Advances in the Molecular Landscape of Lung Cancer Brain Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. NSCLC Brain Metastasis
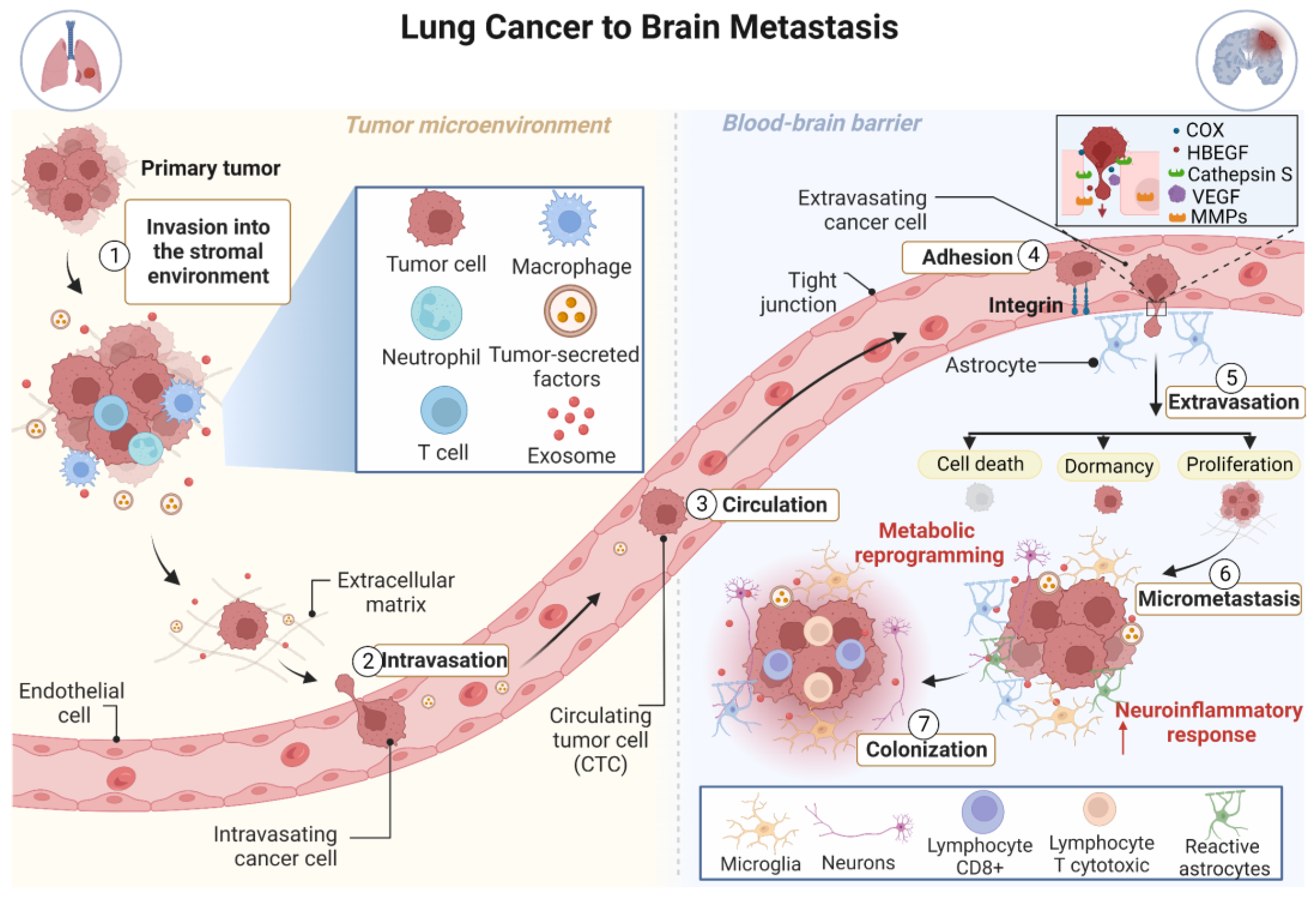
3. The Development of Brain Metastasis Is a Complex, Multistage Process
4. Molecular Determinants of Brain Metastasis in Lung Cancer and Their Implications for Treatment
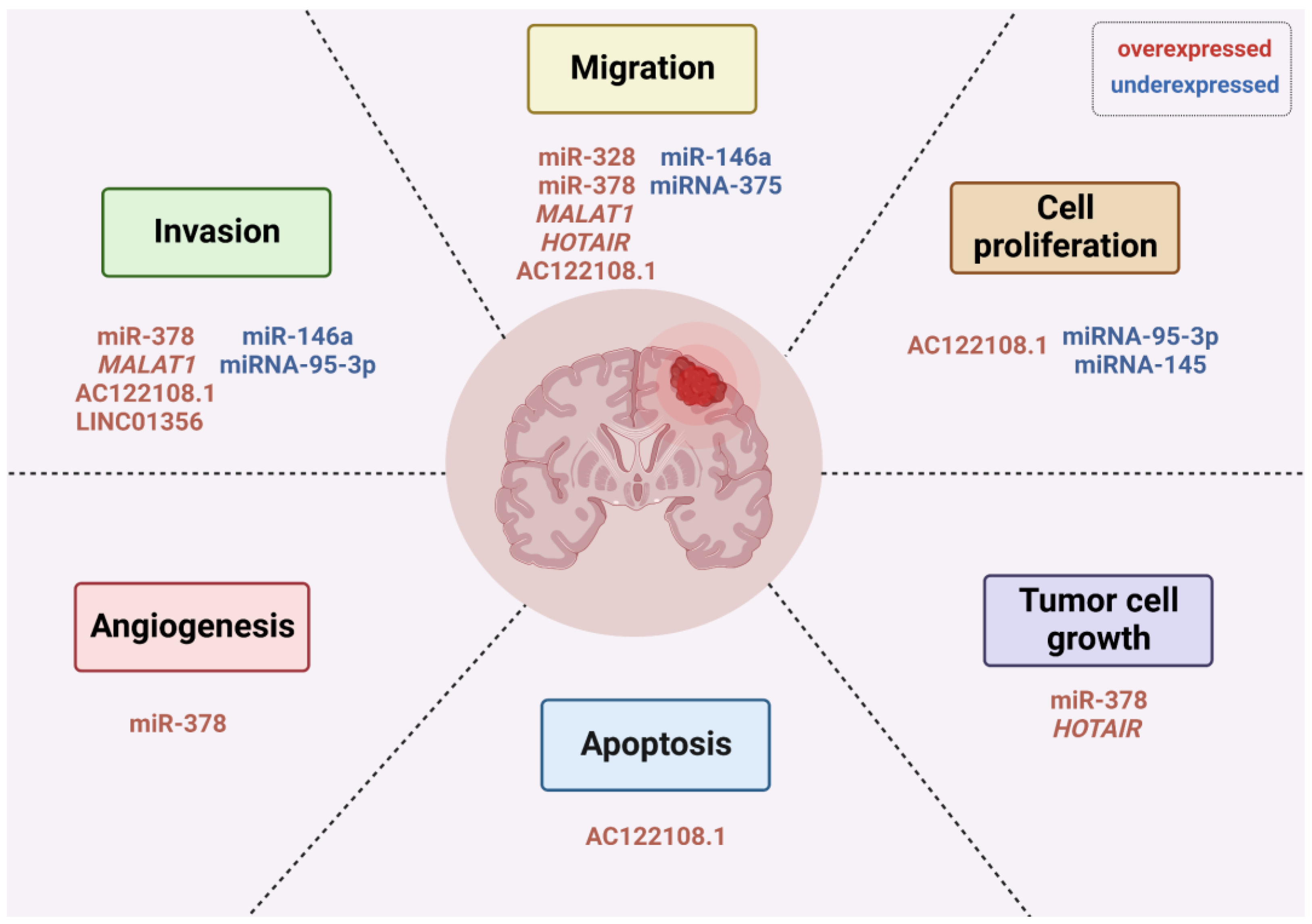
5. Non-Coding RNAs Play Important Roles in Brain Metastasis from Lung Cancer
6. Advances in the Molecular Diagnostics of Brain Metastasis: Liquid Biopsies
7. Treatment of BM from Lung Cancer
8. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The Biology and Management of Non-Small Cell Lung Cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Park, H.K.; Han, J.; Kwon, G.Y.; Yeo, M.-K.; Bae, G.E. Patterns of Extrathoracic Metastasis in Lung Cancer Patients. Curr. Oncol. 2022, 29, 8794–8801. [Google Scholar] [CrossRef]
- Rotow, J.; Bivona, T.G. Understanding and Targeting Resistance Mechanisms in NSCLC. Nat. Rev. Cancer 2017, 17, 637–658. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Suda, K.; Wiens, J.; Bunn, P.A. New and Emerging Targeted Treatments in Advanced Non-Small-Cell Lung Cancer. Lancet 2016, 388, 1012–1024. [Google Scholar] [CrossRef]
- Rodak, O.; Peris-Díaz, M.D.; Olbromski, M.; Podhorska-Okołów, M.; Dzięgiel, P. Current Landscape of Non-Small Cell Lung Cancer: Epidemiology, Histological Classification, Targeted Therapies, and Immunotherapy. Cancers 2021, 13, 4705. [Google Scholar] [CrossRef]
- Hong, D.S.; Fakih, M.G.; Strickler, J.H.; Desai, J.; Durm, G.A.; Shapiro, G.I.; Falchook, G.S.; Price, T.J.; Sacher, A.; Denlinger, C.S.; et al. KRASG12C Inhibition with Sotorasib in Advanced Solid Tumors. N. Engl. J. Med. 2020, 383, 1207–1217. [Google Scholar] [CrossRef]
- Riely, G.J.; Ou, S.-H.I.; Rybkin, I.; Spira, A.; Papadopoulos, K.; Sabari, J.K.; Johnson, M.; Heist, R.S.; Bazhenova, L.; Barve, M.; et al. 99O_PR KRYSTAL-1: Activity and Preliminary Pharmacodynamic (PD) Analysis of Adagrasib (MRTX849) in Patients (Pts) with Advanced Non–Small Cell Lung Cancer (NSCLC) Harboring KRASG12C Mutation. J. Thorac. Oncol. 2021, 16, S751–S752. [Google Scholar] [CrossRef]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F.; et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Shu, C.-L.; Liu, Y.-L. The Path to Personalized Treatment in KRAS-Mutant Non-Small Cell Lung Cancer: A Review of Targeted Therapies and Immunotherapy. Cancer Manag. Res. 2022, 14, 3485–3492. [Google Scholar] [CrossRef]
- Bungaro, M.; Novello, S.; Passiglia, F. Targeting KRASp.G12C Mutation in Advanced Non-Small Cell Lung Cancer: A New Era Has Begun. Curr. Treat. Options Oncol. 2022, 23, 1699–1720. [Google Scholar] [CrossRef]
- Cucurull, M.; Notario, L.; Sanchez-Cespedes, M.; Hierro, C.; Estival, A.; Carcereny, E.; Saigí, M. Targeting KRAS in Lung Cancer Beyond KRAS G12C Inhibitors: The Immune Regulatory Role of KRAS and Novel Therapeutic Strategies. Front. Oncol. 2021, 11, 793121. [Google Scholar] [CrossRef]
- Loriot, Y.; Schuler, M.H.; Iyer, G.; Witt, O.; Doi, T.; Qin, S.; Tabernero, J.; Reardon, D.A.; Massard, C.; Palmer, D.; et al. Tumor Agnostic Efficacy and Safety of Erdafitinib in Patients (Pts) with Advanced Solid Tumors with Prespecified Fibroblast Growth Factor Receptor Alterations (FGFRalt) in RAGNAR: Interim Analysis (IA) Results. J. Clin. Oncol. 2022, 40, 3007. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef]
- Gettinger, S.N.; Horn, L.; Gandhi, L.; Spigel, D.R.; Antonia, S.J.; Rizvi, N.A.; Powderly, J.D.; Heist, R.S.; Carvajal, R.D.; Jackman, D.M.; et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 2004–2012. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Rizvi, N.A.; Goldman, J.W.; Gettinger, S.N.; Borghaei, H.; Brahmer, J.R.; Ready, N.E.; Gerber, D.E.; Chow, L.Q.; Juergens, R.A.; et al. Nivolumab plus Ipilimumab as First-Line Treatment for Advanced Non-Small-Cell Lung Cancer (CheckMate 012): Results of an Open-Label, Phase 1, Multicohort Study. Lancet Oncol. 2017, 18, 31–41. [Google Scholar] [CrossRef]
- Wanleenuwat, P.; Iwanowski, P. Metastases to the Central Nervous System: Molecular Basis and Clinical Considerations. J. Neurol. Sci. 2020, 412, 116755. [Google Scholar] [CrossRef]
- Moravan, M.J.; Fecci, P.E.; Anders, C.K.; Clarke, J.M.; Salama, A.K.S.; Adamson, J.D.; Floyd, S.R.; Torok, J.A.; Salama, J.K.; Sampson, J.H.; et al. Current Multidisciplinary Management of Brain Metastases. Cancer 2020, 126, 1390–1406. [Google Scholar] [CrossRef]
- Cagney, D.N.; Martin, A.M.; Catalano, P.J.; Redig, A.J.; Lin, N.U.; Lee, E.Q.; Wen, P.Y.; Dunn, I.F.; Bi, W.L.; Weiss, S.E.; et al. Incidence and Prognosis of Patients with Brain Metastases at Diagnosis of Systemic Malignancy: A Population-Based Study. Neuro-Oncology 2017, 19, 1511–1521. [Google Scholar] [CrossRef]
- Schoenmaekers, J.J.A.O.; Dingemans, A.-M.C.; Hendriks, L.E.L. Brain Imaging in Early Stage Non-Small Cell Lung Cancer: Still a Controversial Topic? J. Thorac. Dis. 2018, 10, S2168–S2171. [Google Scholar] [CrossRef]
- Hubbs, J.L.; Boyd, J.A.; Hollis, D.; Chino, J.P.; Saynak, M.; Kelsey, C.R. Factors Associated with the Development of Brain Metastases: Analysis of 975 Patients with Early Stage Nonsmall Cell Lung Cancer. Cancer 2010, 116, 5038–5046. [Google Scholar] [CrossRef]
- Achrol, A.S.; Rennert, R.C.; Anders, C.; Soffietti, R.; Ahluwalia, M.S.; Nayak, L.; Peters, S.; Arvold, N.D.; Harsh, G.R.; Steeg, P.S.; et al. Brain Metastases. Nat. Rev. Dis. Primer 2019, 5, 5. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Survival in Patients With Brain Metastases: Summary Report on the Updated Diagnosis-Specific Graded Prognostic Assessment and Definition of the Eligibility Quotient. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3773–3784. [Google Scholar] [CrossRef]
- Levy, A.; Faivre-Finn, C.; Hasan, B.; De Maio, E.; Berghoff, A.S.; Girard, N.; Greillier, L.; Lantuéjoul, S.; O’Brien, M.; Reck, M.; et al. Diversity of Brain Metastases Screening and Management in Non-Small Cell Lung Cancer in Europe: Results of the European Organisation for Research and Treatment of Cancer Lung Cancer Group Survey. Eur. J. Cancer 1990 2018, 93, 37–46. [Google Scholar] [CrossRef]
- Wasp, G.T.; Del Prete, C.; Farrell, J.A.D.; Dragnev, K.H.; Russo, G.; Atkins, G.T.; Phillips, J.D.; Brooks, G.A. Impact of Neuroimaging in the Pretreatment Evaluation of Early Stage Non-Small Cell Lung Cancer. Heliyon 2020, 6, e04319. [Google Scholar] [CrossRef]
- Schoenmaekers, J.; Hofman, P.; Bootsma, G.; Westenend, M.; de Booij, M.; Schreurs, W.; Houben, R.; De Ruysscher, D.; Dingemans, A.-M.; Hendriks, L.E.L. Screening for Brain Metastases in Patients with Stage III Non-Small-Cell Lung Cancer, Magnetic Resonance Imaging or Computed Tomography? A Prospective Study. Eur. J. Cancer 2019, 115, 88–96. [Google Scholar] [CrossRef]
