Effects of Tumor Localization, Age, and Stage on the Outcomes of Gastric and Colorectal Signet Ring Cell Adenocarcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Statistical Analysis
3. Results
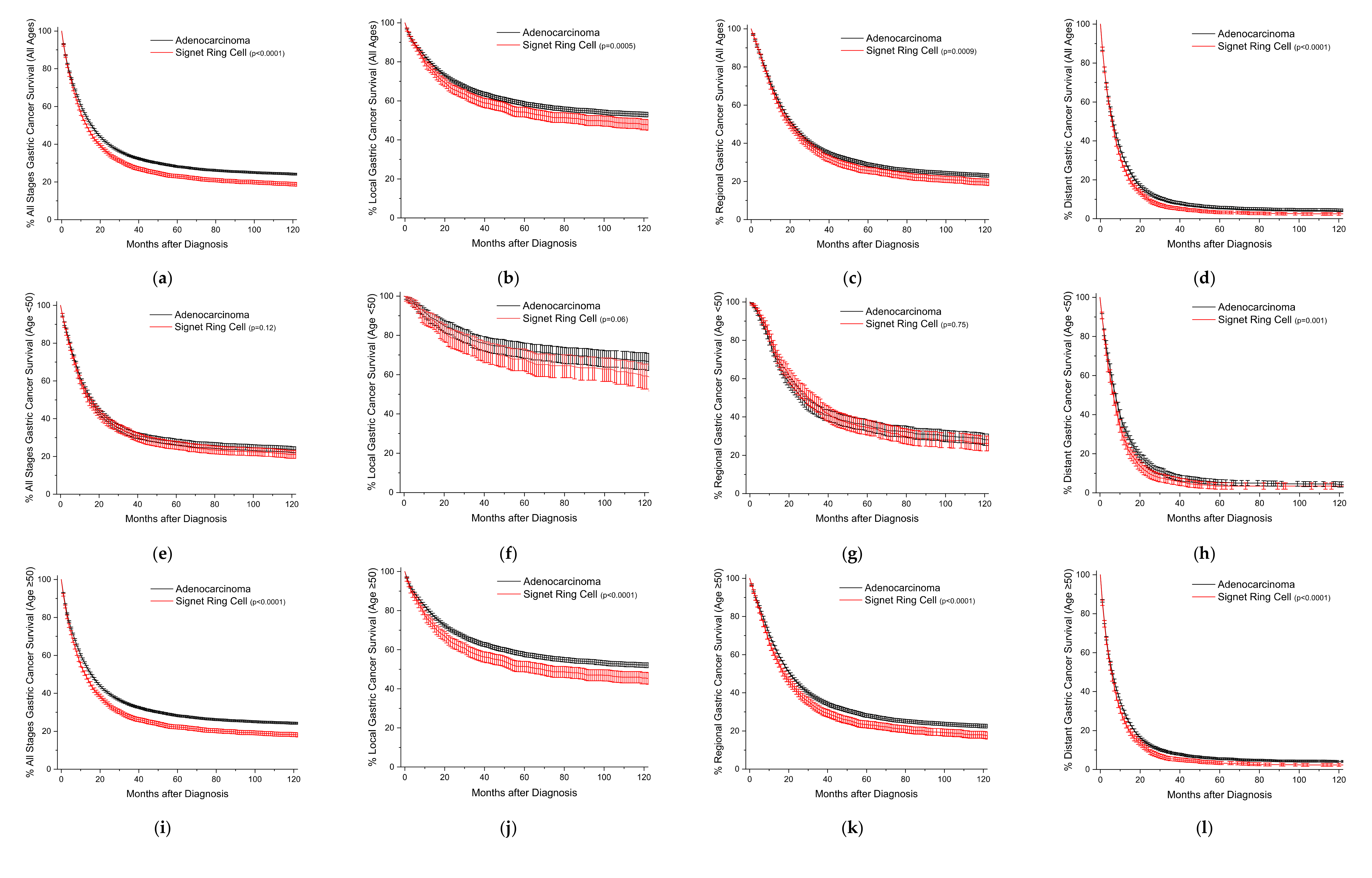
3.1. Frequency, Adjusted Mortality, and Survival Trends for Gastric SRCCs
3.2. Frequency, Adjusted Mortality, and Survival Trends for Colorectal SRCCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; International Agency for Research on Cancer: Lyon, France, 2010. [Google Scholar]
- International Agency for Research on Cancer. WHO Classification of Tumours of the Digestive System, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2019. [Google Scholar]
- Benesch, M.G.K.; Mathieson, A. Epidemiology of signet ring cell adenocarcinomas. Cancers 2020, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.; Nam, K.H.; Choi, S.W.; Hwang, T.S.; Baek, J.H. Comparison of long-term oncologic outcomes between surgical t4 and t3 in patients diagnosed with pathologic stage iia right colon cancer. Front. Oncol. 2022, 12, 931414. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Li, J.; Liu, L.J.; Zheng, N.X.; Zheng, K.; Mei, Z.; Bai, C.G.; Zhang, W. Trends, clinicopathological features, surgical treatment patterns and prognoses of early-onset versus late-onset colorectal cancer: A retrospective cohort study on 34067 patients managed from 2000 to 2021 in a Chinese tertiary center. Int. J. Surg. 2022, 104, 106780. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.G.; Malietzis, G.; Constantinides, V.; Pellino, G.; Tekkis, P.; Kontovounisios, C. Clinicopathological factors and survival outcomes of signet-ring cell and mucinous carcinoma versus adenocarcinoma of the colon and rectum: A systematic review and meta-analysis. Discov. Oncol. 2021, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Meng, Y.; Zhou, X.; Guo, L.; Fu, W. The prognostic significance and gene expression characteristics of gastric signet-ring cell carcinoma: A study based on the seer and tcga databases. Front. Surg. 2022, 9, 819018. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lv, L.; Zheng, K.; Tian, Y.; Zheng, J.-C.; Jiang, C.-G. Prognosis and biological behavior of gastric signet-ring cell carcinoma better or worse: A meta-analysis. Front. Oncol. 2021, 11, 603070. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.H.; Lim, B.J.; Kim, H.; Kim, H.; Park, J.J.; Youn, Y.H.; Park, H.; Noh, S.H.; Kim, J.W.; et al. Sex disparity in gastric cancer: Female sex is a poor prognostic factor for advanced gastric cancer. Ann. Surg. Oncol. 2016, 23, 4344–4351. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, Y.X.; Xu, Q.; Tian, Y.T. Protective effects of female reproductive factors on gastric signet-ring cell carcinoma. World J. Clin. Cases 2022, 10, 5217–5229. [Google Scholar] [CrossRef]
