The Impact of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) versus Conventional Surgery on Patient-Reported Outcomes: A Comparative Cohort Study between the CAIRO6 Trial and the PROCORE Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Treatments
2.4. PROs
2.5. Data Collection
Statistical Analyses
3. Results
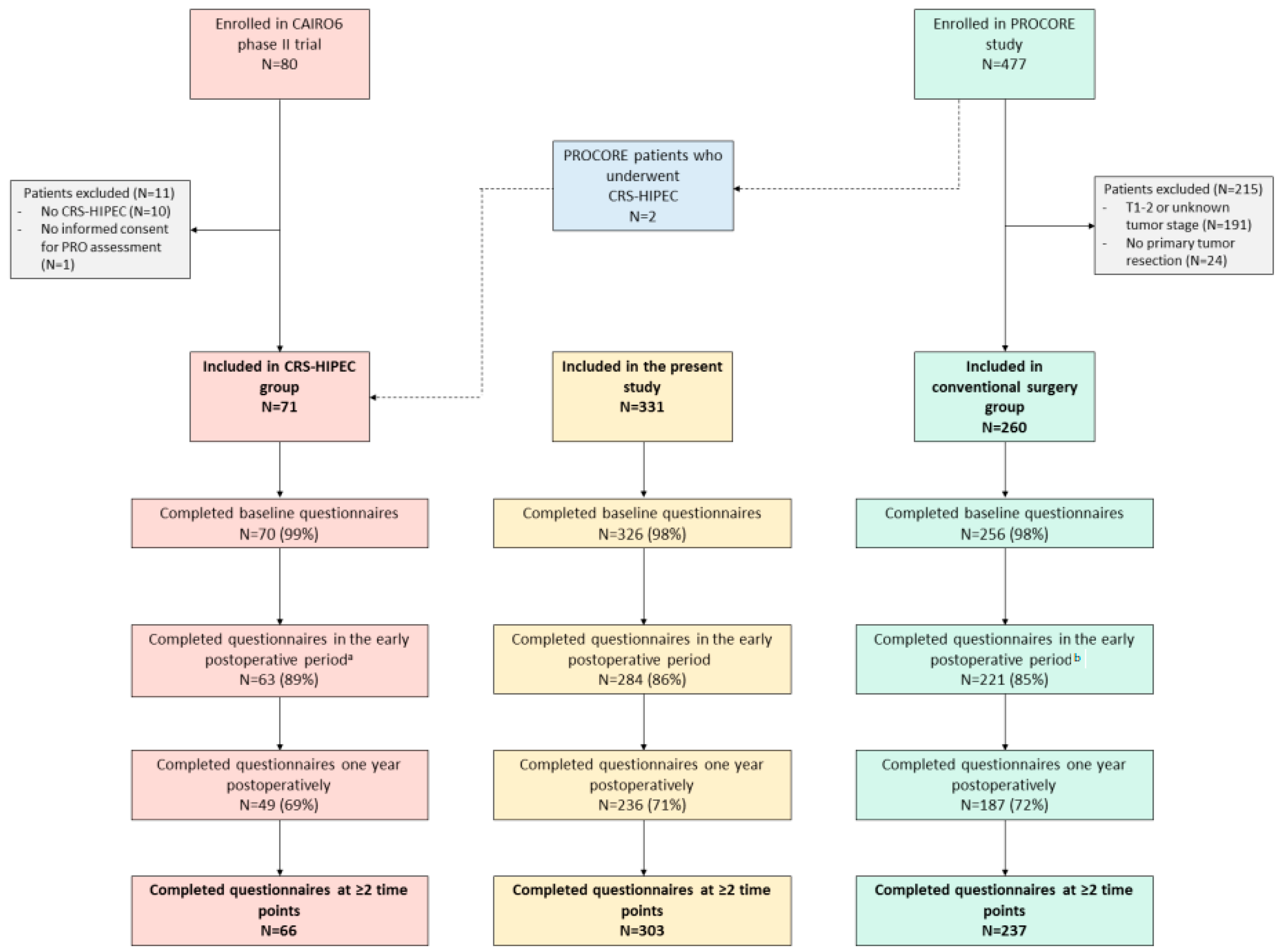
3.1. Patients and Treatments
3.2. Questionnaire Completion Rates
3.3. Comparative Analyses of PROs between Groups and Longitudinal Comparisons within the Groups
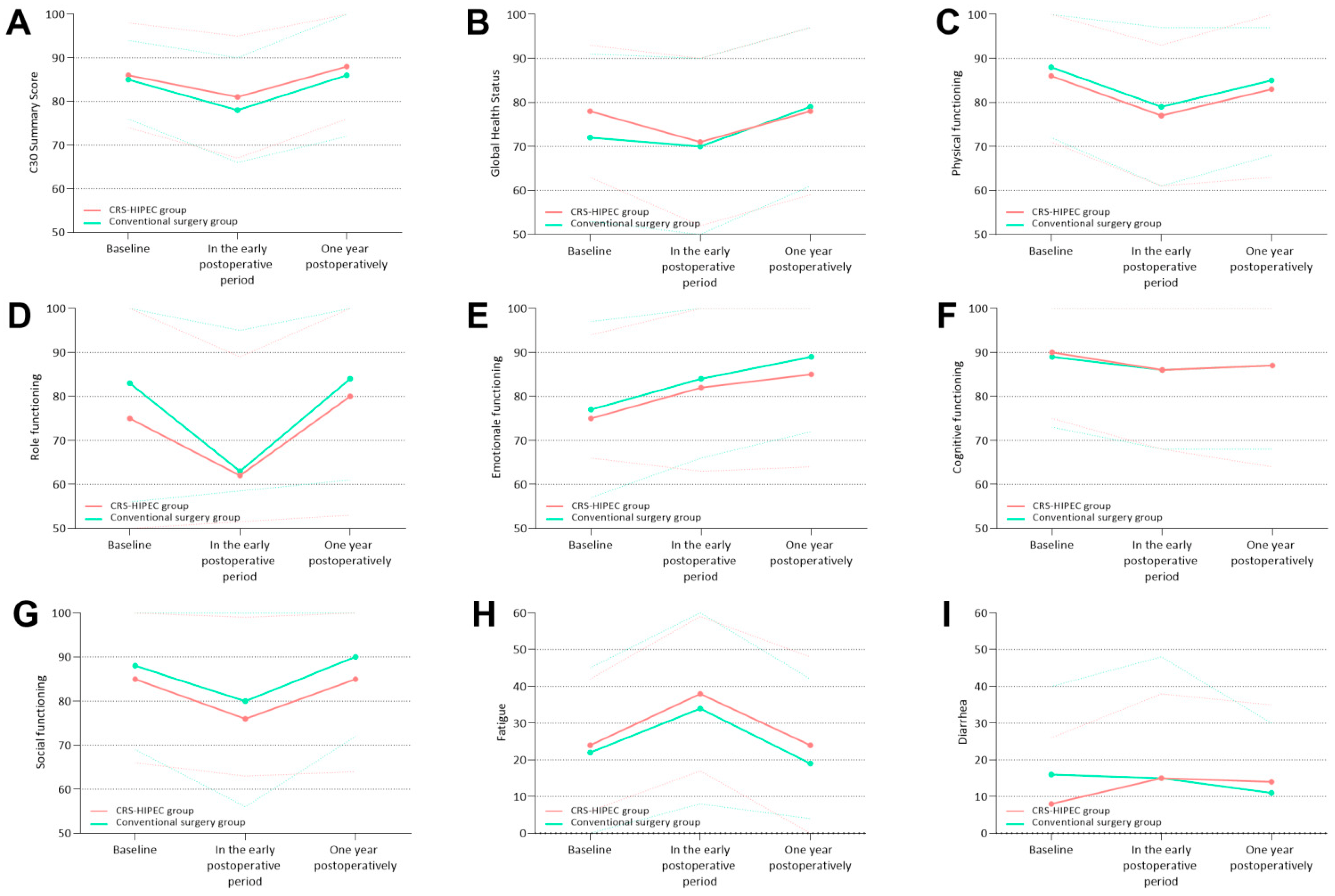
3.4. Functional Scales
3.4.1. C30 Summary Score
3.4.2. Global Health Status
3.4.3. Physical Functioning
3.4.4. Role Functioning
3.4.5. Emotional Functioning
3.4.6. Cognitive Functioning
3.4.7. Social Functioning
3.5. Symptom Scales
3.5.1. Fatigue
3.5.2. Diarrhea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wibe, A. Multidisciplinary Treatment: Influence on Outcomes. Multidisciplinary Treatment of Colorectal Cancer; Springer: Berlin/Heidelberg, Germany, 2021; pp. 11–21. [Google Scholar]
- Verwaal, V.J.; Bruin, S.; Boot, H.; Van Slooten, G.; Van Tinteren, H. 8-year follow-up of randomized trial: Cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann. Surg. Oncol. 2008, 15, 2426–2432. [Google Scholar] [CrossRef] [PubMed]