- Piffko, A.; Asey, B.; Dührsen, L.; Ristow, I.; Salamon, J.; Wikman, H.; Maire, C.L.; Lamszus, K.; Westphal, M.; Sauvigny, T.; et al. Clinical Determinants Impacting Overall Survival of Patients with Operable Brain Metastases from Non-Small Cell Lung Cancer. Front. Oncol. 2022, 12, 951805. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Kinoshita, M.; Takagaki, M.; Sakai, M.; Tateishi, S.; Achiha, T.; Hirayama, R.; Nishino, K.; Uchida, J.; Kumagai, T.; et al. Different Spatial Distributions of Brain Metastases from Lung Cancer by Histological Subtype and Mutation Status of Epidermal Growth Factor Receptor. Neuro-Oncology 2016, 18, 716–724. [Google Scholar] [CrossRef]
- Ozcan, G.; Singh, M.; Vredenburgh, J.J. Leptomeningeal Metastasis from Non-Small Cell Lung Cancer and Current Landscape of Treatments. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 29, 11–29. [Google Scholar] [CrossRef]
- Cheng, H.; Perez-Soler, R. Leptomeningeal Metastases in Non-Small-Cell Lung Cancer. Lancet Oncol. 2018, 19, e43–e55. [Google Scholar] [CrossRef] [PubMed]
- Christopoulos, P.; Endris, V.; Bozorgmehr, F.; Elsayed, M.; Kirchner, M.; Ristau, J.; Buchhalter, I.; Penzel, R.; Herth, F.J.; Heussel, C.P.; et al. EML4-ALK Fusion Variant V3 Is a High-Risk Feature Conferring Accelerated Metastatic Spread, Early Treatment Failure and Worse Overall Survival in ALK+ Non-Small Cell Lung Cancer. Int. J. Cancer 2018, 142, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.-Y.; Na, I.I.; Kim, C.H.; Park, S.; Baek, H.; Yang, S.H. EGFR Mutation and Brain Metastasis in Pulmonary Adenocarcinomas. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2014, 9, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Preusser, M.; Winkler, F.; Valiente, M.; Manegold, C.; Moyal, E.; Widhalm, G.; Tonn, J.-C.; Zielinski, C. Recent Advances in the Biology and Treatment of Brain Metastases of Non-Small Cell Lung Cancer: Summary of a Multidisciplinary Roundtable Discussion. ESMO Open 2018, 3, e000262. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients with Brain Metastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-MolGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-Endothelial Interactions at the Blood-Brain Barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Dejana, E. Endothelial Cell-Cell Junctions: Happy Together. Nat. Rev. Mol. Cell Biol. 2004, 5, 261–270. [Google Scholar] [CrossRef]
- Jia, W.; Lu, R.; Martin, T.A.; Jiang, W.G. The Role of Claudin-5 in Blood-Brain Barrier (BBB) and Brain Metastases (Review). Mol. Med. Rep. 2014, 9, 779–785. [Google Scholar] [CrossRef]
- Fidler, I.J. The Role of the Organ Microenvironment in Brain Metastasis. Semin. Cancer Biol. 2011, 21, 107–112. [Google Scholar] [CrossRef]
- Wrobel, J.K.; Toborek, M. Blood-Brain Barrier Remodeling during Brain Metastasis Formation. Mol. Med. Camb. Mass 2016, 22, 32–40. [Google Scholar] [CrossRef]
- Wilhelm, I.; Molnár, J.; Fazakas, C.; Haskó, J.; Krizbai, I.A. Role of the Blood-Brain Barrier in the Formation of Brain Metastases. Int. J. Mol. Sci. 2013, 14, 1383–1411. [Google Scholar] [CrossRef]
- Reymond, N.; d’Água, B.B.; Ridley, A.J. Crossing the Endothelial Barrier during Metastasis. Nat. Rev. Cancer 2013, 13, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Bos, P.D.; Zhang, X.H.-F.; Nadal, C.; Shu, W.; Gomis, R.R.; Nguyen, D.X.; Minn, A.J.; van de Vijver, M.J.; Gerald, W.L.; Foekens, J.A.; et al. Genes That Mediate Breast Cancer Metastasis to the Brain. Nature 2009, 459, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Díaz, B.; García-Botello, D.R.; Wegman-Ostrosky, T.; Reyes-Soto, G.; Ortiz-Sánchez, E.; Herrera-Montalvo, L.A. Tumor Microenvironment Differences between Primary Tumor and Brain Metastases. J. Transl. Med. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Schulz, M.; Salamero-Boix, A.; Niesel, K.; Alekseeva, T.; Sevenich, L. Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Front. Immunol. 2019, 10, 1713. [Google Scholar] [CrossRef]
- Popper, H. Primary Tumor and Metastasis-Sectioning the Different Steps of the Metastatic Cascade. Transl. Lung Cancer Res. 2020, 9, 2277–2300. [Google Scholar] [CrossRef]
- Luo, L.; Liu, P.; Zhao, K.; Zhao, W.; Zhang, X. The Immune Microenvironment in Brain Metastases of Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 698844. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Yao, J.; Lowery, F.J.; Zhang, Q.; Huang, W.-C.; Li, P.; Li, M.; Wang, X.; Zhang, C.; et al. Microenvironment-Induced PTEN Loss by Exosomal MicroRNA Primes Brain Metastasis Outgrowth. Nature 2015, 527, 100–104. [Google Scholar] [CrossRef]
- Singh, M.; Venugopal, C.; Tokar, T.; McFarlane, N.; Subapanditha, M.K.; Qazi, M.; Bakhshinyan, D.; Vora, P.; Murty, N.K.; Jurisica, I.; et al. Therapeutic Targeting of the Premetastatic Stage in Human Lung-to-Brain Metastasis. Cancer Res. 2018, 78, 5124–5134. [Google Scholar] [CrossRef]
- Valiente, M.; Ahluwalia, M.S.; Boire, A.; Brastianos, P.K.; Goldberg, S.B.; Lee, E.Q.; Le Rhun, E.; Preusser, M.; Winkler, F.; Soffietti, R. The Evolving Landscape of Brain Metastasis. Trends Cancer 2018, 4, 176–196. [Google Scholar] [CrossRef]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martínez, L.; Martínez-Saez, E.; Cajal, S.R.; et al. STAT3 Labels a Subpopulation of Reactive Astrocytes Required for Brain Metastasis. Nat. Med. 2018, 24, 1024–1035. [Google Scholar] [CrossRef]
- Zou, Y.; Watters, A.; Cheng, N.; Perry, C.E.; Xu, K.; Alicea, G.M.; Parris, J.L.D.; Baraban, E.; Ray, P.; Nayak, A.; et al. Polyunsaturated Fatty Acids from Astrocytes Activate PPARγ Signaling in Cancer Cells to Promote Brain Metastasis. Cancer Discov. 2019, 9, 1720–1735. [Google Scholar] [CrossRef]
- Lehuédé, C.; Dupuy, F.; Rabinovitch, R.; Jones, R.G.; Siegel, P.M. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016, 76, 5201–5208. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.-M. The Metabolism of Cancer Cells during Metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef]
- Wang, C.; Luo, D. The Metabolic Adaptation Mechanism of Metastatic Organotropism. Exp. Hematol. Oncol. 2021, 10, 30. [Google Scholar] [CrossRef]
- Ciminera, A.K.; Jandial, R.; Termini, J. Metabolic Advantages and Vulnerabilities in Brain Metastases. Clin. Exp. Metastasis 2017, 34, 401–410. [Google Scholar] [CrossRef]
- Srinivasan, E.S.; Deshpande, K.; Neman, J.; Winkler, F.; Khasraw, M. The Microenvironment of Brain Metastases from Solid Tumors. Neuro-Oncol. Adv. 2021, 3, v121–v132. [Google Scholar] [CrossRef]
- Onwudiwe, K.; Burchett, A.A.; Datta, M. Mechanical and Metabolic Interplay in the Brain Metastatic Microenvironment. Front. Oncol. 2022, 12, 932285. [Google Scholar] [CrossRef]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET Signalling: Principles and Functions in Development, Organ Regeneration and Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef]
