A Point-of-Care Faecal Test Combining Four Biomarkers Allows Avoidance of Normal Colonoscopies and Prioritizes Symptomatic Patients with a High Risk of Colorectal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- FOB+Transferrin+Calprotectin+Lactoferrrin® (CerTest Biotec S.L, Zaragoza, Spain), a one-step chromatographic immunoassay for the simultaneous POC qualitative detection of human haemoglobin (hHb), human transferrin (hTf), human calprotectin (hCp), and human lactoferrin (hLf). Cut-off values of the test are 5.1 μg/g for hHb, 0.4 μg/g for hTf, 50 μg/g for hCp, and 10 μg/g for hLf.
- FIT, by FOB Turbilatex® (CerTest Biotec S.L, Zaragoza, Spain), a latex turbidimetric assay for the immunochemical quantitative detection of haemoglobin. A cut-off of 10 μg/g was chosen.
- FC, by Calprotectin Turbilatex® (CerTest Biotec S.L, Zaragoza, Spain), a latex turbidimetric assay, with a cut-off of 50 μg/g.
3. Results
3.1. Study Population
3.2. Diagnostic Accuracy of Faecal Tests
3.3. Receiver Operator Curves Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adelstein, B.A.; Macaskill, P.; Chan, S.F.; Katelaris, P.H.; Irwig, L. Most bowel cancer symptoms do not indicate colorectal cancer and polyps: A systematic review. BMC Gastroenterol. 2011, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.S.; Aoko, O.; Sihag, S.; Connolly, E.; Omorogbe, J.; Semenov, S.; O’Morain, N.; O’Connor, A.; Breslin, N.; Ryan, B.; et al. Lower gastrointestinal symptoms and symptoms-based triaging systems are poor predictors of clinical significant disease on colonoscopy. BMJ Open Gastroenterol. 2020, 7, e000221. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Brenner, H.; Bouvier, A.M.; Foschi, R.; Hackl, M.; Larsen, I.K.; Lemmens, V.; Mangone, L.; Francisci, S. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: The EUROCARE study. Int. J. Cancer 2012, 131, 1649–1658. [Google Scholar] [CrossRef] [PubMed]
- Mozdiak, E.; Weldeselassie, Y.; McFarlane, M.; Tabuso, M.; Widlak, M.M.; Dunlop, A.; Tsertsvadze, A.; Arasaradnam, R.P. Systematic review with meta-analysis of over 90 000 patients. Does fast-track review diagnose colorectal cancer earlier? Aliment. Pharmacol. Ther. 2019, 50, 348–372. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, H.S.; Park, H.J. Adverse events related to colonoscopy: Global trends and future challenges. World J. Gastroenterol. 2019, 25, 190–204. [Google Scholar] [CrossRef]