- Verwaal, V.J.; van Ruth, S.; de Bree, E.; van Slooten, G.W.; van Tinteren, H.; Boot, H.; Zoetmulder, F.A. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J. Clin. Oncol. 2003, 21, 3737–3743. [Google Scholar] [CrossRef] [PubMed]
- Bushati, M.; Rovers, K.; Sommariva, A.; Sugarbaker, P.; Morris, D.; Yonemura, Y.; Quadros, C.; Somashekhar, S.; Ceelen, W.; Dubé, P.; et al. The current practice of cytoreductive surgery and HIPEC for colorectal peritoneal metastases: Results of a worldwide web-based survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 2018, 44, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Groenen, H.; Morton, D.G.; Laurberg, S.; Bemelman, W.A.; Tanis, P.J. The research committee of the European Society of Coloproctology Recommendations and consensus on the treatment of peritoneal metastases of colorectal origin: A systematic review of national and international guidelines. Color. Dis. 2017, 19, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Baratti, D.; Kusamura, S.; Pietrantonio, F.; Guaglio, M.; Niger, M.; Deraco, M. Progress in treatments for colorectal cancer peritoneal metastases during the years 2010–2015. A systematic review. Crit. Rev. Oncol. Hematol. 2016, 100, 209–222. [Google Scholar] [CrossRef]