- Boccaccio, C.; Comoglio, P.M. Invasive Growth: A MET-Driven Genetic Programme for Cancer and Stem Cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Stella, G.; Corino, A.; Berzero, G.; Kolling, S.; Filippi, A.; Benvenuti, S. Brain Metastases from Lung Cancer: Is MET an Actionable Target? Cancers 2019, 11, 271. [Google Scholar] [CrossRef]
- Stella, G.M.; Senetta, R.; Inghilleri, S.; Verdun di Cantogno, L.; Mantovani, C.; Piloni, D.; Scudeller, L.; Meloni, F.; Papotti, M.; Ricardi, U.; et al. MET Mutations Are Associated with Aggressive and Radioresistant Brain Metastatic Non-Small-Cell Lung Cancer. Neuro-Oncology 2016, 18, 598–599. [Google Scholar] [CrossRef]
- Milan, M.; Benvenuti, S.; Balderacchi, A.M.; Virzì, A.R.; Gentile, A.; Senetta, R.; Cassoni, P.; Comoglio, P.M.; Stella, G.M. RON Tyrosine Kinase Mutations in Brain Metastases from Lung Cancer. ERJ Open Res. 2018, 4, 00083-2017. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Chiang, A.C.; Zhang, X.H.-F.; Kim, J.Y.; Kris, M.G.; Ladanyi, M.; Gerald, W.L.; Massagué, J. WNT/TCF Signaling through LEF1 and HOXB9 Mediates Lung Adenocarcinoma Metastasis. Cell 2009, 138, 51–62. [Google Scholar] [CrossRef]
- Vignot, S.; Frampton, G.M.; Soria, J.-C.; Yelensky, R.; Commo, F.; Brambilla, C.; Palmer, G.; Moro-Sibilot, D.; Ross, J.S.; Cronin, M.T.; et al. Next-Generation Sequencing Reveals High Concordance of Recurrent Somatic Alterations between Primary Tumor and Metastases from Patients with Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2013, 31, 2167–2172. [Google Scholar] [CrossRef]
- Paik, P.K.; Shen, R.; Won, H.; Rekhtman, N.; Wang, L.; Sima, C.S.; Arora, A.; Seshan, V.; Ladanyi, M.; Berger, M.F.; et al. Next-Generation Sequencing of Stage IV Squamous Cell Lung Cancers Reveals an Association of PI3K Aberrations and Evidence of Clonal Heterogeneity in Patients with Brain Metastases. Cancer Discov. 2015, 5, 610–621. [Google Scholar] [CrossRef]
- Shih, D.J.H.; Nayyar, N.; Bihun, I.; Dagogo-Jack, I.; Gill, C.M.; Aquilanti, E.; Bertalan, M.; Kaplan, A.; D’Andrea, M.R.; Chukwueke, U.; et al. Genomic Characterization of Human Brain Metastases Identifies Drivers of Metastatic Lung Adenocarcinoma. Nat. Genet. 2020, 52, 371–377. [Google Scholar] [CrossRef]
- Nicoś, M.; Harbers, L.; Patrucco, E.; Kramer-Drauberg, M.; Zhang, X.; Voena, C.; Kowalczyk, A.; Bożyk, A.; Pęksa, R.; Jarosz, B.; et al. Genomic Profiling Identifies Putative Pathogenic Alterations in NSCLC Brain Metastases. JTO Clin. Res. Rep. 2022, 3, 100435. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, M.; Lei, B.; Zhou, Z.; Huang, Y.; Li, W.; Chen, Q.; Li, P.; Deng, Y. Whole-Exome Sequencing Identifies Somatic Mutations Associated with Lung Cancer Metastasis to the Brain. Ann. Transl. Med. 2021, 9, 694. [Google Scholar] [CrossRef]
- Lee, W.-C.; Reuben, A.; Hu, X.; McGranahan, N.; Chen, R.; Jalali, A.; Negrao, M.V.; Hubert, S.M.; Tang, C.; Wu, C.-C.; et al. Multiomics Profiling of Primary Lung Cancers and Distant Metastases Reveals Immunosuppression as a Common Characteristic of Tumor Cells with Metastatic Plasticity. Genome Biol. 2020, 21, 271. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Carter, S.L.; Santagata, S.; Cahill, D.P.; Taylor-Weiner, A.; Jones, R.T.; Van Allen, E.M.; Lawrence, M.S.; Horowitz, P.M.; Cibulskis, K.; et al. Genomic Characterization of Brain Metastases Reveals Branched Evolution and Potential Therapeutic Targets. Cancer Discov. 2015, 5, 1164–1177. [Google Scholar] [CrossRef]
- Campbell, B.K.; Gao, Z.; Corcoran, N.M.; Stylli, S.S.; Hovens, C.M. Molecular Mechanisms Driving the Formation of Brain Metastases. Cancers 2022, 14, 4963. [Google Scholar] [CrossRef]
- Jiang, T.; Fang, Z.; Tang, S.; Cheng, R.; Li, Y.; Ren, S.; Su, C.; Min, W.; Guo, X.; Zhu, W.; et al. Mutational Landscape and Evolutionary Pattern of Liver and Brain Metastasis in Lung Adenocarcinoma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 237–249. [Google Scholar] [CrossRef]
- Wilson, G.D.; Johnson, M.D.; Ahmed, S.; Cardenas, P.Y.; Grills, I.S.; Thibodeau, B.J. Targeted DNA Sequencing of Non-Small Cell Lung Cancer Identifies Mutations Associated with Brain Metastases. Oncotarget 2018, 9, 25957–25970. [Google Scholar] [CrossRef]
- Kamer, I.; Steuerman, Y.; Daniel-Meshulam, I.; Perry, G.; Izraeli, S.; Perelman, M.; Golan, N.; Simansky, D.; Barshack, I.; Ben Nun, A.; et al. Predicting Brain Metastasis in Early Stage Non-Small Cell Lung Cancer Patients by Gene Expression Profiling. Transl. Lung Cancer Res. 2020, 9, 682–692. [Google Scholar] [CrossRef]
- Wang, H.; Ou, Q.; Li, D.; Qin, T.; Bao, H.; Hou, X.; Wang, K.; Wang, F.; Deng, Q.; Liang, J.; et al. Genes Associated with Increased Brain Metastasis Risk in Non-Small Cell Lung Cancer: Comprehensive Genomic Profiling of 61 Resected Brain Metastases versus Primary Non-Small Cell Lung Cancer (Guangdong Association Study of Thoracic Oncology 1036). Cancer 2019, 125, 3535–3544. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, Y.; Gao, Z.; Zhao, Y.; Wen, Z.; Han, H.; Li, Y.; Chen, H. Development and Validation of a Five-Gene Model to Predict Postoperative Brain Metastasis in Operable Lung Adenocarcinoma. Int. J. Cancer 2020, 147, 584–592. [Google Scholar] [CrossRef]
- Shen, E.; Van Swearingen, A.E.D.; Price, M.J.; Bulsara, K.; Verhaak, R.G.W.; Baëta, C.; Painter, B.D.; Reitman, Z.J.; Salama, A.K.S.; Clarke, J.M.; et al. A Need for More Molecular Profiling in Brain Metastases. Front. Oncol. 2021, 11, 785064. [Google Scholar] [CrossRef]
- Ruan, H.; Zhou, Y.; Shen, J.; Zhai, Y.; Xu, Y.; Pi, L.; Huang, R.; Chen, K.; Li, X.; Ma, W.; et al. Circulating Tumor Cell Characterization of Lung Cancer Brain Metastases in the Cerebrospinal Fluid through Single-Cell Transcriptome Analysis. Clin. Transl. Med. 2020, 10, e246. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, F.; Zhou, M.; Wu, S.; Zou, Q.; Gao, B. Single-Cell RNA Sequencing Analysis Identifies Key Genes in Brain Metastasis from Lung Adenocarcinoma. Curr. Gene Ther. 2021, 21, 338–348. [Google Scholar] [CrossRef]
- Zhang, Q.; Abdo, R.; Iosef, C.; Kaneko, T.; Cecchini, M.; Han, V.K.; Li, S.S.-C. The Spatial Transcriptomic Landscape of Non-Small Cell Lung Cancer Brain Metastasis. Nat. Commun. 2022, 13, 5983. [Google Scholar] [CrossRef] [PubMed]
- Cech, T.R.; Steitz, J.A. The Noncoding RNA Revolution-Trashing Old Rules to Forge New Ones. Cell 2014, 157, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Anastasiadou, E.; Jacob, L.S.; Slack, F.J. Non-Coding RNA Networks in Cancer. Nat. Rev. Cancer 2018, 18, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic Targeting of Non-Coding RNAs in Cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [CrossRef]