- Navarro, M.; Nicolas, A.; Ferrandez, A.; Lanas, A. Colorectal cancer population screening programs worldwide in 2016: An update. World J. Gastroenterol. 2017, 23, 3632–3642. [Google Scholar] [CrossRef]
- Domper-Arnal, M.J.; Hijos-Mallada, G.; Lanas, Á. The impact of COVID-19 pandemic in the diagnosis and management of colorectal cancer patients. Ther. Adv. Gastroenterol. 2022, 15, 17562848221117636. [Google Scholar] [CrossRef]
- Monahan, K.J.; Davies, M.M.; Abulafi, M.; Banerjea, A.; Nicholson, B.D.; Arasaradnam, R.; Barker, N.; Benton, S.; Booth, R.; Burling, D.; et al. Faecal immunochemical testing (FIT) in patients with signs or symptoms of suspected colorectal cancer (CRC): A joint guideline from the Association of Coloproctology of Great Britain and Ireland (ACPGBI) and the British Society of Gastroenterology (BSG). Gut 2022, 71, 1939–1962. [Google Scholar] [CrossRef]
- Cubiella, J.; Marzo-Castillejo, M.; Mascort-Roca, J.J.; Amador-Romero, F.J.; Bellas-Beceiro, B.; Clofent-Vilaplana, J.; Carballal, S.; Ferrándiz-Santos, J.; Gimeno-García, A.Z.; Jover, R.; et al. Clinical practice guideline. Diagnosis and prevention of colorectal cancer. 2018 Update. Gastroenterol. Hepatol. 2018, 41, 585–596. [Google Scholar] [CrossRef]
- Westwood, M.; Lang, S.; Armstrong, N.; van Turenhout, S.; Cubiella, J.; Stirk, L.; Ramos, I.C.; Luyendijk, M.; Zaim, R.; Kleijnen, J.; et al. Faecal immunochemical tests (FIT) can help to rule out colorectal cancer in patients presenting in primary care with lower abdominal symptoms: A systematic review conducted to inform new NICE DG30 diagnostic guidance. BMC Med. 2017, 15, 189. [Google Scholar] [CrossRef]
- Saw, K.S.; Liu, C.; Xu, W.; Varghese, C.; Parry, S.; Bissett, I. Faecal immunochemical test to triage patients with possible colorectal cancer symptoms: Meta-analysis. Br. J. Surg. 2022, 109, 182–190. [Google Scholar] [CrossRef]
- Herrero, J.M.; Vega, P.; Salve, M.; Bujanda, L.; Cubiella, J. Symptom or faecal immunochemical test based referral criteria for colorectal cancer detection in symptomatic patients: A diagnostic tests study. BMC Gastroenterol. 2018, 18, 155. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, N.; Georgiou Delisle, T.; Chen, M.; Benton, S.; Abulafi, M. Faecal immunochemical test is superior to symptoms in predicting pathology in patients with suspected colorectal cancer symptoms referred on a 2WW pathway: A diagnostic accuracy study. Gut 2021, 70, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Booth, R.; Carten, R.; D’Souza, N.; Westwood, M.; Kleijnen, J.; Abulafi, M. Role of the faecal immunochemical test in patients with risk-stratified suspected colorectal cancer symptoms: A systematic review and meta-analysis to inform the ACPGBI/BSG guidelines. Lancet Reg. Health Eur. 2022, 23, 100518. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Levin, T.R. Current and future colorectal cancer screening strategies. Nat. Reviews. Gastroenterol. Hepatol. 2022, 19, 521–531. [Google Scholar] [CrossRef]
- Navarro, M.; Hijos, G.; Sostres, C.; Lué, A.; Puente-Lanzarote, J.J.; Carrera-Lasfuentes, P.; Lanas, A. Reducing the Cut-Off Value of the Fecal Immunochemical Test for Symptomatic Patients Does Not Improve Diagnostic Performance. Front. Med. 2020, 7, 410. [Google Scholar] [CrossRef]
- Loveday, C.; Sud, A.; Jones, M.E.; Broggio, J.; Scott, S.; Gronthound, F.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; et al. Prioritisation by FIT to mitigate the impact of delays in the 2-week wait colorectal cancer referral pathway during the COVID-19 pandemic: A UK modelling study. Gut 2021, 70, 1053–1060. [Google Scholar] [CrossRef]
- Reenaers, C.; Bossuyt, P.; Hindryckx, P.; Vanpoucke, H.; Cremer, A.; Baert, F. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United Eur. Gastroenterol. J. 2018, 6, 1117–1125. [Google Scholar] [CrossRef]
- Ross, F.A.; Park, J.H.; Mansouri, D.; Combet, E.; Horgan, P.G.; McMillan, D.C.; Roxburgh, C.S.D. The role of faecal calprotectin in diagnosis and staging of colorectal neoplasia: A systematic review and meta-analysis. BMC Gastroenterol. 2022, 22, 176. [Google Scholar] [CrossRef]
- Turvill, J.; Aghahoseini, A.; Sivarajasingham, N.; Abbas, K.; Choudhry, M.; Polyzois, K.; Lasithiotakis, K.; Volanaki, D.; Kim, B.; Langlands, F.; et al. Faecal calprotectin in patients with suspected colorectal cancer: A diagnostic accuracy study. Br. J. Gen. Pract. 2016, 66, e499–e506. [Google Scholar] [CrossRef]
- Ross, F.A.; Park, J.H.; Mansouri, D.; Little, C.; Di Rollo, D.G.; Combet, E.; Van Wyk, H.; Horgan, P.G.; McMillan, D.C.; Roxburgh, C.S.D. The role of faecal calprotectin in the identification of colorectal neoplasia in patients attending for screening colonoscopy. Color. Dis. 2022, 24, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Mowat, C.; Digby, J.; Strachan, J.A.; Wilson, R.; Carey, F.A.; Fraser, C.G.; Steele, R.J. Faecal haemoglobin and faecal calprotectin as indicators of bowel disease in patients presenting to primary care with bowel symptoms. Gut 2016, 65, 1463–1469. [Google Scholar] [CrossRef]