- Hall, B.; Padussis, J.; Foster, J.M. Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy in the Management of Colorectal Peritoneal Metastasis. Surg. Clin. N. Am. 2017, 97, 671–682. [Google Scholar] [CrossRef]
- Nakafusa, Y.; Tanaka, T.; Tanaka, M.; Kitajima, Y.; Sato, S.; Miyazaki, K. Comparison of Multivisceral Resection and Standard Operation for Locally Advanced Colorectal Cancer: Analysis of Prognostic Factors for Short-Term and Long-Term Outcome. Dis. Colon Rectum 2004, 47, 2055–2063. [Google Scholar] [CrossRef]
- Simkens, G.A.; Van Oudheusden, T.R.; Braam, H.J.; Luyer, M.D.; Wiezer, M.J.; Van Ramshorst, B.; Nienhuijs, S.W.; De Hingh, I.H. Treatment-Related Mortality After Cytoreductive Surgery and HIPEC in Patients with Colorectal Peritoneal Carcinomatosis is Underestimated by Conventional Parameters. Ann. Surg. Oncol. 2016, 23, 99–105. [Google Scholar] [CrossRef]
- Simkens, G.A.; Van Oudheusden, T.R.; Luyer, M.D.; Nienhuijs, S.W.; Nieuwenhuijzen, G.A.; Rutten, H.J.; De Hingh, I.H. Predictors of Severe Morbidity After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Patients With Colorectal Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2016, 23, 833–841. [Google Scholar] [CrossRef]
- Global Cancer Observatory 2022. Available online: globocan.iarc.fr (accessed on 9 June 2020).