- Beermann, J.; Piccoli, M.-T.; Viereck, J.; Thum, T. Non-Coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Zhang, Z.; Wei, X.; Zhou, Z.-G. Noncoding RNAs in Tumor Metastasis: Molecular and Clinical Perspectives. Cell. Mol. Life Sci. 2021, 78, 6823–6850. [Google Scholar] [CrossRef]
- Solé, C.; Lawrie, C.H. MicroRNAs and Metastasis. Cancers 2019, 12, 96. [Google Scholar] [CrossRef]
- Kim, J.; Yao, F.; Xiao, Z.; Sun, Y.; Ma, L. MicroRNAs and Metastasis: Small RNAs Play Big Roles. Cancer Metastasis Rev. 2018, 37, 5–15. [Google Scholar] [CrossRef]
- Santos, R.M.; Moreno, C.; Zhang, W.C. Non-Coding RNAs in Lung Tumor Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2774. [Google Scholar] [CrossRef]
- Tominaga, N.; Kosaka, N.; Ono, M.; Katsuda, T.; Yoshioka, Y.; Tamura, K.; Lötvall, J.; Nakagama, H.; Ochiya, T. Brain Metastatic Cancer Cells Release MicroRNA-181c-Containing Extracellular Vesicles Capable of Destructing Blood-Brain Barrier. Nat. Commun. 2015, 6, 6716. [Google Scholar] [CrossRef] [PubMed]
- Fong, M.Y.; Zhou, W.; Liu, L.; Alontaga, A.Y.; Chandra, M.; Ashby, J.; Chow, A.; O’Connor, S.T.F.; Li, S.; Chin, A.R.; et al. Breast-Cancer-Secreted MiR-122 Reprograms Glucose Metabolism in Premetastatic Niche to Promote Metastasis. Nat. Cell Biol. 2015, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Alsidawi, S.; Malek, E.; Driscoll, J.J. MicroRNAs in Brain Metastases: Potential Role as Diagnostics and Therapeutics. Int. J. Mol. Sci. 2014, 15, 10508–10526. [Google Scholar] [CrossRef]
- Singh, M.; Garg, N.; Venugopal, C.; Hallett, R.; Tokar, T.; McFarlane, N.; Mahendram, S.; Bakhshinyan, D.; Manoranjan, B.; Vora, P.; et al. STAT3 Pathway Regulates Lung-Derived Brain Metastasis Initiating Cell Capacity through MiR-21 Activation. Oncotarget 2015, 6, 27461–27477. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Ranade, A.R.; Tran, N.L.; Nasser, S.; Sridhar, S.; Korn, R.L.; Ross, J.T.D.; Dhruv, H.; Foss, K.M.; Sibenaller, Z.; et al. MicroRNA-328 Is Associated with (Non-Small) Cell Lung Cancer (NSCLC) Brain Metastasis and Mediates NSCLC Migration. Int. J. Cancer 2011, 129, 2621–2631. [Google Scholar] [CrossRef]
- Chen, L.; Xu, S.; Xu, H.; Zhang, J.; Ning, J.; Wang, S. MicroRNA-378 Is Associated with Non-Small Cell Lung Cancer Brain Metastasis by Promoting Cell Migration, Invasion and Tumor Angiogenesis. Med. Oncol. 2012, 29, 1673–1680. [Google Scholar] [CrossRef]
- Lee, D.Y.; Deng, Z.; Wang, C.-H.; Yang, B.B. MicroRNA-378 Promotes Cell Survival, Tumor Growth, and Angiogenesis by Targeting SuFu and Fus-1 Expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef]
- Long, H.; Wang, Z.; Chen, J.; Xiang, T.; Li, Q.; Diao, X.; Zhu, B. MicroRNA-214 Promotes Epithelial-Mesenchymal Transition and Metastasis in Lung Adenocarcinoma by Targeting the Suppressor-of-Fused Protein (Sufu). Oncotarget 2015, 6, 38705–38718. [Google Scholar] [CrossRef]
- Remon, J.; Alvarez-Berdugo, D.; Majem, M.; Moran, T.; Reguart, N.; Lianes, P. MiRNA-197 and MiRNA-184 Are Associated with Brain Metastasis in EGFR-Mutant Lung Cancers. Clin. Transl. Oncol. 2016, 18, 153–159. [Google Scholar] [CrossRef]
- Hwang, S.J.; Seol, H.J.; Park, Y.M.; Kim, K.H.; Gorospe, M.; Nam, D.-H.; Kim, H.H. MicroRNA-146a Suppresses Metastatic Activity in Brain Metastasis. Mol. Cells 2012, 34, 329–334. [Google Scholar] [CrossRef]
- Hwang, S.J.; Lee, H.W.; Kim, H.R.; Song, H.J.; Lee, D.H.; Lee, H.; Shin, C.H.; Joung, J.-G.; Kim, D.-H.; Joo, K.M.; et al. Overexpression of MicroRNA-95-3p Suppresses Brain Metastasis of Lung Adenocarcinoma through Downregulation of Cyclin D1. Oncotarget 2015, 6, 20434–20448. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, Y.; Zhang, Y.; Tan, W.; Xue, J.; Yang, Z.; Zhang, Y.; Lu, Y.; Hu, X. Downregulation of MiR-145 Contributes to Lung Adenocarcinoma Cell Growth to Form Brain Metastases. Oncol. Rep. 2013, 30, 2027–2034. [Google Scholar] [CrossRef]
- Chen, L.-J.; Li, X.-Y.; Zhao, Y.-Q.; Liu, W.-J.; Wu, H.-J.; Liu, J.; Mu, X.-Q.; Wu, H.-B. Down-Regulated MicroRNA-375 Expression as a Predictive Biomarker in Non-Small Cell Lung Cancer Brain Metastasis and Its Prognostic Significance. Pathol. Res. Pract. 2017, 213, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Alsidawi, S.; Jagannathan, S.; Sumita, K.; Sasaki, A.T.; Aronow, B.; Warnick, R.E.; Lawler, S.; Driscoll, J.J. The Brain Microenvironment Negatively Regulates MiRNA-768-3p to Promote K-Ras Expression and Lung Cancer Metastasis. Sci. Rep. 2013, 3, 2392. [Google Scholar] [CrossRef]
- Lu, Y.; Govindan, R.; Wang, L.; Liu, P.; Goodgame, B.; Wen, W.; Sezhiyan, A.; Pfeifer, J.; Li, Y.; Hua, X.; et al. MicroRNA Profiling and Prediction of Recurrence/Relapse-Free Survival in Stage I Lung Cancer. Carcinogenesis 2012, 33, 1046–1054. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, T.; Chen, X.; Gu, J.; Huang, J.; Lu, B.; Wang, Z. Long Non-Coding RNA in Lung Cancer. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 504, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Meng, H.; Bai, Y.; Wang, K. Regulation of LncRNA and Its Role in Cancer Metastasis. Oncol. Res. 2016, 23, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, L.; Wang, Y.; Jiang, X.; Xia, H.; Zhuang, Z. Long Noncoding RNA MALAT1 Promotes Brain Metastasis by Inducing Epithelial-Mesenchymal Transition in Lung Cancer. J. Neurooncol. 2015, 121, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Xiao, G.; Chen, Y.; Deng, Y. LncRNA MALAT1 Promotes Migration and Invasion of Non-Small-Cell Lung Cancer by Targeting MiR-206 and Activating Akt/MTOR Signaling. Anticancer Drugs 2018, 29, 725–735. [Google Scholar] [CrossRef]
- Nakagawa, T.; Endo, H.; Yokoyama, M.; Abe, J.; Tamai, K.; Tanaka, N.; Sato, I.; Takahashi, S.; Kondo, T.; Satoh, K. Large Noncoding RNA HOTAIR Enhances Aggressive Biological Behavior and Is Associated with Short Disease-Free Survival in Human Non-Small Cell Lung Cancer. Biochem. Biophys. Res. Commun. 2013, 436, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Pan, Q.; Gao, H.; Wang, Y.; Zhong, X. MiR-17-5p/HOXA7 Is a Potential Driver for Brain Metastasis of Lung Adenocarcinoma Related to Ferroptosis Revealed by Bioinformatic Analysis. Front. Neurol. 2022, 13, 878947. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Liu, H.; Du, P.; Dong, X.; Pang, Q.; Guo, H. Long Non-Coding RNA AC122108.1 Promotes Lung Adenocarcinoma Brain Metastasis and Progression through the Wnt/β-Catenin Pathway by Directly Binding to Aldolase A. Ann. Transl. Med. 2021, 9, 1729. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jing, W.; Jia, W.; Zhai, X.; Zhu, H.; Yu, J. Downregulation of LncRNA XR_429159.1 Linked to Brain Metastasis in Patients With Limited-Stage Small-Cell Lung Cancer. Front. Oncol. 2021, 11, 603271. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, G.-Y.; Jiang, L.; Jia, L.; Zhang, Z.; Li, X.; Wang, R.; Zhou, M.; Zhou, Y.; Zeng, Z.; et al. Long Noncoding RNA CAR10 Promotes Lung Adenocarcinoma Metastasis via MiR-203/30/SNAI Axis. Oncogene 2019, 38, 3061–3076. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Lu, Y.-R. BCYRN1, a c-MYC-Activated Long Non-Coding RNA, Regulates Cell Metastasis of Non-Small-Cell Lung Cancer. Cancer Cell Int. 2015, 15, 36. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, L.; Li, L.; Cao, Y. Exosomes Derived from Brain Metastatic Breast Cancer Cells Destroy the Blood-Brain Barrier by Carrying LncRNA GS1-600G8.5. BioMed Res. Int. 2020, 2020, 7461727. [Google Scholar] [CrossRef]