- Widlak, M.M.; Thomas, C.L.; Thomas, M.G.; Tomkins, C.; Smith, S.; O’Connell, N.; Wurie, S.; Burns, L.; Harmston, C.; Evans, C.; et al. Diagnostic accuracy of faecal biomarkers in detecting colorectal cancer and adenoma in symptomatic patients. Aliment. Pharmacol. Ther. 2017, 45, 354–363. [Google Scholar] [CrossRef]
- Turvill, J.; Mellen, S.; Jeffery, L.; Bevan, S.; Keding, A.; Turnock, D. Diagnostic accuracy of one or two faecal haemoglobin and calprotectin measurements in patients with suspected colorectal cancer. Scand. J. Gastroenterol. 2018, 53, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Fan, L.; Han, M.; Zhu, S.; Zhang, S.; Shi, H. The usefulness of fecal hemoglobin and calprotectin tests in diagnosing significant bowel diseases: A prospective study. Scand. J. Gastroenterol. 2022, 1–7. [Google Scholar] [CrossRef]
- Lué, A.; Hijos, G.; Sostres, C.; Perales, A.; Navarro, M.; Barra, M.V.; Mascialino, B.; Andalucia, C.; Puente, J.J.; Lanas, Á.; et al. The combination of quantitative faecal occult blood test and faecal calprotectin is a cost-effective strategy to avoid colonoscopies in symptomatic patients without relevant pathology. Ther. Adv. Gastroenterol. 2020, 13, 1756284820920786. [Google Scholar] [CrossRef] [PubMed]
- Laserna-Mendieta, E.J.; Lucendo, A.J. Faecal calprotectin in inflammatory bowel diseases: A review focused on meta-analyses and routine usage limitations. Clin. Chem. Lab. Med. 2019, 57, 1295–1307. [Google Scholar] [CrossRef]
- D’Amico, F.; Rubin, D.T.; Kotze, P.G.; Magro, F.; Siegmund, B.; Kobayashi, T.; Olivera, P.A.; Bossuyt, P.; Pouillon, L.; Louis, E.; et al. International consensus on methodological issues in standardization of fecal calprotectin measurement in inflammatory bowel diseases. United Eur. Gastroenterol. J. 2021, 9, 451–460. [Google Scholar] [CrossRef]
- Chen, J.G.; Cai, J.; Wu, H.L.; Xu, H.; Zhang, Y.X.; Chen, C.; Wang, Q.; Xu, J.; Yuan, X.L. Colorectal cancer screening: Comparison of transferrin and immuno fecal occult blood test. World J. Gastroenterol. 2012, 18, 2682–2688. [Google Scholar] [CrossRef]