- Reale, M.L.; De Luca, E.; Lombardi, P.; Marandino, L.; Zichi, C.; Pignataro, D.; Ghisoni, E.; Di Stefano, R.F.; Mariniello, A.; Trevisi, E.; et al. Quality of life analysis in lung cancer: A systematic review of phase III trials published between 2012 and 2018. Lung Cancer 2020, 139, 47–54. [Google Scholar] [CrossRef]
- Wiltink, L.M.; White, K.; King, M.T.; Rutherford, C. Systematic review of clinical practice guidelines for colorectal and anal cancer: The extent of recommendations for managing long-term symptoms and functional impairments. Support. Care Cancer 2020, 28, 2523–2532. [Google Scholar] [CrossRef]
- da Silva, R.G.; Sugarbaker, P.H. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J. Am. Coll. Surg. 2006, 203, 878–886. [Google Scholar] [CrossRef]
- Hill, A.R.; Mcquellon, R.P.; Russell, G.B.; Shen, P.; Stewart, J.H.; Levine, E.A. Survival and Quality of Life Following Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colonic Origin. Ann. Surg. Oncol. 2011, 18, 3673–3679. [Google Scholar] [CrossRef]
- Ihemelandu, C.U.; Mcquellon, R.; Shen, P.; Stewart, J.H.; Votanopoulos, K.; Levine, E.A. Predicting Postoperative Morbidity Following Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (CS+HIPEC) with Preoperative FACT-C (Functional Assessment of Cancer Therapy) and Patient-Rated Performance Status. Ann. Surg. Oncol. 2013, 20, 3519–3526. [Google Scholar] [CrossRef]
- Mcquellon, R.P.; Danhauer, S.C.; Russell, G.B.; Shen, P.; Fenstermaker, J.; Stewart, J.H.; Levine, E.A. Monitoring Health Outcomes Following Cytoreductive Surgery Plus Intraperitoneal Hyperthermic Chemotherapy for Peritoneal Carcinomatosis. Ann. Surg. Oncol. 2007, 14, 1105–1113. [Google Scholar] [CrossRef]
- Passot, G.; Bakrin, N.; Roux, A.; Vaudoyer, D.; Gilly, F.-N.; Glehen, O.; Cotte, E. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: A prospective study of 216 patients. Eur. J. Surg. Oncol. 2014, 40, 529–535. [Google Scholar] [CrossRef]
- Steffens, D.; Koh, C.; Ansari, N.; Solomon, M.J.; Brown, K.; McBride, K.; Young, J.; Young, C.J.; Moran, B. Quality of Life After Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Early Results from a Prospective Cohort Study of 115 Patients. Ann. Surg. Oncol. 2020, 27, 3986–3994. [Google Scholar] [CrossRef]
- Tsilimparis, N.; Bockelmann, C.; Raue, W.; Menenakos, C.; Perez, S.; Rau, B.; Hartmann, J. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: Is it worth the risk? Ann. Surg. Oncol. 2013, 20, 226–232. [Google Scholar] [CrossRef]
- Cleeland, C.S. Symptom Burden: Multiple Symptoms and Their Impact as Patient-Reported Outcomes. JNCI Monogr. 2007, 2007, 16–21. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of Cancer-Related Fatigue on the Lives of Patients: New Findings From the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef]
- Mols, F.; Beijers, T.; Lemmens, V.; Hurk, C.J.V.D.; Vreugdenhil, G.; van de Poll-Franse, L.V. Chemotherapy-Induced Neuropathy and Its Association With Quality of Life Among 2- to 11-Year Colorectal Cancer Survivors: Results From the Population-Based PROFILES Registry. J. Clin. Oncol. 2013, 31, 2699–2707. [Google Scholar] [CrossRef] [PubMed]
- Rovers, K.P.; Bakkers, C.; Simkens, G.A.A.M.; Burger, J.W.A.; Nienhuijs, S.W.; Creemers, G.-J.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; Ayez, N.; et al. Perioperative systemic therapy and cytoreductive surgery with HIPEC versus upfront cytoreductive surgery with HIPEC alone for isolated resectable colorectal peritoneal metastases: Protocol of a multicentre, open-label, parallel-group, phase II-III, randomised, superiority study (CAIRO6). BMC Cancer 2019, 19, 390. [Google Scholar]