- Guo, J.; Shen, S.; Liu, X.; Ruan, X.; Zheng, J.; Liu, Y.; Liu, L.; Ma, J.; Ma, T.; Shao, L.; et al. Role of Linc00174/MiR-138-5p (MiR-150-5p)/FOSL2 Feedback Loop on Regulating the Blood-Tumor Barrier Permeability. Mol. Ther. Nucleic Acids 2019, 18, 1072–1090. [Google Scholar] [CrossRef]
- Ma, J.; Wang, P.; Yao, Y.; Liu, Y.; Li, Z.; Liu, X.; Li, Z.; Zhao, X.; Xi, Z.; Teng, H.; et al. Knockdown of Long Non-Coding RNA MALAT1 Increases the Blood-Tumor Barrier Permeability by up-Regulating MiR-140. Biochim. Biophys. Acta 2016, 1859, 324–338. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Y.; Wang, P.; Liu, X.; Ma, J.; Zheng, J.; Li, Z.; Li, Z.; Cai, H.; Liu, Y. Knockdown of Long Non-Coding RNA XIST Increases Blood-Tumor Barrier Permeability and Inhibits Glioma Angiogenesis by Targeting MiR-137. Oncogenesis 2017, 6, e303. [Google Scholar] [CrossRef]
- Geng, S.; Tu, S.; Bai, Z.; Geng, Y. Exosomal LncRNA LINC01356 Derived From Brain Metastatic Nonsmall-Cell Lung Cancer Cells Remodels the Blood-Brain Barrier. Front. Oncol. 2022, 12, 825899. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Deng, S.; Li, L.; Liu, T.; Zhang, T.; Li, J.; Yu, Y.; Xu, Y. TGF-Β1-Mediated Exosomal Lnc-MMP2-2 Increases Blood-Brain Barrier Permeability via the MiRNA-1207-5p/EPB41L5 Axis to Promote Non-Small Cell Lung Cancer Brain Metastasis. Cell Death Dis. 2021, 12, 721, Erratum in Cell Death Dis. 2021, 12, 916. [Google Scholar] [CrossRef] [PubMed]
- Katsushima, K.; Jallo, G.; Eberhart, C.G.; Perera, R.J. Long Non-Coding RNAs in Brain Tumors. NAR Cancer 2021, 3, zcaa041. [Google Scholar] [CrossRef]
- Cheng, J.; Meng, J.; Zhu, L.; Peng, Y. Exosomal Noncoding RNAs in Glioma: Biological Functions and Potential Clinical Applications. Mol. Cancer 2020, 19, 66. [Google Scholar] [CrossRef]
- Xing, F.; Liu, Y.; Wu, S.-Y.; Wu, K.; Sharma, S.; Mo, Y.-Y.; Feng, J.; Sanders, S.; Jin, G.; Singh, R.; et al. Loss of XIST in Breast Cancer Activates MSN-c-Met and Reprograms Microglia via Exosomal MiRNA to Promote Brain Metastasis. Cancer Res. 2018, 78, 4316–4330. [Google Scholar] [CrossRef]
- Wang, S.; Liang, K.; Hu, Q.; Li, P.; Song, J.; Yang, Y.; Yao, J.; Mangala, L.S.; Li, C.; Yang, W.; et al. JAK2-Binding Long Noncoding RNA Promotes Breast Cancer Brain Metastasis. J. Clin. Investig. 2017, 127, 4498–4515. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, P.; Xia, L.; He, X.; Xia, Z.; Huang, Y.; Liu, W.; Li, L.; Chen, L. A Brain-Enriched LncRNA Shields Cancer Cells from Immune-Mediated Killing for Metastatic Colonization in the Brain. Proc. Natl. Acad. Sci. USA 2022, 119, e2200230119. [Google Scholar] [CrossRef]
- Le, P.; Romano, G.; Nana-Sinkam, P.; Acunzo, M. Non-Coding RNAs in Cancer Diagnosis and Therapy: Focus on Lung Cancer. Cancers 2021, 13, 1372. [Google Scholar] [CrossRef]
- Xu, K.; Jiang, X.; Ariston Gabriel, A.N.; Li, X.; Wang, Y.; Xu, S. Evolving Landscape of Long Non-Coding RNAs in Cerebrospinal Fluid: A Key Role From Diagnosis to Therapy in Brain Tumors. Front. Cell Dev. Biol. 2021, 9, 737670. [Google Scholar] [CrossRef]
- Kalita-de Croft, P.; Joshi, V.; Saunus, J.M.; Lakhani, S.R. Emerging Biomarkers for Diagnosis, Prevention and Treatment of Brain Metastases-From Biology to Clinical Utility. Diseases 2022, 10, 11. [Google Scholar] [CrossRef]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating Liquid Biopsies into the Management of Cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Bracht, J.W.P.; Mayo-de-Las-Casas, C.; Berenguer, J.; Karachaliou, N.; Rosell, R. The Present and Future of Liquid Biopsies in Non-Small Cell Lung Cancer: Combining Four Biosources for Diagnosis, Prognosis, Prediction, and Disease Monitoring. Curr. Oncol. Rep. 2018, 20, 70. [Google Scholar] [CrossRef]
- Bratulic, S.; Gatto, F.; Nielsen, J. The Translational Status of Cancer Liquid Biopsies. Regen. Eng. Transl. Med. 2021, 7, 312–352. [Google Scholar] [CrossRef]
- Lu, Y.-T.; Delijani, K.; Mecum, A.; Goldkorn, A. Current Status of Liquid Biopsies for the Detection and Management of Prostate Cancer. Cancer Manag. Res. 2019, 11, 5271–5291. [Google Scholar] [CrossRef] [PubMed]
- Kwapisz, D. The First Liquid Biopsy Test Approved. Is It a New Era of Mutation Testing for Non-Small Cell Lung Cancer? Ann. Transl. Med. 2017, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Doyle, G.V.; Terstappen, L.W.M.M. Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J. Oncol. 2010, 2010, 617421. [Google Scholar] [CrossRef]
- Tong, B.; Wang, M. Circulating Tumor Cells in Patients with Lung Cancer: Developments and Applications for Precision Medicine. Future Oncol. 2019, 15, 2531–2542. [Google Scholar] [CrossRef]
- Lindsay, C.R.; Faugeroux, V.; Michiels, S.; Pailler, E.; Facchinetti, F.; Ou, D.; Bluthgen, M.V.; Pannet, C.; Ngo-Camus, M.; Bescher, G.; et al. A Prospective Examination of Circulating Tumor Cell Profiles in Non-Small-Cell Lung Cancer Molecular Subgroups. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Satelli, A.; Li, S. Vimentin in Cancer and Its Potential as a Molecular Target for Cancer Therapy. Cell. Mol. Life Sci. 2011, 68, 3033–3046. [Google Scholar] [CrossRef]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; Schuuring, E.; Timens, W.; Terstappen, L.W.M.M.; Hiltermann, T.J.N.; Groen, H.J.M. Circulating Tumor Cells in Lung Cancer Are Prognostic and Predictive for Worse Tumor Response in Both Targeted- and Chemotherapy. Transl. Lung Cancer Res. 2019, 8, 854–861. [Google Scholar] [CrossRef]
- Aljohani, H.M.; Aittaleb, M.; Furgason, J.M.; Amaya, P.; Deeb, A.; Chalmers, J.J.; Bahassi, E.M. Genetic Mutations Associated with Lung Cancer Metastasis to the Brain. Mutagenesis 2018, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Hernández, L.E.; Eslami, S.Z.; Alix-Panabières, C. Circulating Tumor Cell as the Functional Aspect of Liquid Biopsy to Understand the Metastatic Cascade in Solid Cancer. Mol. Asp. Med. 2020, 72, 100816. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Clinical Applications of Circulating Tumor Cells and Circulating Tumor DNA as Liquid Biopsy. Cancer Discov. 2016, 6, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Yang, X.; Xing, W.; Yu, H.; Si, T.; Guo, Z. Detection of Circulating Tumor DNA from Non-Small Cell Lung Cancer Brain Metastasis in Cerebrospinal Fluid Samples. Thorac. Cancer 2020, 11, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, Z.; Huang, T.; Wang, Y.; Song, M.M.; Song, T.; Long, G.; Zhang, X.; Li, X.; Zhang, L. Cerebrospinal Fluid Circulating Tumor DNA Depicts Profiling of Brain Metastasis in NSCLC. Mol. Oncol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Darlix, A.; Cayrefourcq, L.; Pouderoux, S.; Menjot de Champfleur, N.; Bievelez, A.; Jacot, W.; Leaha, C.; Thezenas, S.; Alix-Panabières, C. Detection of Circulating Tumor Cells in Cerebrospinal Fluid of Patients with Suspected Breast Cancer Leptomeningeal Metastases: A Prospective Study. Clin. Chem. 2022, 68, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Buscail, E.; Alix-Panabières, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdon, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656. [Google Scholar] [CrossRef]