- Gies, A.; Cuk, K.; Schrotz-King, P.; Brenner, H. Fecal immunochemical test for hemoglobin in combination with fecal transferrin in colorectal cancer screening. United Eur. Gastroenterol. J. 2018, 6, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Jiang, M.; Sun, M.J.; Cao, Q. Fecal Lactoferrin for Assessment of Inflammatory Bowel Disease Activity: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2020, 54, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Hirata, I.; Hoshimoto, M.; Saito, O.; Kayazawa, M.; Nishikawa, T.; Murano, M.; Toshina, K.; Wang, F.Y.; Matsuse, R. Usefulness of fecal lactoferrin and hemoglobin in diagnosis of colorectal diseases. World J. Gastroenterol. 2007, 13, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Maclean, W.; Zahoor, Z.; O’Driscoll, S.; Piggott, C.; Whyte, M.B.; Rockall, T.; Jourdan, I.; Benton, S.C. Comparison of the QuikRead go(®) point-of-care faecal immunochemical test for haemoglobin with the FOB Gold Wide(®) laboratory analyser to diagnose colorectal cancer in symptomatic patients. Clin. Chem. Lab. Med. 2022, 60, 101–108. [Google Scholar] [CrossRef]
- Kok, L.; Elias, S.G.; Witteman, B.J.; Goedhard, J.G.; Muris, J.W.; Moons, K.G.; de Wit, N.J. Diagnostic accuracy of point-of-care fecal calprotectin and immunochemical occult blood tests for diagnosis of organic bowel disease in primary care: The Cost-Effectiveness of a Decision Rule for Abdominal Complaints in Primary Care (CEDAR) study. Clin. Chem. 2012, 58, 989–998. [Google Scholar] [CrossRef]
- Hassan, C.; Antonelli, G.; Dumonceau, J.M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Farrugia, A.; Widlak, M.; Evans, C.; Smith, S.C.; Arasaradnam, R. Faecal immunochemical testing (FIT) in symptomatic patients: What are we missing? Frontline Gastroenterol. 2020, 11, 28–33. [Google Scholar] [CrossRef]
- Alonso-Abreu, I.; Alarcón-Fernández, O.; Gimeno-García, A.Z.; Romero-García, R.; Carrillo-Palau, M.; Nicolás-Pérez, D.; Jiménez, A.; Quintero, E. Early Colonoscopy Improves the Outcome of Patients with Symptomatic Colorectal Cancer. Dis. Colon Rectum 2017, 60, 837–844. [Google Scholar] [CrossRef]
- Dobrusin, A.; Hawa, F.; Montagano, J.; Walsh, C.X.; Ellimoottil, C.; Gunaratnam, N.T. Patients with Gastrointestinal Conditions Consider Telehealth Equivalent to In-Person Care. Gastroenterology 2023, 164, 156–158.e2. [Google Scholar] [CrossRef]
- Gies, A.; Niedermaier, T.; Alwers, E.; Hielscher, T.; Weigl, K.; Heisser, T.; Schrotz-King, P.; Hoffmeister, M.; Brenner, H. Consistent Major Differences in Sex- and Age-Specific Diagnostic Performance among Nine Faecal Immunochemical Tests Used for Colorectal Cancer Screening. Cancers 2021, 13, 3574. [Google Scholar] [CrossRef] [PubMed]