- Bonhof, C.S.; van de Poll-Franse, L.V.; Wasowicz, D.K.; Beerepoot, L.V.; Vreugdenhil, G.; Mols, F. The course of peripheral neuropathy and its association with health-related quality of life among colorectal cancer patients. J. Cancer Surviv. 2021, 15, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Vereniging Klinische Genetica Nederland; Landelijke Werkgroep Gastrointestinale Tumoren. Oncoline Landelijke Richtlijn Erfelijke Darmkanker Versie.2.0; Integraal Kanker Centrum Nederland: Utrecht, The Netherlands, 2015. [Google Scholar]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; De Haes, J.C.J.M.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Giesinger, J.M.; Kieffer, J.M.; Fayers, P.M.; Groenvold, M.; Petersen, M.A.; Scott, N.W.; Sprangers, M.A.; Velikova, G.; Aaronson, N.K. Replication and validation of higher order models demonstrated that a summary score for the EORTC QLQ-C30 is robust. J. Clin. Epidemiol. 2016, 69, 79–88. [Google Scholar] [CrossRef]
- Braun, M.S.; Seymour, M.T. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther. Adv. Med. Oncol. 2011, 3, 43–52. [Google Scholar] [CrossRef]
- Vardy, J.L.; Dhillon, H.M.; Pond, G.R.; Rourke, S.B.; Bekele, T.; Renton, C.; Dodd, A.; Zhang, H.; Beale, P.; Clarke, S.; et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: A prospective, longitudinal, controlled study. J. Clin. Oncol. 2015, 33, 4085. [Google Scholar] [CrossRef]
| CRS-HIPEC Group N = 71 | Conventional Surgery Group N = 260 | p-Value | |
|---|---|---|---|
| Sex, n (%) | 0.176 | ||
| Male | 36 (51) | 156 (60) | |
| Female | 35 (49) | 104 (40) | |
| Age, n (%) | <0.001 | ||
| ≤50 | 13 (18) | 11 (4) | |
| 51–70 | 40 (56) | 135 (52) | |
| >70 | 18 (25) | 114 (44) | |
| ASA, n (%) | 0.112 | ||
| 1–2 | 65 (92) | 211 (82) | |
| 3–4 | 6 (8) | 40 (15) | |
| Unknown | - | 9 (3) | |
| Primary tumor location, n (%) | <0.001 | ||
| Right colon | 24 (34) | 96 (37) | |
| Left colon | 42 (59) | 94 (36) | |
| Rectum | 3 (4) | 68 (26) | |
| Unknown | 2 (3) | 2 (1) | |
| Any (neo)adjuvant systemic therapy, n (%) | 0.133 | ||
| No | 38 (54) | 165 (64) | |
| Yes | 33 (47) | 95 (36) | |
| Neoadjuvant systemic therapy, n (%) | <0.001 | ||
| No | 37 (52) | 235 (90) | |
| Yes | 34 (48) | 25 (10) | |
| Adjuvant systemic therapy, n (%) | 0.553 | ||
| No | 49 (69) | 189 (73) | |
| Yes | 22 (31) | 71 (27) |
| Unadjusted | Adjusted for Systemic Therapy | ||||
|---|---|---|---|---|---|
| PRO | Mean Difference d | 95% CI | p-Value | 95% CI | p-Value |
| C30 summary score a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.015 | NA | 0.012 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −1 | −6–2 | 0.337 | −6–2 | 0.312 |
| Early postoperative period d | −3 | −8–−1 | 0.024 | −8–−1 | 0.021 |
| One year postoperatively | −2 | −9–−1 | 0.020 | −9–−1 | 0.017 |
| Global Health Status a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.811 | NA | 0.838 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | +6 | −2–9 | 0.199 | −2–9 | 0.206 |
| Early postoperative periodd | +1 | −5–6 | 0.736 | −5–6 | 0.753 |
| One year postoperatively | −1 | −10–2 | 0.232 | −10–2 | 0.222 |
| Physical functioning a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.033 | NA | 0.026 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −2 | −9–1 | 0.116 | −9–1 | 0.102 |
| Early postoperative period d | −3 | −9–1 | 0.105 | −9–1 | 0.090 |
| One year postoperatively | −2 | −11–0 | 0.069 | −11–0 | 0.057 |