- Rehman, A.U.; Khan, P.; Maurya, S.K.; Siddiqui, J.A.; Santamaria-Barria, J.A.; Batra, S.K.; Nasser, M.W. Liquid Biopsies to Occult Brain Metastasis. Mol. Cancer 2022, 21, 113. [Google Scholar] [CrossRef]
- Füzéry, A.K.; Levin, J.; Chan, M.M.; Chan, D.W. Translation of Proteomic Biomarkers into FDA Approved Cancer Diagnostics: Issues and Challenges. Clin. Proteom. 2013, 10, 13. [Google Scholar] [CrossRef]
- Borrebaeck, C.A.K. Precision Diagnostics: Moving towards Protein Biomarker Signatures of Clinical Utility in Cancer. Nat. Rev. Cancer 2017, 17, 199–204. [Google Scholar] [CrossRef]
- Arrieta, O.; Saavedra-Perez, D.; Kuri, R.; Aviles-Salas, A.; Martinez, L.; Mendoza-Posada, D.; Castillo, P.; Astorga, A.; Guzman, E.; De la Garza, J. Brain Metastasis Development and Poor Survival Associated with Carcinoembryonic Antigen (CEA) Level in Advanced Non-Small Cell Lung Cancer: A Prospective Analysis. BMC Cancer 2009, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-S.; Kim, Y.-S.; Jung, S.-L.; Lee, K.-Y.; Kang, J.-H.; Park, S.; Kim, Y.-K.; Yoo, I.-R.; Choi, B.-O.; Jang, H.-S.; et al. The Relevance of Serum Carcinoembryonic Antigen as an Indicator of Brain Metastasis Detection in Advanced Non-Small Cell Lung Cancer. Tumour Biol. 2012, 33, 1065–1073. [Google Scholar] [CrossRef]
- Nazmeen, A.; Maiti, S.; Mandal, K.; Roy, S.K.; Ghosh, T.K.; Sinha, N.K.; Mandal, K. Better Predictive Value of Cancer Antigen125 (CA125) as Biomarker in Ovary and Breast Tumors and Its Correlation with the Histopathological Type/Grade of the Disease. Med. Chem. 2017, 13, 796–804. [Google Scholar] [CrossRef] [PubMed]
- Pollán, M.; Varela, G.; Torres, A.; de la Torre, M.; Ludeña, M.D.; Ortega, M.D.; Pac, J.; Freixenet, J.; Gómez, G.; Sebastián, F.; et al. Clinical Value of P53, c-ErbB-2, CEA and CA125 Regarding Relapse, Metastasis and Death in Resectable Non-Small Cell Lung Cancer. Int. J. Cancer 2003, 107, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhang, Y.; Lyu, Y.; Jin, B.; Guo, H.; Wu, J.; Li, X.; Liu, X. Lactate Dehydrogenase and Serum Tumor Markers for Predicting Metastatic Status in Geriatric Patients with Lung Adenocarcinoma. Cancer Biomark. Sect. Dis. Markers 2019, 26, 139–150. [Google Scholar] [CrossRef]
- Vogelbaum, M.A.; Brown, P.D.; Messersmith, H.; Brastianos, P.K.; Burri, S.; Cahill, D.; Dunn, I.F.; Gaspar, L.E.; Gatson, N.T.N.; Gondi, V.; et al. Treatment for Brain Metastases: ASCO-SNO-ASTRO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 492–516. [Google Scholar] [CrossRef]
- Yang, G.; Xing, L.; Sun, X. Navigate Towards the Immunotherapy Era: Value of Immune Checkpoint Inhibitors in Non-Small Cell Lung Cancer Patients With Brain Metastases. Front. Immunol. 2022, 13, 852811. [Google Scholar] [CrossRef]
- Aizer, A.A.; Lamba, N.; Ahluwalia, M.S.; Aldape, K.; Boire, A.; Brastianos, P.K.; Brown, P.D.; Camidge, D.R.; Chiang, V.L.; Davies, M.A.; et al. Brain Metastases: A Society for Neuro-Oncology (SNO) Consensus Review on Current Management and Future Directions. Neuro-Oncology 2022, 24, 1613–1646. [Google Scholar] [CrossRef]
- Tsui, D.C.C.; Camidge, D.R.; Rusthoven, C.G. Managing Central Nervous System Spread of Lung Cancer: The State of the Art. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 642–660. [Google Scholar] [CrossRef]
- Lauko, A.; Thapa, B.; Venur, V.A.; Ahluwalia, M.S. Management of Brain Metastases in the New Era of Checkpoint Inhibition. Curr. Neurol. Neurosci. Rep. 2018, 18, 70. [Google Scholar] [CrossRef]
- Ulahannan, D.; Khalifa, J.; Faivre-Finn, C.; Lee, S.-M. Emerging Treatment Paradigms for Brain Metastasis in Non-Small-Cell Lung Cancer: An Overview of the Current Landscape and Challenges Ahead. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 2923–2931. [Google Scholar] [CrossRef]
- Yousefi, M.; Bahrami, T.; Salmaninejad, A.; Nosrati, R.; Ghaffari, P.; Ghaffari, S.H. Lung Cancer-Associated Brain Metastasis: Molecular Mechanisms and Therapeutic Options. Cell. Oncol. Dordr. 2017, 40, 419–441. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, W.; Li, Z.; Wei, H.; Cai, Q.; Chen, Q.; Liu, Z.; Ye, H.; Song, P.; Cheng, L.; et al. Clinical Application of 3D-Slicer + 3D Printing Guide Combined with Transcranial Neuroendoscopic in Minimally Invasive Neurosurgery. Sci. Rep. 2022, 12, 20421. [Google Scholar] [CrossRef]
- Romero, I.A.; Radewicz, K.; Jubin, E.; Michel, C.C.; Greenwood, J.; Couraud, P.-O.; Adamson, P. Changes in Cytoskeletal and Tight Junctional Proteins Correlate with Decreased Permeability Induced by Dexamethasone in Cultured Rat Brain Endothelial Cells. Neurosci. Lett. 2003, 344, 112–116. [Google Scholar] [CrossRef]
- Shibata, S. Ultrastructure of Capillary Walls in Human Brain Tumors. Acta Neuropathol. 1989, 78, 561–571. [Google Scholar] [CrossRef]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-Term Side Effects of Glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Ryken, T.C.; McDermott, M.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; Kondziolka, D.; et al. The Role of Steroids in the Management of Brain Metastases: A Systematic Review and Evidence-Based Clinical Practice Guideline. J. Neurooncol. 2010, 96, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Walbert, T.; Harrison, R.A.; Schiff, D.; Avila, E.K.; Chen, M.; Kandula, P.; Lee, J.W.; Le Rhun, E.; Stevens, G.H.J.; Vogelbaum, M.A.; et al. SNO and EANO Practice Guideline Update: Anticonvulsant Prophylaxis in Patients with Newly Diagnosed Brain Tumors. Neuro-Oncology 2021, 23, 1835–1844. [Google Scholar] [CrossRef]