- Kaneko, S.; Tamakoshi, A.; Ohno, Y.; Mizoue, T.; Yoshimura, T. Menstrual and reproductive factors and the mortality risk of gastric cancer in japanese menopausal females. Cancer Causes Control. 2003, 14, 53–59. [Google Scholar] [CrossRef]
- Hu, B.; Zou, R.P.; Gan, Y.W.; Zhu, Y.H.; Ren, S.M.; Hou, W.Z.; Xie, Z.X.; Wang, R.; Yang, W.T.; Lin, P.J.; et al. A population-based predictive model to identify patients with signet ring cell carcinoma of the stomach who are most suitable for primary tumor resection. World J. Surg. Oncol. 2022, 20, 87. [Google Scholar] [CrossRef]
- Taghavi, S.; Jayarajan, S.N.; Davey, A.; Willis, A.I. Prognostic significance of signet ring gastric cancer. J. Clin. Oncol. 2012, 30, 3493–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Shang, M.-Y.; Dong, Z.; Zhang, J.; Wang, Y.; Zheng, Z.-C.; Zhao, Y. Clinicopathological features and prognostic analysis of signet ring cell gastric carcinoma: A population-based study. Transl. Cancer Res. 2019, 8, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Lloyd, S.; Decker, R.H.; Wilson, L.D.; Yu, J.B. Overview of the surveillance, epidemiology, and end results database: Evolution, data variables, and quality assurance. Curr. Probl. Cancer 2012, 36, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Benesch, M.G.K.; Mathieson, A. Epidemiology of mucinous adenocarcinomas. Cancers 2020, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Hu, F.; Li, C.; Yao, Q.; Zhang, H.; Xue, Y. Clinicopathologic characteristics and prognosis of proximal and distal gastric cancer. OncoTargets Ther. 2018, 11, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Chon, H.J.; Hyung, W.J.; Kim, C.; Park, S.; Kim, J.-H.; Park, C.H.; Ahn, J.B.; Kim, H.; Chung, H.C.; Rha, S.Y.; et al. Differential prognostic implications of gastric signet ring cell carcinoma: Stage adjusted analysis from a single high-volume center in asia. Ann. Surg. 2017, 265, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Korphaisarn, K.; Morris, V.; Davis, J.S.; Overman, M.J.; Fogelman, D.R.; Kee, B.K.; Dasari, A.; Raghav, K.P.S.; Shureiqi, I.; Trupti, M.; et al. Signet ring cell colorectal cancer: Genomic insights into a rare subpopulation of colorectal adenocarcinoma. Br. J. Cancer 2019, 121, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, U.; Zimmermann, A.; Späth, C.; Müller, T.; Maak, M.; Schuster, T.; Slotta-Huspenina, J.; Käser, S.A.; Michalski, C.W.; Janssen, K.-P.; et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann. Surg. 2013, 258, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Tajiri, K.; Sudo, T.; Ishi, K.; Kawahara, A.; Nagasu, S.; Shimomura, S.; Yuge, K.; Katagiri, M.; Yomoda, T.; Fujiyoshi, K.; et al. Investigation of clinicopathological characters and gene expression features in colorectal signet-ring cell carcinoma utilizing cms classification. Mol. Clin. Oncol. 2021, 14, 98. [Google Scholar] [CrossRef]