| Role functioning a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.029 | NA | 0.029 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −9 | −16–0 | 0.057 | −16–0 | 0.059 |
| Early postoperative period d | −1 | −11–5 | 0.496 | −11–5 | 0.501 |
| One year postoperatively | −6 | −16–2 | 0.115 | −16–2 | 0.118 |
| Emotional functioning a | |||||
| Comparison of differential effects over time between both groups | NA | 0.059 | NA | 0.048 | |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −1 | −8–3 | 0.359 | −8–3 | 0.329 |
| Early postoperative periodd | −2 | −9–2 | 0.229 | −9–2 | 0.205 |
| One year postoperatively | −4 | −13–−1 | 0.017 | −14–−2 | 0.014 |
| Cognitive functioning a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.701 | NA | 0.690 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −1 | −5–5 | 0.940 | −5–5 | 0.932 |
| Early postoperative periodd | 0 | −6–4 | 0.610 | −6–4 | 0.603 |
| One year postoperatively | 0 | −7–5 | 0.721 | −7–5 | 0.711 |
| Social functioning a | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.006 | NA | 0.006 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −3 | −12–−0 | 0.042 | −12 –−0 | 0.044 |
| Early postoperative periodd | −4 | −11–1 | 0.090 | −11–1 | 0.094 |
| One year postoperatively | −5 | −15–−2 | 0.015 | −15–−2 | 0.016 |
| Fatigue b | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.047 | NA | 0.048 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | +2 | 3–10 | 0.248 | −3–10 | 0.251 |
| Early postoperative periodd | +4 | −1–12 | 0.105 | −1–12 | 0.107 |
| One year postoperatively | +5 | −1–14 | 0.096 | −1–14 | 0.099 |
| Diarrhea b | |||||
| Comparison of differential effects over time between both groups | NA | NA | 0.976 | NA | 0.958 |
| Comparisons between the groups at each measurement c | |||||
| Baseline | −7 | −12–1 | 0.083 | −12–1 | 0.086 |
| Early postoperative periodd | 0 | −4–9 | 0.495 | −4–9 | 0.486 |
| One year postoperatively | +3 | −2–12 | 0.189 | −2–12 | 0.183 |
| CRS-HIPEC N = 66 | Conventional Surgery N = 237 | |||||||
|---|---|---|---|---|---|---|---|---|
| PRO | Mean Difference c | 95% CI | p-Value | Cohen’s D e | Mean Difference c | 95% CI | p-Value | Cohen’s D e |
| C30 summary score a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −7 | −11–−4 | <0.001 | 0.66 | −5 | −7–−3 | <0.001 | 0.38 |
| Early postoperative period d vs. one year postoperatively | +8 | 3–10 | 0.002 | 0.61 | +7 | 5–9 | <0.001 | 0.54 |
| Baseline vs. one year postoperatively | +1 | −5–−3 | 0.701 | NA | +2 | 0–4 | 0.018 | NA |
| Global Health Status a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −7 | −10–0 | 0.066 | NA | −2 | −5–1 | 0.119 | NA |
| Early postoperative period d vs. one year postoperatively | +7 | −2–10 | 0.163 | NA | +9 | 6–12 | <0.001 | 0.47 |
| Baseline vs. one year postoperatively | 0 | −6–5 | 0.815 | NA | +7 | 4–10 | <0.001 | 0.38 |
| Physical functioning a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −9 | −14–−4 | <0.001 | 0.58 | −8 | −11–−7 | <0.001 | 0.53 |
| Early postoperative period d vs. one year postoperatively | +6 | −1–10 | 0.146 | NA | +5 | 3–8 | <0.001 | 0.34 |
| Baseline vs. one year postoperatively | −3 | −10–0 | 0.074 | NA | −3 | −6–−1 | 0.003 | 0.18 |
| Role functioning a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −12 | −25–−7 | <0.001 | 0.50 | −20 | −24–−16 | <0.001 | 0.68 |
| Early postoperative period d vs. one year postoperatively | +18 | 6–26 | 0.002 | 0.67 | +21 | 15–25 | <0.001 | 0.75 |
| Baseline vs. one year postoperatively | +6 | −10–9 | 0.913 | NA | +1 | −5–5 | 0.968 | NA |