- Georgiou Delisle, T.; D’Souza, N.; Davies, B.; Benton, S.; Chen, M.; Ward, H.; Abulafi, M. Faecal immunochemical test for suspected colorectal cancer symptoms: Patient survey of usability and acceptability. BJGP Open 2022, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenburg, S.A.V.; Vuik, F.E.R.; Kruip, M.; Kuipers, E.J.; Spaander, M.C.W. Effect of anticoagulants and NSAIDs on accuracy of faecal immunochemical tests (FITs) in colorectal cancer screening: A systematic review and meta-analysis. Gut 2019, 68, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Hicks, G.; D’Souza, N.; Georgiou Delisle, T.; Chen, M.; Benton, S.C.; Abulafi, M. Using the faecal immunochemical test in patients with rectal bleeding: Evidence from the NICE FIT study. Color. Dis. 2021, 23, 1630–1638. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data | Significant Pathology n = 118 | Non-Significant Findings n = 453 | p-Value Univariant | p-Value Multivariant OR (CI 95%) 2 | |
|---|---|---|---|---|---|
| Median age in years (interquartile range) | 70 (59.5–80.5) | 60 (48.5–71.5) | p < 0.01 | p < 0.01 1.04 (1.02–1.05) | |
| Gender | Male Female | 67 (24.6%) 51 (17.1%) | 205 (75.4%) 248 (82.9%) | p = 0.017 | p = 0.039 1.57 (1.03–2.42) |
| Concomitant treatments | Any of the following NSAIDs 1 Acetylsalicylic acid Other antiplatelets Vitamin K antagonist Direct oral anticoagulants | 42 (26.6%) 9 (18%) 21 (27.3%) 4 (22.2%) 7 (43.8%) 6 (42.9%) | 116 (73.4%) 41 (82%) 56 (72.7%) 14 (77.8%) 9 (56.3%) 8 (57.1%) | p = 0.02 | p = 0.984 |
| Department requesting colonoscopy | Primary Care Gastroenterology General Surgery Other | 89 (24.1%) 18 (12.9%) 4 (10.8%) 7 (29.2%) | 281 (75.9%) 122 (87.1%) 33 (89.2%) 17 (70.8%) | p = 0.019 | p = 0.229 |
| Indication | Rectal bleeding Chronic diarrhoea Abdominal pain Change in bowel habits Anaemia / Iron deficiency Other | 36 (21.8%) 18 (15.8%) 17 (17.5%) 16 (17.6%) 29 (32.6%) 2 (20%) | 129 (78.2%) 96 (84.2%) 80 (82.5%) 75 (82.4%) 60 (67.4%) 13 (80%) | p = 0.064 | - |
| Diagnosis | Test | True Positives | False Negatives | Sensitivity | Specificity | PPV | NPV | OR (95%CI) 1 |
|---|---|---|---|---|---|---|---|---|
| Significant Pathology (n = 118) | FIT FC FIT or FC | 68 83 95 | 50 35 23 | 57.6% 70.3% 80.5% | 87.9% 54.8% 50.1% | 55.3% 28.8% 29.6% | 88.4% 87.6% 90.8% | 8.7 (5.4–13.9) 2.3 (1.5–3.7) 3.5 (2.1–5.8) |