- Liu, X.; Huang, L.; Liu, M.; Wang, Z. The molecular associations of signet-ring cell carcinoma in colorectum: Meta-analysis and system review. Medicina 2022, 58, 836. [Google Scholar] [CrossRef]
- Remo, A.; Fassan, M.; Vanoli, A.; Bonetti, L.R.; Barresi, V.; Tatangelo, F.; Gafà, R.; Giordano, G.; Pancione, M.; Grillo, F.; et al. Morphology and molecular features of rare colorectal carcinoma histotypes. Cancers 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef] [PubMed]
- Corso, G.; Intra, M.; Trentin, C.; Veronesi, P.; Galimberti, V. Cdh1 germline mutations and hereditary lobular breast cancer. Fam. Cancer 2016, 15, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. Cdh1 (e-cadherin) mutation and gastric cancer: Genetics, molecular mechanisms and guidelines for management. Cancer Manag. Res. 2019, 11, 10477–10486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepard, B.; Yoder, L.; Holmes, C. Prophylactic total gastrectomy for hereditary diffuse gastric cancer. ACG Case Rep. J. 2016, 3, e179. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Adar, T.; Patel, D.; Lauwers, G.Y.; Yoon, S.S.; Mullen, J.T.; Chung, D.C. Surveillance endoscopy in the management of hereditary diffuse gastric cancer syndrome. Clin. Gastroenterol. Hepatol. 2019, 19, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Lennerz, J.K.; Chung, D.C.; Patel, D.; Deshpande, V.; Yoon, S.S.; Lauwers, G.Y. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: Biopsy recommendations after topographic distribution of cancer foci in a series of 10 cdh1-mutated gastrectomies. Am. J. Surg. Pathol. 2012, 36, 1709–1717. [Google Scholar] [CrossRef]
- Kumar, S.; Katona, B.W.; Long, J.M.; Domchek, S.; Rustgi, A.K.; Roses, R.; Ginsberg, G.G. Endoscopic ultrasound has limited utility in diagnosis of gastric cancer in carriers of cdh1 mutations. Clin. Gastroenterol. Hepatol. 2020, 18, 505–508.E1. [Google Scholar] [CrossRef]
- Van Dieren, J.M.; Kodach, L.L.; den Hartog, P.; van der Kolk, L.E.; Sikorska, K.; van Velthuysen, M.F.; van Sandick, J.W.; Koemans, W.J.; Snaebjornsson, P.; Cats, A. Gastroscopic surveillance with targeted biopsies compared with random biopsies in cdh1 mutation carriers. Endoscopy 2020, 52, 839–846. [Google Scholar] [CrossRef]
- Castro, R.; Lobo, J.; Pita, I.; Videira, F.; Pedro-Afonso, L.; Dinis-Ribeiro, M.; Brandão, C. Random biopsies in patients harboring a cdh1 mutation: Time to change the approach? Rev. Esp. Enferm. Dig. 2020, 112, 367–372. [Google Scholar] [CrossRef]
