radiative, MW | n.r. | Grants by Deutsche Krebshilfe, Deutsche Forschungsgemeinschaft (DFG), Cancer Research Institute (NY, USA) |
| Puthawala et al. [37] | 2001 | HN | Single institution, prospective phase I/II, single arm | 133 | Salvage interstitial-LDR-BT + HT +/− CT | Interstitial MW | n.r. | n.r. |
| Geiger et al. [38] | 2002 | HN | Single institution, prospective phase I/II, single arm | 15 | Interstitial-PDR-BT + HT + CT | Interstitial, MW | n.r. | n.r. |
| Gabriele et al. [39] | 2009 | HN | Two-center, retrospective | 14 | EBRT + HT | Superficial, radiative, MW | none | none |
| Bartochowska et al. [40] | 2012 | HN | Two-center, retrospective | 16 | Palliative interstitial-HDR/PDR-BT + iHT | Interstitial, MW | n.r. | n.r. |
| Verduijn et al. [41] | 2018 | HN | Single institution, retrospective | 18 | RT(IMRT/CBK/BT) + HT +/− OP | Deep local, radiative, RF | Co-founders of Sensius BV | Sensius BV and KWF Kankerbestrijding (Dutch Cancer Society) grant |
| Kroesen et al. [42] | 2021 | HN | Single institution, retrospective | 22 | RT(CBK/IMRT/VMAT) + HT +/− OP | Deep local, radiative, MW | Co-founders of Sensius BV | Sensius BV and KWF Kankerbestrijding (Dutch Cancer Society) grant |
| Zschaeck et al. [43] | 2021 | HN | Single institution, prospective phase I | 10 | EBRT + FRWBH +/− CT +/− OP | Whole body, wIRA | none | Dr. med. h.c. Erwin Braun Stiftung |
| Juffermans et al. [44] | 2003 | REC | Single institution, retrospective | 54 | EBRT + HT | Deep regional, radiative, RF | n.r. | n.r. |
| Milani et al. [45] | 2008 | REC | Single institution, prospective phase I/II | 24 | EBRT + HT + CT | Deep regional, radiative, RF | n.r. | n.r. |
| Ott et al. [46] | 2021 | REC | Multi-center, prospective, phase I/II | 10 | EBRT + HT + CT | Deep regional, radiative, RF | none | none |
| Maier-Hauff et al. [47] | 2007 | CNS | Single institution, prospective, single arm | 11 | EBRT + HT | Internal, Fe3O4 (magnetite nanoparticles) | Employee/co-founder of MagForce AG, patents | n.r. |
| Maier-Hauff et al. [48] | 2011 | CNS | Prospective, single arm, two-center phase II | 59 | EBRT + HT | Internal, Fe3O4 (magnetite nanoparticles) | Employee/co-founder of MagForce AG, patents | MagForce AG |
| Heo et al. [49] | 2017 | CNS | Single institution, retrospective | 20 | EBRT + HT +/− CT, OP | External, capacitive, RF | n.r. | n.r. |
| de Jong et al. [50] | 2012 | RAS | Two-center, retrospective | 16 | EBRT + HT | Superficial, radiative, MW | none | none |
| Linthorst et al. [51] | 2013 | RAS | Two-center, retrospective | 24 | EBRT + HT +/− OP | Superficial, radiative, MW | none | n.r. |
| Notter et al. [52] | 2021 | RAS | Multi-center, retrospective | 10 | EBRT + HT | Superficial, wIRA | none | none |
| Surwit et al [53] | 1983 | CER | Single institution, prospective phase I | 12 | Interstitial-LDR-BT + iHT | Interstitial, RF | n.r. | n.r. |
| Gupta et al. [54] | 1999 | ENDO | Single institution, retrospective | 15 | Interstitial-LDR-BT + iHT | Interstitial, RF | n.r. | n.r. |
| Yamaguchi et al. [55] | 2011 | ESO | Single institution, retrospective | 14 | EBRT + HT | Deep, capacitive, RF | none | n.r. |
| Ohguri et al. [56] | 2012 | LU | Single institution, retrospective | 33 | EBRT + HT | Deep, capacitive, RF | none | n.r. |
| Author | Year | Study Type | N | Entity | Treatment | Re-RT Dose (Mean/Median(Range), Gy) | HT | Response | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Petrovich et al. [34] | 1989 | Single institution, prospective phase I/II, single arm | 20 | HN | Interstitial BT + iHT | 40 Gy or 50 Gy dep. on prior RT | Interstitial, MW | CR 68%, PR 32%, median OS 8.5 mo, 2y-OS 18%, 95% palliation | Acute: G3 aspiration pneumonia (n = 1), G4 soft tissue necrosis (n = 1) |