| Colorectal Cancer (n = 30) | FIT FC FIT or FC | 24 25 28 | 6 5 2 | 80% 83.3% 93.3% | 81.7% 51.4% 45.9%% | 19.5% 8.7% 8.7%% | 98.7% 98.2% 99.2% | 15.3 (6.1–38.9) 4.2 (1.6–11.3) 9.6 (2.3–41.2) |
| Inflammatory Bowel Disease (n = 15) | FIT FC FIT or FC | 11 13 14 | 4 2 1 | 73.3% 86.7% 93.3% | 79.9% 50.5% 44.8% | 8.9% 4.5% 4.4% | 99.1% 99.3% 99.6% | 16.3 (4.8–55.9) 9 (2–41.4) 15.3 (2–119.4) |
| Adenoma Requiring Surveillance (n = 53) | FIT FC FIT or FC | 25 32 39 | 28 21 14 | 47.2% 60.4% 73.6% | 81.1% 50.6% 45.6% | 20.3% 11.1% 12.1% | 93.8% 92.6% 94.4% | 3 (1.7–5.6) 1.2 (0.7–2.1) 1.8 (1.1–3.2) |
| Diagnosis | Test | True Positives | False Negatives | Sensitivity | Specificity | PPV | NPV | OR (95%CI) 1 |
|---|---|---|---|---|---|---|---|---|
| Significant Pathology (n = 118) | hHb hTf hCp HLf | 79 53 98 41 | 39 65 20 77 | 66.9% 44.9% 83.1% 34.8% | 87.4% 85.4% 48.1% 92.3% | 58.1% 44.5% 29.5% 53.9% | 91% 85.6% 91.6% 84.4% | 12.6 (7.8–20.3) 5.3 (3.3–8.6) 3.7 (2.2–6.3) 5.7 (3.3–9.7) |
| Colorectal Cancer (n = 30) | hHb hTf hCp HLf | 26 18 28 18 | 4 12 2 12 | 86.7% 60% 93.3% 60% | 79.7% 81.3% 43.6% 89.3% | 19.1% 15.1% 8.4% 23.7% | 99.1% 97.4% 99.2% 97.6% | 22.2 (7.5–65.7) 6.7 (3.1–14.6) 8.4 (1.9–36.4) 10.8 (4.9–24) |
| Inflammatory Bowel Disease (n = 15) | hHb hTf hCp HLf | 14 10 15 14 | 1 5 0 1 | 93.3% 66.7% 100% 93.3% | 78.1% 80.4% 42.8% 90.5% | 10.3% 8.4% 4.5% 21.2% | 99.8% 98.9% 100% 99.8% | 75.3 (9.4–600.6) 8.1 (2.7–24.3) - 142.6 (18–1128.2) |
| Adenoma Requiring Surveillance (n = 53) | hHb hTf hCp HLf | 31 20 38 6 | 22 33 15 47 | 58.5% 37.7% 71.7% 11.3% | 79.7% 80.9% 43% 86.5% | 22.8% 16.8% 11.4% 7.9% | 94.9% 92.7% 93.7% 90.5% | 4.4 (2.4–8.1) 2.7 (1.4–5) 1.4 (0.7–2.7) 0.5 (0.2–1.4) |
| Diagnosis | Positive Tests | True Positives | False Negatives | Sensitivity | Specificity | PPV | NPV | OR (95%CI) 1 |
|---|---|---|---|---|---|---|---|---|
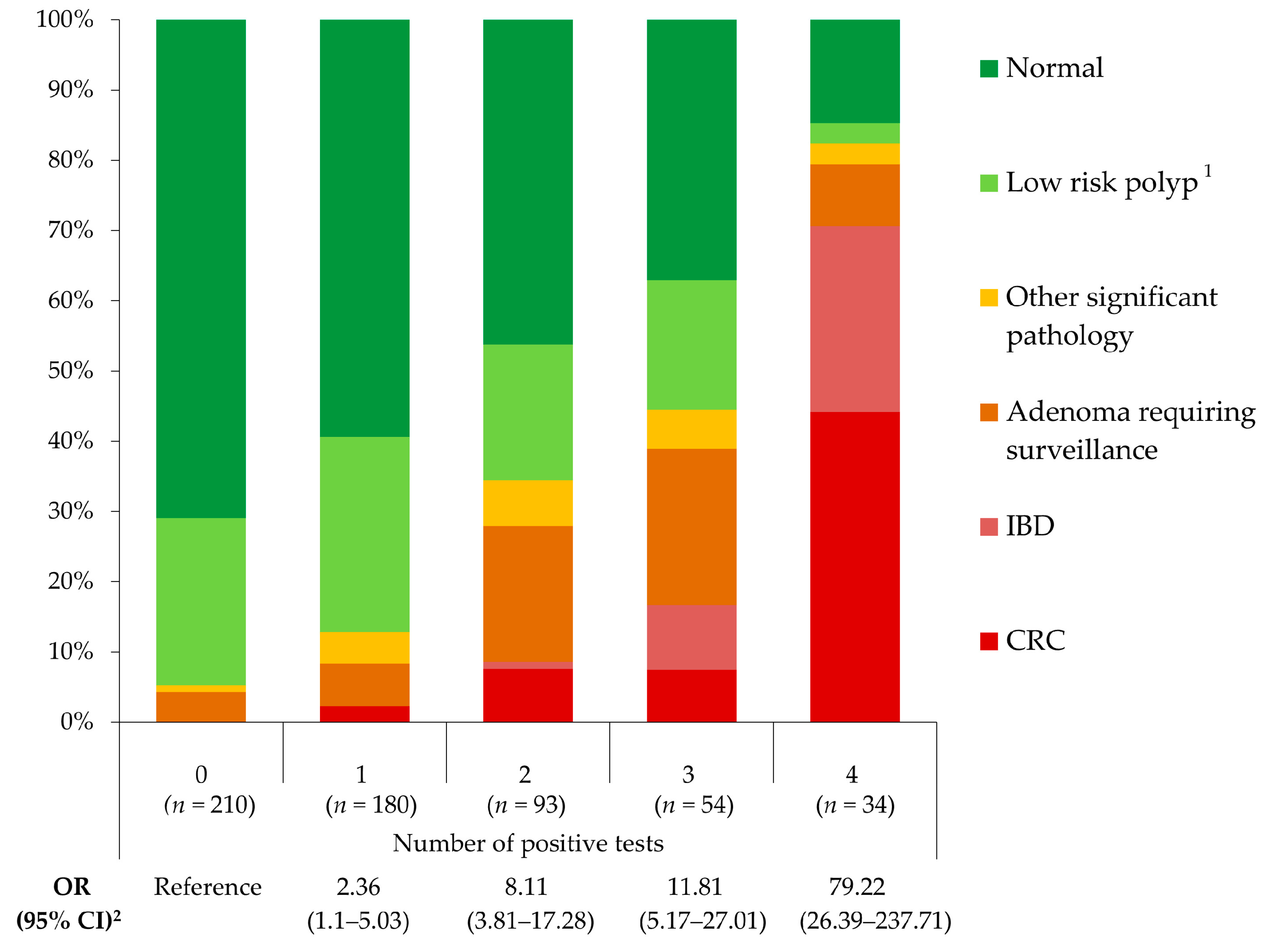
| Significant Pathology (n = 118) | ≥1 test ≥2 tests ≥3 tests 4 tests | 107 84 52 28 | 11 34 66 90 | 90.7% 71.2% 44.1% 23.7% | 43.9% 78.6% 92% 98.7% | 29.6% 46.4% 59.1% 82.3% | 94.8% 91.3% 86.3% 83.2% | 6.5 (3.4–12.6) 8.1 (5.1–12.9) 8.3 (5–13.9) 25 (9.5–65.7) |
| Colorectal Cancer (n = 30) | ≥1 test ≥2 tests ≥3 tests 4 tests | 30 26 19 15 | 0 4 11 15 | 100% 86.7% 63.3% 50% | 38.8% 71.4% 87.3% 96.5% | 8.3% 14.4% 21.6% 44.1% | 100% 99% 97.7% 97.2% | - 13.9 (4.7–40.9) 10.2 (4.6–22.7) 24.9 (10.4–59.5) |