- Mi, E.Z.; Mi, E.Z.; di Pietro, M.; O’Donovan, M.; Hardwick, R.H.; Richardson, S.; Ziauddeen, H.; Fletcher, P.C.; Caldas, C.; Tischkowitz, M.; et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to cdh1 mutation status. Gastrointest. Endosc. 2018, 87, 408–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benesch, M.G.K.; Bursey, S.R.; O’Connell, A.C.; Ryan, M.G.; Howard, C.L.; Stockley, C.C.; Mathieson, A. Cdh1 gene mutation hereditary diffuse gastric cancer outcomes: Analysis of a large cohort, systematic review of endoscopic surveillance, and secondary cancer risk postulation. Cancers 2021, 13, 2622. [Google Scholar] [CrossRef] [PubMed]
- Adib, E.; El Zarif, T.; Nassar, A.H.; Akl, E.W.; Abou Alaiwi, S.; Mouhieddine, T.H.; Esplin, E.D.; Hatchell, K.; Nielsen, S.M.; Rana, H.Q.; et al. Cdh1 germline variants are enriched in patients with colorectal cancer, gastric cancer, and breast cancer. Br. J. Cancer 2022, 126, 797–803. [Google Scholar] [CrossRef] [PubMed]

| Gastric Location | Adenocarcinoma | Signet Ring Cell |
|---|---|---|
| All Sites | 44,239 (100) | 9972 (100) |
| Proximal | 29,519 (66.7) | 5486 (55.0) |
| Cardia | 21,828 (49.3) | 2934 (29.4) |
| Fundus | 2454 (5.5) | 549 (5.5) |
| Body | 5237 (11.8) | 2003 (20.1) |
| Distal | 14,720 (33.3) | 4486 (45.0) |
| Antrum | 12,783 (28.9) | 3927 (39.4) |
| Pylorus | 1937 (4.4) | 559 (5.6) |
| Gastric Location | Adenocarcinoma | Signet Ring Cell | ||||||
|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | ||||
| Age (Years) | <50 | ≥50 | <50 | ≥50 | <50 | ≥50 | <50 | ≥50 |
| All Sites | ||||||||
| N (%) | 2782 [9.2] | 27,380 [90.8] | 1086 [7.7] | 12,991 [92.3] | 1031 [18.8] | 4466 [81.2] | 984 [22.0] | 3491 [78.0] |
| (100) | (100) | (100) | (100) | (100) | (100) | (100) | (100) | |
| Stage (%) | ||||||||
| In Situ | 9 (0.3) | 214 (0.8) | 5 (0.5) | 120 (0.9) | 0 (0) | 1 (<0.1) | 0 (0) | 2 (<0.1) |
| Localized | 408 (14.7) | 6711 (24.5) | 181 (16.7) | 3533 (27.2) | 146 (14.2) | 892 (20.0) | 171 (17.4) | 786 (22.5) |
| Regional | 899 (32.3) | 8308 (30.3) | 367 (33.8) | 3622 (27.9) | 360 (34.9) | 1585 (35.5) | 309 (31.4) | 1085 (31.1) |
| Distant | 1354 (48.7) | 9842 (35.9) | 501 (46.1) | 4033 (31.0) | 480 (46.6) | 1638 (36.7) | 477 (48.5) | 1254 (35.9) |
| Unstaged | 114 (4.1) | 2305 (8.4) | 32 (2.9) | 1683 (13.0) | 4 (4.4) | 350 (7.8) | 27 (2.7) | 364 (10.4) |
| Incidence | 4.12 (3.95–4.29) | 135 (133–137) | 1.55 (1.45–1.66) | 44.5 (43.7–45.3) | 1.83 (1.72–1.95) | 25.0 (24.3–25.7) | 1.82 (1.71–1.94) | 15.9 (15.5–16.4) |
| Proximal | ||||||||
| N (%) | 2118 (76.1) | 19,806 (72.3) | 613 (56.4) | 6982 (53.7) | 564 (54.7) | 2738 (61.3) | 480 (48.8) | 1707 (48.8) |
| Stage (%) | ||||||||
| In Situ | 6 (0.3) | 128 (0.6) | 2 (0.3) | 60 (0.9) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) |
| Localized | 290 (13.7) | 4697 (13.7) | 102 (16.6) | 1798 (25.8) | 71 (12.6) | 517 (18.9) | 70 (14.6) | 357 (21.0) |