| Emotional functioning a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | +6 | 1–11 | 0.012 | 0.37 | +7 | 4 –9 | <0.001 | 0.37 |
| Early postoperative period d vs. one year postoperatively | +7 | −4–7 | 0.635 | NA | +1 | 3–8 | <0.001 | 0.29 |
| Baseline vs. one year postoperatively | +13 | 9–15 | 0.007 | 0.50 | +8 | 2–13 | <0.001 | 0.65 |
| Cognitive functioning a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −3 | −9–0 | 0.031 | NA | −4 | −6–−1 | 0.003 | 0.18 |
| Early postoperative period d vs. one year postoperatively | +1 | −3–6 | 0.525 | NA | +1 | −1–34 | <0.001 | 0.05 |
| Baseline vs. one year postoperatively | −2 | −8–2 | 0.193 | NA | −3 | −5–0 | <0.001 | 0.11 |
| Social functioning a | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | −9 | −15–−1 | 0.018 | NA | −8 | −12–−6 | <0.001 | 0.37 |
| Early postoperative period d vs. one year postoperatively | +9 | −9–6 | 0.749 | NA | +10 | 7–14 | <0.001 | 0.47 |
| Baseline vs. one year postoperatively | 0 | −1–15 | 0.073 | NA | +2 | −2–5 | 0.323 | NA |
| Fatigue b | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | +14 | 8–20 | <0.001 | 0.72 | +12 | 9–16 | <0.001 | 0.49 |
| Early postoperative period d vs. one year postoperatively | −14 | −20–7 | <0.001 | 0.62 | −15 | −18–−11 | <0.001 | 0.61 |
| Baseline vs. one year postoperatively | 0 | −6–7 | 0.908 | NA | −1 | −6–2 | 0.279 | NA |
| Diarrhea b | ||||||||
| Comparisons between time points within groups | ||||||||
| Baseline vs. early postoperative period d | +7 | 2–14 | 0.012 | NA | 0 | −4–3 | 0.781 | NA |
| Early postoperative period d vs. one year postoperatively | −1 | −9–6 | 0.669 | NA | −4 | −8–0 | 0.049 | NA |
| Baseline vs. one year postoperatively | +6 | 0–14 | 0.064 | NA | −4 | −8–−1 | 0.022 | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakkers, C.; van de Vlasakker, V.C.J.; Rovers, K.P.B.; Lurvink, R.J.; Nienhuijs, S.W.; Burger, J.W.A.; Creemers, G.-J.M.; Bonhof, C.S.; Mols, F.; de Hingh, I.H.J.T. The Impact of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) versus Conventional Surgery on Patient-Reported Outcomes: A Comparative Cohort Study between the CAIRO6 Trial and the PROCORE Study. Cancers 2023, 15, 788. https://doi.org/10.3390/cancers15030788
Bakkers C, van de Vlasakker VCJ, Rovers KPB, Lurvink RJ, Nienhuijs SW, Burger JWA, Creemers G-JM, Bonhof CS, Mols F, de Hingh IHJT. The Impact of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) versus Conventional Surgery on Patient-Reported Outcomes: A Comparative Cohort Study between the CAIRO6 Trial and the PROCORE Study. Cancers. 2023; 15(3):788. https://doi.org/10.3390/cancers15030788
Chicago/Turabian StyleBakkers, Checca, Vincent C. J. van de Vlasakker, Koen P. B. Rovers, Robin J. Lurvink, Simon W. Nienhuijs, Jacobus W. A. Burger, Geert-Jan M. Creemers, Cynthia S. Bonhof, Floortje Mols, and Ignace H. J. T. de Hingh. 2023. "The Impact of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) versus Conventional Surgery on Patient-Reported Outcomes: A Comparative Cohort Study between the CAIRO6 Trial and the PROCORE Study" Cancers 15, no. 3: 788. https://doi.org/10.3390/cancers15030788
APA StyleBakkers, C., van de Vlasakker, V. C. J., Rovers, K. P. B., Lurvink, R. J., Nienhuijs, S. W., Burger, J. W. A., Creemers, G.-J. M., Bonhof, C. S., Mols, F., & de Hingh, I. H. J. T. (2023). The Impact of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) versus Conventional Surgery on Patient-Reported Outcomes: A Comparative Cohort Study between the CAIRO6 Trial and the PROCORE Study. Cancers, 15(3), 788. https://doi.org/10.3390/cancers15030788