- Mut, M. Surgical Treatment of Brain Metastasis: A Review. Clin. Neurol. Neurosurg. 2012, 114, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Lee, S.H.; Kim, S.; Joo, J.; Yoo, H.; Lee, S.H.; Shin, S.H.; Gwak, H.-S. Risk for Leptomeningeal Seeding after Resection for Brain Metastases: Implication of Tumor Location with Mode of Resection: Clinical Article. J. Neurosurg. 2012, 116, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Winther, R.R.; Hjermstad, M.J.; Skovlund, E.; Aass, N.; Helseth, E.; Kaasa, S.; Yri, O.E.; Vik-Mo, E.O. Surgery for Brain Metastases-Impact of the Extent of Resection. Acta Neurochir. 2022, 164, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- She, C.; Wang, R.; Lu, C.; Sun, Z.; Li, P.; Yin, Q.; Liu, Q.; Wang, P.; Li, W. Prognostic Factors and Outcome of Surgically Treated Patients with Brain Metastases of Non-Small Cell Lung Cancer: Post-Metastasectomy Survival. Thorac. Cancer 2019, 10, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chow, K.K.H.; Aredo, J.V.; Padda, S.K.; Han, S.S.; Kakusa, B.W.; Hayden Gephart, M. Epidermal Growth Factor Receptor Mutation Status Confers Survival Benefit in Patients with Non-Small-Cell Lung Cancer Undergoing Surgical Resection of Brain Metastases: A Retrospective Cohort Study. World Neurosurg. 2019, 125, e487–e496. [Google Scholar] [CrossRef]
- Fuchs, J.; Früh, M.; Papachristofilou, A.; Bubendorf, L.; Häuptle, P.; Jost, L.; Zippelius, A.; Rothschild, S.I. Resection of Isolated Brain Metastases in Non-Small Cell Lung Cancer (NSCLC) Patients—Evaluation of Outcome and Prognostic Factors: A Retrospective Multicenter Study. PLoS ONE 2021, 16, e0253601. [Google Scholar] [CrossRef]
- Antuña, A.; Vega, M.; Sanchez, C.; Fernandez, V. Brain Metastases of Non–Small Cell Lung Cancer: Prognostic Factors in Patients with Surgical Resection. J. Neurol. Surg. Part Cent. Eur. Neurosurg. 2018, 79, 101–107. [Google Scholar] [CrossRef]
- Jünger, S.T.; Reinecke, D.; Meissner, A.-K.; Goldbrunner, R.; Grau, S. Resection of Symptomatic Non–Small Cell Lung Cancer Brain Metastasis in the Setting of Multiple Brain Metastases. J. Neurosurg. 2022, 136, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Franke, J.L.; Medikonda, R.; Jackson, C.M.; Srivastava, S.; Choi, J.; Forde, P.M.; Brahmer, J.R.; Ettinger, D.S.; Feliciano, J.L.; et al. Mutation Status and Postresection Survival of Patients with Non–Small Cell Lung Cancer Brain Metastasis: Implications of Biomarker-Driven Therapy. J. Neurosurg. 2022, 136, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Song, K.; Zhou, Z.; Yu, Z.; Lv, Y.; Xu, H. Survival and Prognostic Factors in Patients Undergoing the Resection of Solitary Brain Metastasis from Non-Small Cell Lung Cancer: A Retrospective Cohort Study. J. Thorac. Dis. 2022, 14, 4113–4124. [Google Scholar] [CrossRef] [PubMed]
- Nakao, T.; Okuda, T.; Yoshioka, H.; Fujita, M. Clinical Outcomes of Surgical Resection for Brain Metastases from Non-Small Cell Lung Cancer. Anticancer Res. 2020, 40, 4801–4804. [Google Scholar] [CrossRef]
- Jeene, P.M.; de Vries, K.C.; van Nes, J.G.H.; Kwakman, J.J.M.; Wester, G.; Rozema, T.; Braam, P.M.; Zindler, J.D.; Koper, P.; Nuyttens, J.J.; et al. Survival after Whole Brain Radiotherapy for Brain Metastases from Lung Cancer and Breast Cancer Is Poor in 6325 Dutch Patients Treated between 2000 and 2014. Acta Oncol. 2018, 57, 637–643. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current Approaches to the Management of Brain Metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Klamer, B.G.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Whitton, A.C.; Greenspoon, J.; Parney, I.F.; et al. Association of Long-Term Outcomes With Stereotactic Radiosurgery vs Whole-Brain Radiotherapy for Resected Brain Metastasis: A Secondary Analysis of The N107C/CEC.3 (Alliance for Clinical Trials in Oncology/Canadian Cancer Trials Group) Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1809. [Google Scholar] [CrossRef]
- Yang, J.-J.; Zhou, C.; Huang, Y.; Feng, J.; Lu, S.; Song, Y.; Huang, C.; Wu, G.; Zhang, L.; Cheng, Y.; et al. Icotinib versus Whole-Brain Irradiation in Patients with EGFR-Mutant Non-Small-Cell Lung Cancer and Multiple Brain Metastases (BRAIN): A Multicentre, Phase 3, Open-Label, Parallel, Randomised Controlled Trial. Lancet Respir. Med. 2017, 5, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Ahn, M.-J.; Garassino, M.C.; Han, J.-Y.; Katakami, N.; Kim, H.R.; Hodge, R.; Kaur, P.; Brown, A.P.; Ghiorghiu, D.; et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2702–2709. [Google Scholar] [CrossRef]
- Reungwetwattana, T.; Nakagawa, K.; Cho, B.C.; Cobo, M.; Cho, E.K.; Bertolini, A.; Bohnet, S.; Zhou, C.; Lee, K.H.; Nogami, N.; et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Goss, G.; Tsai, C.-M.; Shepherd, F.A.; Ahn, M.-J.; Bazhenova, L.; Crinò, L.; de Marinis, F.; Felip, E.; Morabito, A.; Hodge, R.; et al. CNS Response to Osimertinib in Patients with T790M-Positive Advanced NSCLC: Pooled Data from Two Phase II Trials. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 687–693. [Google Scholar] [CrossRef]
- Gadgeel, S.M.; Shaw, A.T.; Govindan, R.; Gandhi, L.; Socinski, M.A.; Camidge, D.R.; De Petris, L.; Kim, D.-W.; Chiappori, A.; Moro-Sibilot, D.L.; et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 4079–4085. [Google Scholar] [CrossRef] [PubMed]
- Camidge, D.R.; Kim, D.-W.; Tiseo, M.; Langer, C.J.; Ahn, M.-J.; Shaw, A.T.; Huber, R.M.; Hochmair, M.J.; Lee, D.H.; Bazhenova, L.A.; et al. Exploratory Analysis of Brigatinib Activity in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2693–2701. [Google Scholar] [CrossRef]
- Ando, K.; Akimoto, K.; Sato, H.; Manabe, R.; Kishino, Y.; Homma, T.; Kusumoto, S.; Yamaoka, T.; Tanaka, A.; Ohmori, T.; et al. Brigatinib and Alectinib for ALK Rearrangement-Positive Advanced Non-Small Cell Lung Cancer With or Without Central Nervous System Metastasis: A Systematic Review and Network Meta-Analysis. Cancers 2020, 12, 942. [Google Scholar] [CrossRef]
- Kim, D.-W.; Mehra, R.; Tan, D.S.W.; Felip, E.; Chow, L.Q.M.; Camidge, D.R.; Vansteenkiste, J.; Sharma, S.; De Pas, T.; Riely, G.J.; et al. Activity and Safety of Ceritinib in Patients with ALK-Rearranged Non-Small-Cell Lung Cancer (ASCEND-1): Updated Results from the Multicentre, Open-Label, Phase 1 Trial. Lancet Oncol. 2016, 17, 452–463. [Google Scholar] [CrossRef]
- Garon, E.B.; Heist, R.S.; Seto, T.; Han, J.-Y.; Reguart, N.; Groen, H.J.; Tan, D.S.; Hida, T.; de Jonge, M.J.; Orlov, S.V.; et al. Abstract CT082: Capmatinib in MET Ex14-Mutated (Mut) Advanced Non-Small Cell Lung Cancer (NSCLC): Results from the Phase II GEOMETRY Mono-1 Study, Including Efficacy in Patients (Pts) with Brain Metastases (BM). Cancer Res. 2020, 80, CT082. [Google Scholar] [CrossRef]