| Regional | 637 (30.1) | 5684 (28.7) | 184 (30.0) | 1699 (24.3) | 178 (31.6) | 913 (33.3) | 125 (26.0) | 444 (26.1) |
| Distant | 1090 (51.5) | 7616 (38.5) | 310 (50.6) | 2493 (35.7) | 290 (51.4) | 1094 (40.0) | 269 (56.0) | 708 (41.5) |
| Unstaged | 95 (4.5) | 1681 (8.5) | 15 (2.4) | 932 (13.3) | 25 (4.4) | 214 (7.8) | 16 (3.3) | 194 (11.4) |
| Incidence | 3.35 (3.20–3.50) | 104 (103–106) | 0.98 (0.90–1.07) | 27.0 (26.4–27.6) | 1.02 (0.93–1.10) | 15.6 (15.1–16.2) | 0.94 (0.86–1.03) | 8.0 (7.7–8.3) |
| Distal | ||||||||
| N (%) | 664 (23.9) | 7574 (27.7) | 473 (43.6) | 6009 (46.3) | 467 (45.3) | 1728 (38.7) | 504 (51.2) | 1787 (51.2) |
| Stage (%) | ||||||||
| In Situ | 1 (0.2) | 86 (1.1) | 3 (0.6) | 60 (1.0) | 0 (0) | 1 (0.1) | 0 (0) | 1 (0.1) |
| Localized | 118 (178) | 2014 (26.6) | 79 (16.7) | 1735 (28.9) | 75 (16.1) | 375 (21.7) | 101 (20.0) | 429 (24.0) |
| Regional | 262 (39.5) | 2624 (34.6) | 183 (38.7) | 1923 (32.0) | 182 (39.0) | 672 (38.9) | 184 (36.5) | 641 (35.9) |
| Distant | 264 (39.8) | 2226 (29.4) | 191 (40.4) | 1540 (25.6) | 190 (40.7) | 544 (31.5) | 208 (41.3) | 546 (30.6) |
| Unstaged | 19 (2.9) | 624 (8.2) | 17 (3.6) | 751 (12.5) | 20 (4.3) | 136 (7.9) | 11 (2.2) | 170 (9.5) |
| Incidence | 0.77 (0.70–0.85) | 30.9 (30.1–31.7) | 0.56 (0.50–0.63) | 17.5 (17.0–18.0) | 0.82 (0.74–0.90) | 9.4 (9.0–9.9) | 0.89 (0.80–0.96) | 8.0 (7.6–8.3) |
| Gastric Location | Signet Ring Cell vs. Adenocarcinoma (All Ages) | Signet Ring Cell vs. Adenocarcinoma (Age < 50) | Signet Ring Cell vs. Adenocarcinoma (Age ≥ 50) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable |
| All Sites | 1.14 (1.11–1.18) | 1.11 (1.08–1.15) | 1.05 (0.99–1.12) | 1.05 (0.98–1.13) | 1.17 (1.14–1.21) | 1.12 (1.08–1.15) |
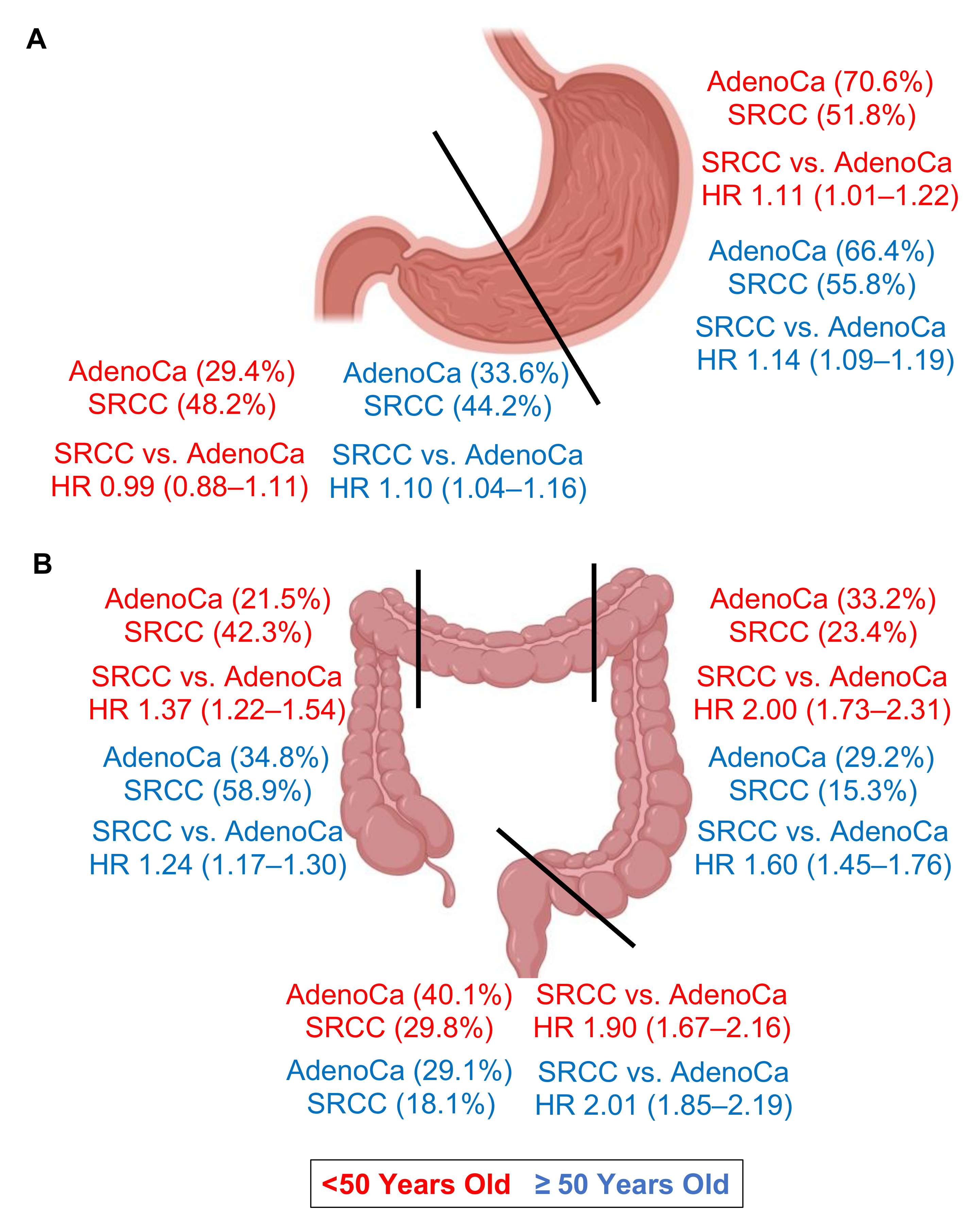
| Proximal Cardia Fundus Body | 1.21 (1.17–1.25) 1.30 (1.24–1.36) 1.04 (0.93–1.17) 1.14 (1.07–1.22) | 1.14 (1.10–1.18) 1.20 (1.14–1.26) 1.07 (0.95–1.20) 1.03 (0.96–1.11) | 1.17 (1.08–1.28) 1.21 (1.07–1.36) 1.24 (0.96–1.61) 1.08 (0.92–1.27) | 1.11 (1.01–1.22) 1.10 (0.97–1.25) 1.52 (1.12–2.06) 0.99 (0.83–1.17) | 1.23 (1.18–1.27) 1.33 (1.27–1.40) 1.01 (0.89–1.15) 1.16 (1.07–1.24) | 1.14 (1.09–1.19) 1.21 (1.15–1.28) 1.01 (0.89–1.16) 1.04 (0.96–1.13) |
| Distal Antrum Pylorus | 1.14 (1.09–1.19) 1.17 (1.12–1.22) 0.98 (0.87–1.11) | 1.08 (1.03–1.13) 1.09 (1.04–1.15) 0.99 (0.87–1.13) | 1.03 (0.92–1.14) 1.01 (0.90–1.14) 1.10 (0.82–1.48) | 0.99 (0.88–1.11) 0.99 (0.88–1.12) 0.87 (0.64–1.19) | 1.18 (1.12–1.24) 1.21 (1.15–1.28) 0.98 (0.85–1.12) | 1.10 (1.04–1.16) 1.11 (1.05–1.18) 0.98 (0.85–1.15) |
| Colorectal Location | Adenocarcinoma | Signet Ring Cell |
|---|---|---|
| All Sites | 393,879 (100) | 6291 (100) |
| Right Colon | 131,482 (33.4) | 3481 (55.3) |
| Appendix | 1761 (0.4) | 669 (10.6) |
| Cecum | 62,888 (16.0) | 1480 (23.5) |