| Inflammatory Bowel Disease (n = 15) | ≥1 test ≥2 tests ≥3 tests 4 tests | 15 15 14 9 | 0 0 1 6 | 100% 100% 93.3% 60% | 37.8% 70.1% 86.7% 95.5% | 4.2% 8.3% 15.9% 26.5% | 100% 100% 99.8% 98.9% | - - 126.5 (15.9–1008) 41.5 (12.6–136.9) |
| Adenoma Requiring Surveillance (n = 53) | ≥1 test ≥2 tests ≥3 tests 4 tests | 44 33 15 3 | 9 20 38 50 | 83% 62.3% 28.3% 5.7% | 38.8% 71.4% 85.9% 94% | 12.2% 18.2% 17.1% 8.8% | 95.7% 94.9% 92.1% 90.7% | 2.5 (1.2–5.3) 3.3 (1.8–6.1) 1.8 (0.9–3.6) 0.7 (0.2–2.5) |
| Diagnosis | hHb | hTf | hCp | hLf | Combination of Four Tests |
|---|---|---|---|---|---|
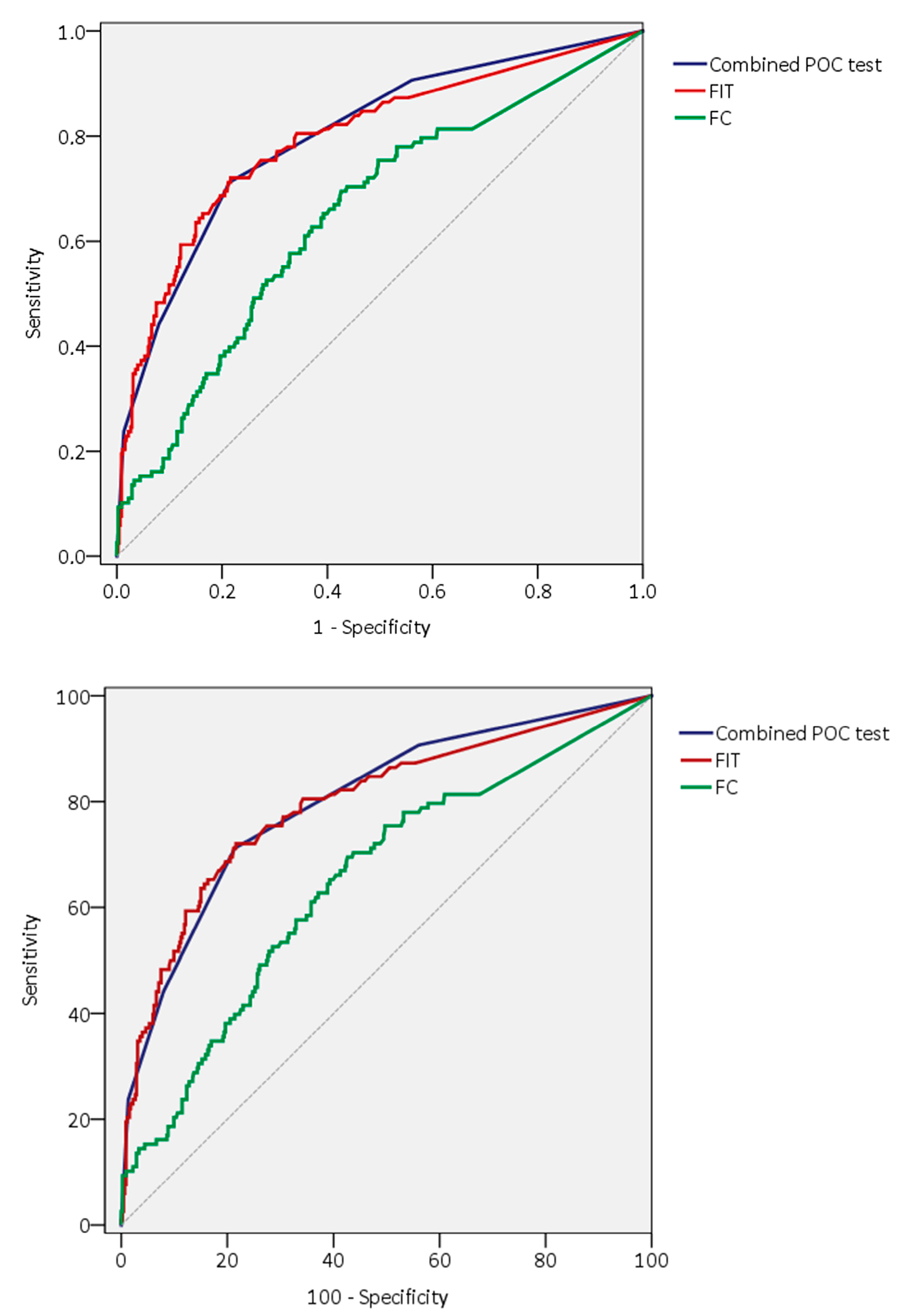
| Significant pathology | 0.772 (0.718–0.825) | 0.652 (0.592–0.712) | 0.656 (0.604–0.707) | 0.635 (0.573–0.696) | 0.801 (0.754–0.848) |
| p value 1 | p = 0.076 | p < 0.01 | p < 0.01 | p < 0.01 | Reference |
| p value 1 | Reference | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.076 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hijos-Mallada, G.; Saura, N.; Lué, A.; Velamazan, R.; Nieto, R.; Navarro, M.; Arechavaleta, S.; Chueca, E.; Gomollon, F.; Lanas, A.; et al. A Point-of-Care Faecal Test Combining Four Biomarkers Allows Avoidance of Normal Colonoscopies and Prioritizes Symptomatic Patients with a High Risk of Colorectal Cancer. Cancers 2023, 15, 721. https://doi.org/10.3390/cancers15030721
Hijos-Mallada G, Saura N, Lué A, Velamazan R, Nieto R, Navarro M, Arechavaleta S, Chueca E, Gomollon F, Lanas A, et al. A Point-of-Care Faecal Test Combining Four Biomarkers Allows Avoidance of Normal Colonoscopies and Prioritizes Symptomatic Patients with a High Risk of Colorectal Cancer. Cancers. 2023; 15(3):721. https://doi.org/10.3390/cancers15030721
Chicago/Turabian StyleHijos-Mallada, Gonzalo, Nuria Saura, Alberto Lué, Raúl Velamazan, Rocío Nieto, Mercedes Navarro, Samantha Arechavaleta, Eduardo Chueca, Fernando Gomollon, Angel Lanas, and et al. 2023. "A Point-of-Care Faecal Test Combining Four Biomarkers Allows Avoidance of Normal Colonoscopies and Prioritizes Symptomatic Patients with a High Risk of Colorectal Cancer" Cancers 15, no. 3: 721. https://doi.org/10.3390/cancers15030721
APA StyleHijos-Mallada, G., Saura, N., Lué, A., Velamazan, R., Nieto, R., Navarro, M., Arechavaleta, S., Chueca, E., Gomollon, F., Lanas, A., & Sostres, C. (2023). A Point-of-Care Faecal Test Combining Four Biomarkers Allows Avoidance of Normal Colonoscopies and Prioritizes Symptomatic Patients with a High Risk of Colorectal Cancer. Cancers, 15(3), 721. https://doi.org/10.3390/cancers15030721