| Emami et al. [35] | 1996 | Multi-center, prospective, randomized, phase III, two arms, multiple entities, 75/176 HN | 40 ITRT vs. 35 IRT (176), 84% re-RT | HN | Interstitial BT + iHT vs. interstitial BT | n.r. | Interstitial, MW/RF | CR 62% vs. 52% n.s., PR 4% vs. 13% n.s., 2y-LC (43%) vs. (37%) n.s. | Acute: ≥G3 22% vs. 12%, G4 10% vs. 3% (skin/subcutaneous/mucosal), late: ≥G3 20% vs. 15% n.s. |
| Feyerabend et al. [36] | 1997 | Single institution, prospective phase I/II, single arm | 13 | HN | EBRT + HT + CT | 36(30–50) | Superficial, radiative, MW | CR 8%, PR 84% | Acute: G3 skin reaction (n = 1) |
| Puthawala et al. [37] | 2001 | Single institution, prospective phase I/II, single arm, 133/220 with HT | 133 (220) | HN | Salvage interstitial-LDR-BT + iHT +/− CT | 53(35–65) | Interstitial, MW | CR (77%), 2y-LC (69%), +/− HT n.s. | n.r. |
| Geiger et al. [38] | 2002 | Single institution, prospective phase I/II, single arm | 15 | HN | Interstitial-PDR-BT + iHT + CT | 55(34–60) | Interstitial, MW | 2y-LC 68%, 2y-OS 67% | Acute: G3 soft tissue ulceration (n = 1) |
| Gabriele et al. [39] | 2009 | Two-center, retrospective, multiple entities, 14/51 HN | 14 (51) | HN | EBRT + HT | n.r. | Superficial, radiative, MW | CR 33%, PR 25%, NR 41.7%, 18-mo-LC 50% | No acute/late ≥G3 toxicity observed |
| Bartochowska et al. [40] | 2012 | Two-center, retrospective, 16/156 with HT | 16 (156) | HN | Palliative interstitial-HDR/PDR-BT + iHT | HDR (12–20), PDR 20(20–40) | Interstitial, MW | Median OS: (7 mo), 2y-OS: (17%), +/− HT n.s. | No excess toxicity +/− HT |
| Verduijn et al. [41] | 2018 | Single institution, retrospective, 18/27 with re-RT | 18 (27) | HN | RT(IMRT/CBK/BT) + HT +/− OP | IMRT (40–70 Gy/2 Gy), CBK (5 × 5.5 Gy, 6 × 5 Gy, 6 × 5.5 Gy or 6 × 6 Gy 2×/wk), BT (38 Gy in 12 Fx) | Deep local, radiative, RF | CR 39%, 2y-LC: 36%, 2y-OS: 33% | Tube feeding (n = 11), radiation dermatitis (n = 2), pneumonitis (n = 2), fibrosis (n = 1) for all patients |
| Kroesen et al. [42] | 2021 | Single institution, retrospective | 22 | HN | RT(CBK/IMRT/VMAT) + HT +/− OP | IMRT/VMAT 60(20–60), CBK (6 × 5.5 Gy) | Deep local, radiative, MW | 2y-LC: 36.4%, 2y-OS 54.6% after definitive therapy (11/22) | lLte: ≥G3 39.2% (xerostomia, dysphagia, osteoradionecrosis and trismus) |
| Zschaeck et al. [43] | 2021 | Single institution, prospective phase I | 10 | HN | EBRT + FRWBH +/− CT | 66 Gy/1.2 Gy bi-daily | FRWBH wIRA | Completion rate 50%, median OS 10 mo | No increased toxicity with more HT sessions |
| Author | Year | Study Type | N | Entity | Treatment | Re-RT Dose (Mean/Median(Range), Gy) | HT | Response | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Maier-Hauff et al. [47] | 2007 | Single institution, prospective, single arm, 11/14 with re-RT | 11 (14) | CNS (GBM) | EBRT + HT +/− adjuvant CT | (20–30) | Internal, Fe3O4 (magnetite nanoparticles) | Median OS: 14.5 mo from primary diagnosis, 7.6 mo “after reintervention” | No treatment-related toxicity observed |
| Maier-Hauff et al. [48] | 2011 | Prospective, single arm, two-center phase II | 59 | CNS (GBM) | EBRT + HT | 30 Gy/2 Gy | Internal, Fe3O4 (magnetite nanoparticles) | Median OS: 13.4 mo from recurrence diagnosis | 23.7% seizures, 21% motor disturbances |
| Heo et al. [49] | 2017 | Single institution, retrospective | 20 | CNS (HGG, 80% III, 20% IV) | EBRT + HT +/− CT, OP | 30(16–40) | External, capacitive, RF | Median OS: 8.4 mo from start of re-RT | No ≥G3 toxicity reported during treatment |
| Author | Year | Study Type | N | Entity | Treatment | Re-RT Dose (Mean/Median(Range), Gy) | HT | Response | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| de Jong et al. [50] | 2012 | Two-center, retrospective | 16 (13 unresectable, 3 surgery) | RAS | EBRT + HT +/− OP | 32(6–36) | Superficial, radiative, MW | Median OS: 9 mo; unresectable: 3y-LC: 31% | Late: G4 peripheral limb ischemia (n = 1) |