| Ascending Colon | 51,752 (13.1) | 1017 (16.2) |
| Hepatic Flexure | 15,081 (3.8) | 315 (5.0) |
| Transverse Colon | 26,492 (6.7) | 442 (7.0) |
| Left | 116,632 (29.6) | 1073 (17.1) |
| Splenic Flexure | 10,985 (2.8) | 134 (2.1) |
| Descending Colon | 18,161 (4.6) | 204 (3.2) |
| Sigmoid Colon | 87,486 (22.2) | 735 (11.7) |
| Rectal | 119,273 (30.3) | 1295 (20.6) |
| Rectosigmoid | 37,657 (9.6) | 327 (5.2) |
| Rectum | 81,616 (20.7) | 968 (15.4) |
| Colorectal Location | Adenocarcinoma | Signet Ring Cell | ||||||
|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Male | Female | ||||
| Age (Years) | <50 | ≥50 | <50 | ≥50 | <50 | ≥50 | <50 | ≥50 |
| All Sites | ||||||||
| N (%) | 22,579 [11.1] | 181,541 [88.9] | 19,470 [10.3] | 170,289 [89.7] | 764 [23.7] | 2464 [76.3] | 585 [19.1] | 2478 [80.9] |
| (100) | (100) | (100) | (100) | (100) | (100) | (100) | (100) | |
| Stage | ||||||||
| In Situ | 101 (0.4) | 1292 (0.7) | 95 (0.5) | 1091 (0.6) | 0 (0) | 3 (0.1) | 0 (0) | 2 (0.1) |
| Localized | 5263 (23.3) | 58,867 (32.4) | 4618 (23.7) | 55,919 (32.8) | 64 (8.4) | 316 (12.8) | 35 (6.0) | 368 (14.9) |
| Regional | 10,294 (45.6) | 75,339 (41.5) | 8895 (45.7) | 72,384 (42.5) | 342 (44.8) | 1158 (47.0) | 231 (39.5) | 1086 (43.8) |
| Distant | 6410 (28.4) | 39,790 (21.9) | 5545 (28.5) | 34,201 (20.1) | 341 (44.6) | 926 (37.6) | 310 (53.0) | 944 (38.1) |
| Unstaged | 511 (2.3) | 6253 (3.4) | 317 (1.6) | 6694 (3.9) | 17 (2.2) | 61 (2.5) | 9 (1.5) | 78 (3.1) |
| Incidence | 4.43 (4.38–4.49) | 104.8 (104.4–105.3) | 3.80 (3.75–3.85) | 75.0 (74.7–75.3) | 1.38 (1.29–1.49) | 15.0 (14.5–15.6) | 1.08 (1.00–1.17) | 11.5 (11.1–12.0) |
| Right | ||||||||
| N (%) | 4780 (21.2) | 54,707 (30.1) | 4171 (21.4) | 67,824 (39.8) | 281 (36.8) | 1297 (52.6) | 289 (49.4) | 1614 (65.1) |
| Stage | ||||||||
| In Situ | 23 (0.5) | 401 (0.7) | 19 (0.5) | 417 (0.6) | 0 (0) | 1 (0.1) | 0 (0) | 1 (0.1) |
| Localized | 1188 (24.9) | 18,183 (33.2) | 1010 (24.2) | 23,069 (34.0) | 26 (9.3) | 163 (12.6) | 20 (6.9) | 247 (15.3) |
| Regional | 2206 (46.2) | 22,580 (41.3) | 1787 (42.8) | 28,555 (42.1) | 113 (40.2) | 603 (46.5) | 103 (35.6) | 702 (43.5) |
| Distant | 1310 (27.4) | 12,093 (22.1) | 1308 (31.4) | 13,618 (20.1) | 138 (49.1) | 511 (39.4) | 163 (56.4) | 633 (39.2) |
| Unstaged | 53 (1.1) | 1450 (2.7) | 47 (1.1) | 2165 (3.2) | 4 (1.4) | 19 (1.5) | 3 (1.0) | 31 (1.9) |
| Incidence | 0.92 (0.90–0.95) | 34.0 (33.7–34.2) | 0.80 (0.78–0.82) | 30.8 (30.6–31.0) | 0.52 (0.46–0.59) | 7.82 (7.44–8.23) | 0.55 (0.49–0.62) | 7.34 (7.03–7.70) |
| Transverse | ||||||||
| N (%) | 1188 (5.3) | 11,318 (6.2) | 1062 (5.5) | 12,924 (7.6) | 32 (4.2) | 158 (6.4) | 28 (4.8) | 224 (9.0) |
| Stage | ||||||||