- Le, X.; Sakai, H.; Felip, E.; Veillon, R.; Garassino, M.C.; Raskin, J.; Cortot, A.B.; Viteri, S.; Mazieres, J.; Smit, E.F.; et al. Tepotinib Efficacy and Safety in Patients with MET Exon 14 Skipping NSCLC: Outcomes in Patient Subgroups from the VISION Study with Relevance for Clinical Practice. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Gainor, J.F.; Oxnard, G.R.; Tan, D.S.W.; Owen, D.H.; Cho, B.C.; Loong, H.H.; McCoach, C.E.; Weiss, J.; Kim, Y.J.; et al. Intracranial Efficacy of Selpercatinib in RET Fusion-Positive Non-Small Cell Lung Cancers on the LIBRETTO-001 Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4160–4167. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Subbiah, V.; Gautschi, O.; Tomasini, P.; de Braud, F.; Solomon, B.J.; Shao-Weng Tan, D.; Alonso, G.; Wolf, J.; Park, K.; et al. Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 41, JCO2200393. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in Patients with Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Drilon, A.; Tan, D.S.W.; Lassen, U.N.; Leyvraz, S.; Liu, Y.; Patel, J.D.; Rosen, L.; Solomon, B.; Norenberg, R.; Dima, L.; et al. Efficacy and Safety of Larotrectinib in Patients With Tropomyosin Receptor Kinase Fusion-Positive Lung Cancers. JCO Precis. Oncol. 2022, 6, e2100418. [Google Scholar] [CrossRef]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in Patients with TRK Fusion-Positive Solid Tumours: A Pooled Analysis of Three Phase 1/2 Clinical Trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in Advanced ROS1-Positive Non-Small-Cell Lung Cancer: A Multicentre, Open-Label, Single-Arm, Phase 1-2 Trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef]
- Planchard, D.; Besse, B.; Groen, H.J.M.; Souquet, P.-J.; Quoix, E.; Baik, C.S.; Barlesi, F.; Kim, T.M.; Mazieres, J.; Novello, S.; et al. Dabrafenib plus Trametinib in Patients with Previously Treated BRAF(V600E)-Mutant Metastatic Non-Small Cell Lung Cancer: An Open-Label, Multicentre Phase 2 Trial. Lancet Oncol. 2016, 17, 984–993. [Google Scholar] [CrossRef]
- Alvarez-Breckenridge, C.; Remon, J.; Piña, Y.; Nieblas-Bedolla, E.; Forsyth, P.; Hendriks, L.; Brastianos, P.K. Emerging Systemic Treatment Perspectives on Brain Metastases: Moving Toward a Better Outlook for Patients. Am. Soc. Clin. Oncol. Educ. Book Am. Soc. Clin. Oncol. Annu. Meet. 2022, 42, 1–19. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Dziadziuszko, R.; Barlesi, F.; Krebs, M.G.; Shaw, A.T.; de Braud, F.; Rolfo, C.; Ahn, M.-J.; Wolf, J.; et al. Entrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol. 2020, 21, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Dziadziuszko, R.; Krebs, M.G.; De Braud, F.; Siena, S.; Drilon, A.; Doebele, R.C.; Patel, M.R.; Cho, B.C.; Liu, S.V.; Ahn, M.-J.; et al. Updated Integrated Analysis of the Efficacy and Safety of Entrectinib in Locally Advanced or Metastatic ROS1 Fusion-Positive Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Buriolla, S.; Pelizzari, G.; Corvaja, C.; Alberti, M.; Targato, G.; Bortolot, M.; Torresan, S.; Cortiula, F.; Fasola, G.; Follador, A. Immunotherapy in NSCLC Patients with Brain Metastases. Int. J. Mol. Sci. 2022, 23, 7068. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Yu, Y.; Shao, S.; Deng, R.; Yu, J.; Wang, X.; She, X.; Huang, D.; Shen, X.; Duan, W.; et al. The Prospect of Immunotherapy Combined with Chemotherapy in Patients with Advanced Non-Small Cell Lung Cancer: A Narrative Review. Ann. Transl. Med. 2021, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Negrao, M.V.; Skoulidis, F.; Montesion, M.; Schulze, K.; Bara, I.; Shen, V.; Xu, H.; Hu, S.; Sui, D.; Elamin, Y.Y.; et al. Oncogene-Specific Differences in Tumor Mutational Burden, PD-L1 Expression, and Outcomes from Immunotherapy in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2021, 9, e002891. [Google Scholar] [CrossRef]
- Liu, C.; Zheng, S.; Jin, R.; Wang, X.; Wang, F.; Zang, R.; Xu, H.; Lu, Z.; Huang, J.; Lei, Y.; et al. The Superior Efficacy of Anti-PD-1/PD-L1 Immunotherapy in KRAS-Mutant Non-Small Cell Lung Cancer That Correlates with an Inflammatory Phenotype and Increased Immunogenicity. Cancer Lett. 2020, 470, 95–105. [Google Scholar] [CrossRef]
- Otano, I.; Ucero, A.C.; Zugazagoitia, J.; Paz-Ares, L. At the Crossroads of Immunotherapy for Oncogene-Addicted Subsets of NSCLC. Nat. Rev. Clin. Oncol. 2023. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Schalper, K.A.; Gettinger, S.N.; Mahajan, A.; Herbst, R.S.; Chiang, A.C.; Lilenbaum, R.; Wilson, F.H.; Omay, S.B.; Yu, J.B.; et al. Pembrolizumab for Management of Patients with NSCLC and Brain Metastases: Long-Term Results and Biomarker Analysis from a Non-Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2020, 21, 655–663. [Google Scholar] [CrossRef]
- Mansfield, A.S.; Herbst, R.S.; de Castro, G.; Hui, R.; Peled, N.; Kim, D.-W.; Novello, S.; Satouchi, M.; Wu, Y.-L.; Garon, E.B.; et al. Outcomes With Pembrolizumab Monotherapy in Patients With Programmed Death-Ligand 1-Positive NSCLC With Brain Metastases: Pooled Analysis of KEYNOTE-001, 010, 024, and 042. JTO Clin. Res. Rep. 2021, 2, 100205. [Google Scholar] [CrossRef]
- Powell, S.F.; Rodríguez-Abreu, D.; Langer, C.J.; Tafreshi, A.; Paz-Ares, L.; Kopp, H.-G.; Rodríguez-Cid, J.; Kowalski, D.M.; Cheng, Y.; Kurata, T.; et al. Outcomes With Pembrolizumab Plus Platinum-Based Chemotherapy for Patients With NSCLC and Stable Brain Metastases: Pooled Analysis of KEYNOTE-021, -189, and -407. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2021, 16, 1883–1892. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-Line Nivolumab plus Ipilimumab Combined with Two Cycles of Chemotherapy in Patients with Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.A.; Caridà, G.; Ciliberto, D.; d’Apolito, M.; Pelaia, C.; Caracciolo, D.; Riillo, C.; Correale, P.; Galvano, A.; Russo, A.; et al. Efficacy and Safety of First-Line Checkpoint Inhibitors-Based Treatments for Non-Oncogene-Addicted Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. ESMO Open 2022, 7, 100465. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, V.G.P.; de Araújo, R.P.; Santesso, M.R.; Seneda, A.L.; Minutentag, I.W.; Felix, T.F.; Hamamoto Filho, P.T.; Pewarchuk, M.E.; Brockley, L.J.; Marchi, F.A.; et al. Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers 2023, 15, 722. https://doi.org/10.3390/cancers15030722
Souza VGP, de Araújo RP, Santesso MR, Seneda AL, Minutentag IW, Felix TF, Hamamoto Filho PT, Pewarchuk ME, Brockley LJ, Marchi FA, et al. Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers. 2023; 15(3):722. https://doi.org/10.3390/cancers15030722
Chicago/Turabian StyleSouza, Vanessa G. P., Rachel Paes de Araújo, Mariana R. Santesso, Ana Laura Seneda, Iael W. Minutentag, Tainara Francini Felix, Pedro Tadao Hamamoto Filho, Michelle E. Pewarchuk, Liam J. Brockley, Fábio A. Marchi, and et al. 2023. "Advances in the Molecular Landscape of Lung Cancer Brain Metastasis" Cancers 15, no. 3: 722. https://doi.org/10.3390/cancers15030722
APA StyleSouza, V. G. P., de Araújo, R. P., Santesso, M. R., Seneda, A. L., Minutentag, I. W., Felix, T. F., Hamamoto Filho, P. T., Pewarchuk, M. E., Brockley, L. J., Marchi, F. A., Lam, W. L., Drigo, S. A., & Reis, P. P. (2023). Advances in the Molecular Landscape of Lung Cancer Brain Metastasis. Cancers, 15(3), 722. https://doi.org/10.3390/cancers15030722