| Linthorst et al. [51] | 2013 | Two-center, retrospective | 24 (13 unresectable, 11 surgery) | RAS | EBRT + HT +/− OP | 32(32–54) | Superficial, radiative, MW | Median OS: 12 mo; unresectable: OS 5 mo, 3y-LC: 22%; surgery: OS 13 mo, 3y-LC: 46% | Acute: G3 wound infection (n = 1), late: G4 osteonecrosis (n = 1), G4 chronic wound (n = 1) |
| Notter et al. [52] | 2021 | Multi-center, retrospective | 10 | RAS | EBRT + wIRA HT | 5 × 4 Gy 1 Fx/wk, 1 patient 25 × 2 Gy 5 Fx/wk | Superficial, wIRA | Median OS: 17 mo | No acute/late ≥G3 toxicity observed |
| Author | Year | Study Type | N | Entity | Treatment | Re-RT Dose (Mean/Median(Range), Gy) | HT | Response | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Juffermans et al. [44] | 2003 | Single institution, retrospective | 54 | REC | EBRT + HT | 32(24–32) | Deep regional, radiative, RF | Completion rate 87%, median OS 10 mo, palliative effect 83%, duration of palliation 6 mo | No acute/late ≥G3 toxicity observed |
| Milani et al. [45] | 2008 | Single institution, prospective phase I/II | 24 | REC | EBRT + HT + CT | 39.6(30–45) | Deep regional, radiative, RF | Completion rate 92%, palliative effect 70% of responding patients, 3y-LPFS 15%, median OS 27 mo, 3y-OS 30% | Acute G3 diarrhea 12.5% of all patients |
| Ott et al. [46] | 2021 | Multi-center, prospective, phase II, LARC and LRRC, 10/16 LRRC with re-RT | 10 (16) | REC | EBRT + HT + CT +/− OP | 45 Gy/1.8 Gy | Deep regional, radiative, RF | Completion rate RT (99%), HT (90%), LRRC: 3y-LPFS (49%), 3y-OS (85%), pCR (19%) | G3 toxicity n.r. for LRRC patients separately, no G4/5 toxicity observed |
| Author | Year | Study Type | N | Entity | Treatment | Re-RT Dose (Mean/Median(Range), Gy) | HT | Response | Toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Surwit et al. [53] | 1983 | Single institution, prospective phase I | 21 | 12 cervical, 2 vaginal/urethral, 3 uterine, 4 ovarian | Interstitial-LDR-BT + iHT | 22(15–45.6) | Interstitial RF | CR+PR 81%, duration of response 4 mo, palliation effect 76% among patients with tumor response | Fistulae in 4/21 patients |
| Gupta et al. [54] | 1999 | Single institution, retrospective, 15/69 with re-RT | 15 (69) | 10 endometrial, 2 vaginal/urethral, 3 cervical | Interstitial-LDR-BT + iHT | 35(25–55) | Interstitial RF | 3y-LC 49% | G4: (14%), no excess toxicity +/− HT |
| Yamaguchi et al. [55] | 2011 | Single institution, retrospective, 14/31 with HT | 14 (31) | Esophageal | EBRT (3D-CRT) + HT +/− CT | 40 Gy (curative), 36 Gy (palliative) | Deep, capacitive, RF | median OS: 8.1 mo, +/− HT n.s. | ≥G3 esophageal complications: 6/31 patients |
| Ohguri et al. [56] | 2012 | Single institution, retrospective | 33 | NSCLC | EBRT+HT | 50(29–70) | Deep, capacitive, RF | RT completion rate 97%, median OS: 18.1 mo, DLC: 12.1 mo, PFS: 6.7 mo | ≥G3: acute: thrombocytopenia (n = 1), pleuritis (n = 1); late: brachial plexus neuropathy (n = 1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Zschaeck, S.; Debus, J.; Weykamp, F. Combined Hyperthermia and Re-Irradiation in Non-Breast Cancer Patients: A Systematic Review. Cancers 2023, 15, 742. https://doi.org/10.3390/cancers15030742
Kim J-Y, Zschaeck S, Debus J, Weykamp F. Combined Hyperthermia and Re-Irradiation in Non-Breast Cancer Patients: A Systematic Review. Cancers. 2023; 15(3):742. https://doi.org/10.3390/cancers15030742
Chicago/Turabian StyleKim, Ji-Young, Sebastian Zschaeck, Jürgen Debus, and Fabian Weykamp. 2023. "Combined Hyperthermia and Re-Irradiation in Non-Breast Cancer Patients: A Systematic Review" Cancers 15, no. 3: 742. https://doi.org/10.3390/cancers15030742
APA StyleKim, J.-Y., Zschaeck, S., Debus, J., & Weykamp, F. (2023). Combined Hyperthermia and Re-Irradiation in Non-Breast Cancer Patients: A Systematic Review. Cancers, 15(3), 742. https://doi.org/10.3390/cancers15030742