| In Situ | 2 (0.2) | 64 (0.6) | 6 (0.6) | 64 (0.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Localized | 285 (24.0) | 3750 (33.1) | 244 (23.0) | 4224 (32.7) | 2 (6.2) | 17 (10.8) | 2 (7.1) | 45 (20.1) |
| Regional | 588 (49.5) | 4983 (44.0) | 475 (44.7) | 5899 (45.6) | 18 (56.2) | 89 (56.3) | 16 (57.1) | 112 (50.0) |
| Distant | 297 (25.0) | 2242 (19.8) | 327 (30.8) | 2360 (18.3) | 12 (37.5) | 47 (29.7) | 10 (35.7) | 56 (25.0) |
| Unstaged | 16 (1.3) | 279 (2.5) | 10 (0.9) | 10 (0.9) | 0 (0) | 5 (3.2) | 0 (0) | 11 (4.9) |
| Incidence | 0.24 (0.23–0.25) | 7.48 (7.36–7.60) | 0.21 (0.20–0.22) | 6.13 (6.03–6.22) | 0.07 (0.05–0.10) | 1.04 (0.90–1.20) | 0.06 (0.04–0.08) | 1.17 (1.04–1.31) |
| Left | ||||||||
| N (%) | 6760 (29.9) | 55,415 (30.5) | 7205 (37.0) | 47,252 (27.7) | 190 (24.9) | 425 (17.2) | 127 (21.7) | 331 (13.4) |
| Stage | ||||||||
| In Situ | 39 (0.6) | 453 (0.8) | 36 (0.5) | 325 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Localized | 1437 (21.3) | 17,279 (31.2) | 1593 (22.1) | 14,719 (31.2) | 16 (8.4) | 51 (12.0) | 1 (0.8) | 37 (11.2) |
| Regional | 2922 (43.2) | 22,701 (41.0) | 3250 (45.1) | 20,191 (42.7) | 72 (37.9) | 169 (39.8) | 48 (37.8) | 129 (39.0) |
| Distant | 2262 (33.5) | 13,432 (24.2) | 2261 (31.4) | 10,464 (22.1) | 99 (52.1) | 196 (46.1) | 77 (60.6) | 152 (45.9) |
| Unstaged | 100 (1.5) | 1550 (2.8) | 65 (0.9) | 1553 (3.3) | 3 (1.6) | 9 (2.1) | 1 (0.8) | 12 (3.9) |
| Incidence | 1.33 (1.30–1.36) | 30.4 (30.2–30.6) | 1.41 (1.38–1.44) | 19.9 (19.7–20.0) | 0.35 (0.30–0.40) | 2.61 (2.40–2.85) | 0.22 (0.18–0.26) | 1.55 (1.40–1.71) |
| Rectal | ||||||||
| N (%) | 9851 (43.6) | 60,101 (33.1) | 7032 (36.1) | 42,289 (24.8) | 261 (34.2) | 584 (23.7) | 141 (24.1) | 309 (12.5) |
| Stage | ||||||||
| In Situ | 37 (0.4) | 374 (0.6) | 34 (0.5) | 285 (0.7) | 0 (0) | 2 (0.3) | 0 (0) | 1 (0.3) |
| Localized | 2353 (23.9) | 19,655 (32.7) | 1771 (25.2) | 13,907 (32.9) | 20 (7.7) | 85 (14.6) | 12 (8.5) | 39 (12.6) |
| Regional | 4578 (46.5) | 25,075 (41.7) | 3383 (48.1) | 17,739 (41.9) | 139 (53.3) | 297 (50.9) | 64 (45.4) | 143 (46.3) |
| Distant | 2541 (25.8) | 12,023 (20.0) | 1649 (23.4) | 7759 (18.3) | 92 (35.2) | 172 (29.5) | 60 (42.6) | 103 (33.3) |
| Unstaged | 342 (3.5) | 2974 (4.9) | 195 (2.8) | 2599 (6.1) | 10 (3.8) | 28 (4.8) | 5 (3.5) | 23 (7.4) |
| Incidence | 1.94 (1.90–1.98) | 32.9 (32.7–33.2) | 1.39 (1.36–1.42) | 18.2 (18.1–18.4) | 0.44 (0.39–0.50) | 3.52 (3.27–3.80) | 0.25 (0.21–0.30) | 1.46 (1.32–1.62) |
| Colorectal Location | Signet Ring Cell vs. Adenocarcinoma (All Ages) | Signet Ring Cell vs. Adenocarcinoma (Age < 50) | Signet Ring Cell vs. Adenocarcinoma (Age ≥ 50) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | Univariate | Multivariable | Univariate | Multivariable | Univariate | Multivariable |
| All Sites | 2.39 (2.31–2.47) | 1.55 (1.50–1.60) | 2.99 (2.79–3.20) | 1.78 (1.66–1.91) | 2.30 (2.21–2.39) | 1.46 (1.40–1.52) |
| Transverse Colon | 2.01 (1.75–2.30) | 1.38 (1.20–1.58) | 2.47 (1.78–3.43) | 1.90 (1.34–2.70) | 1.94 (1.67–2.25) | 1.29 (1.11–1.50) |
| Right Colon | 2.17 (2.07–2.27) | 1.28 (1.22–1.34) | 2.66 (2.39–2.96) | 1.37 (1.22–1.54) | 2.10 (1.99–2.21) | 1.24 (1.17–1.30) |
| Appendix | 2.00 (1.77–2.26) | 0.95 (0.82–1.09) | 2.47 (1.95–3.12) | 0.99 (0.74–1.30) | 1.85 (1.60–2.14) | 0.91 (0.77–1.08) |
| Cecum | 2.28 (2.13–2.44) | 1.35 (1.26–1.45) | 2.80 (2.34–3.34) | 1.45 (1.20–1.75) | 2.24 (2.08–2.40) | 1.33 (1.29–1.43) |
| Ascending Colon | 1.85 (1.69–2.03) | 1.24 (1.12–1.36) | 2.34 (1.84–2.98) | 1.54 (1.20–1.99) | 1.79 (1.62–1.99) | 1.19 (1.07–1.31) |
| Hepatic Flexure | 2.23 (1.91–2.61) | 1.83 (1.56–2.15) | 2.40 (1.72–3.35) | 1.81 (1.25–2.63) | 2.18 (1.83–2.60) | 1.73 (1.44–2.07) |
| Left | 2.88 (2.67–3.11) | 1.78 (1.64–1.92) | 3.42 (2.98–3.92) | 2.00 (1.73–2.31) | 2.75 (2.51–3.03) | 1.60 (1.45–1.76) |
| Splenic Flexure | 1.86 (1.46–2.36) | 1.45 (1.14–1.86) | 2.04 (1.33–3.13) | 1.25 (0.79–1.98) | 1.89 (1.41–2.52) | 1.36 (1.01–1.82) |
| Descending Colon | 2.88 (2.42–3.42) | 1.84 (1.54–2.20) | 3.97 (3.01–5.24) | 2.05 (1.52–2.78) | 2.55 (2.03–3.20) | 1.56 (1.24–1.97) |
| Sigmoid Colon | 3.12 (2.84–3.42) | 1.82 (1.65–2.00) | 3.67 (3.10–4.35) | 2.11 (1.78–2.53) | 2.99 (2.68–3.34) | 1.66 (1.49–1.86) |
| Rectal | 2.70 (2.53–2.90) | 2.10 (1.96–2.25) | 3.10 (2.74–3.50) | 1.90 (1.67–2.16) | 2.73 (2.51–2.96) | 2.01 (1.85–2.19) |
| Rectosigmoid | 2.98 (2.60–3.40) | 2.02 (1.77–2.32) | 3.32 (2.58–4.28) | 1.70 (1.30–2.21) | 2.96 (2.53–3.47) | 1.98 (1.69–2.33) |
| Rectum | 2.61 (2.41–2.82) | 2.11 (1.94–2.27) | 3.05 (2.65–3.51) | 1.95 (1.68–2.27) | 2.63 (2.39–2.90) | 2.00 (1.81–2.20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benesch, M.G.K.; Mathieson, A.; O’Brien, S.B.L. Effects of Tumor Localization, Age, and Stage on the Outcomes of Gastric and Colorectal Signet Ring Cell Adenocarcinomas. Cancers 2023, 15, 714. https://doi.org/10.3390/cancers15030714
Benesch MGK, Mathieson A, O’Brien SBL. Effects of Tumor Localization, Age, and Stage on the Outcomes of Gastric and Colorectal Signet Ring Cell Adenocarcinomas. Cancers. 2023; 15(3):714. https://doi.org/10.3390/cancers15030714
Chicago/Turabian StyleBenesch, Matthew G. K., Alexander Mathieson, and Shalana B. L. O’Brien. 2023. "Effects of Tumor Localization, Age, and Stage on the Outcomes of Gastric and Colorectal Signet Ring Cell Adenocarcinomas" Cancers 15, no. 3: 714. https://doi.org/10.3390/cancers15030714
APA StyleBenesch, M. G. K., Mathieson, A., & O’Brien, S. B. L. (2023). Effects of Tumor Localization, Age, and Stage on the Outcomes of Gastric and Colorectal Signet Ring Cell Adenocarcinomas. Cancers, 15(3), 714. https://doi.org/10.3390/cancers15030714







