Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials
Abstract
Simple Summary
Abstract
1. Introduction
2. From Botanical Foods to Anticancer Therapeutics: The Science beyond the Myth
3. Designing the Preclinical Study: Consideration of the Regulatory, Scientific, and Clinical Settings Prior to Human Studies
3.1. Establishment of a Mechanism-Based and Clinically Relevant In Vitro Bioassay
3.2. Selection of Clinically Representative Preclinical Models: From Wet (In Vitro, Ex Vivo, and In Vivo) to Dry (In Silico)
3.2.1. In Vitro Models: Choose and Use the Cancer Cell Lines Wisely
3.2.2. Ex Vivo Models: Patient-Derived Organoids and Organs on a Chip
3.2.3. In Vivo Models: The Bridge between Cells and Human
3.2.4. In Silico Models: Multi-Omics and Systems Biology Approaches Reinforce Anticancer Foods Research
4. Critical Considerations in Clinical Studies under IND Regulations
4.1. The “Totality-of-the-Evidence” Approach for Reliable Consistency of IND
4.2. Choosing the Appropriate Patient Population
4.3. Critical Safety Concerns in Early-Phase Clinical Studies
4.4. Diversity of Research Participants and Their Dietary Background
4.5. The Challenges of Using a Placebo in Botanical Foods Intervention Studies
5. Case Study: Development of White Button Mushroom as Anticancer Botanical Medicine at the City of Hope
5.1. “Prior Human Experience” of Mushroom Products as An Anticancer Medicine
5.2. Clinically Relevant In Vitro Bioassay Established in Our Lab
5.3. Various Preclinical Models and MultiTarget Profiling Approaches Applied in Our Study
5.4. The “Totality-of-the-Evidence” Approach for WBM Product as IND
5.5. Designed and Conducted Clinical Interventional Studies on Human
6. Discussion and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tannock, I.F. Conventional cancer therapy: Promise broken or promise delayed? Lancet 1998, 351 (Suppl. 2), SII9–SII16. [Google Scholar] [CrossRef]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef]
- Chakraborty, S.; Hosen, M.I.; Ahmed, M.; Shekhar, H.U. Onco-Multi-OMICS Approach: A New Frontier in Cancer Research. Biomed. Res Int. 2018, 2018, 9836256. [Google Scholar] [CrossRef]
- Benz, E.J.J. The Jeremiah Metzger Lecture Cancer in the Twenty-First Century: An Inside View from an Outsider. Trans. Am. Clin. Climatol. Assoc. 2017, 128, 275–297. [Google Scholar] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.L.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.V.; Papneja, N.; Miller, W.H.J. Review of cancer immunotherapy: From the past to the present, to the future. Curr. Oncol. 2020, 27 (Suppl. 2), S87–S97. [Google Scholar] [CrossRef]
- Keefe, D.M.K.; Bateman, E.H. Potential Successes and Challenges of Targeted Cancer Therapies. J. Natl. Cancer Inst. Monogr. 2019, 53, lgz008. [Google Scholar] [CrossRef]
- Tan, S.; Li, D.; Zhu, X. Cancer immunotherapy: Pros, cons and beyond. Biomed. Pharmacother. 2020, 124, 109821. [Google Scholar] [CrossRef]
- Kuperstein, I.; Bonnet, E.; Nguyen, H.-A.; Cohen, D.; Viara, E.; Grieco, L.; Fourquet, S.; Calzone, L.; Russo, C.; Kondratova, M.; et al. Atlas of Cancer Signalling Network: A systems biology resource for integrative analysis of cancer data with Google Maps. Oncogenesis 2015, 4, e160. [Google Scholar] [CrossRef] [PubMed]
- Vanneman, M.; Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 2012, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Gilad, Y.; Gellerman, G.; Lonard, D.M.; O’Malley, B.W. Drug Combination in Cancer Treatment-From Cocktails to Conjugated Combinations. Cancers 2021, 13, 669. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Dow, M.; Carlin, D.E.; Bejar Carter, H. The Emerging Potential for Network Analysis to Inform Precision Cancer Medicine. J. Mol. Biol. 2018, 430, 2875–2899. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Bourne, P.E. Developing multi-target therapeutics to fine-tune the evolutionary dynamics of the cancer ecosystem. Front. Pharmacol. 2015, 6, 209. [Google Scholar] [CrossRef]
- Petrelli, A.; Giordano, S. From single- to multi-target drugs in cancer therapy: When aspecificity becomes an advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar] [CrossRef]
- Shi, D.; Khan, F.; Abagyan, R. Extended Multitarget Pharmacology of Anticancer Drugs. J. Chem. Inf. Model. 2019, 59, 3006–3017. [Google Scholar] [CrossRef]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural Product Target Network Reveals Potential for Cancer Combination Therapies. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The Multitarget Activity of Natural Extracts on Cancer: Synergy and Xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Usmani, D.; Tarique, M.; Naz, H.; Ashraf, M.; Raliya, R.; Tabrez, S.; Zughaibi, T.A.; Alsaieedi, A.; Hakeem, I.J.; et al. The Role of Natural Products and Their Multitargeted Approach to Treat Solid Cancer. Cells 2022, 11, 2209. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Salamanca-Fernández, E.; Garcia-Villanova, B.; Sánchez, M.J. The Impact of Plant-Based Dietary Patterns on Cancer-Related Outcomes: A Rapid Review and Meta-Analysis. Nutrients 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; George, J. Combinatorial strategies employing nutraceuticals for cancer development. Ann. N. Y. Acad. Sci. 2011, 1229, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Lefranc, F.; Tabanca, N.; Kiss, R. Assessing the anticancer effects associated with food products and/or nutraceuticals using in vitro and in vivo preclinical development-related pharmacological tests. Semin. Cancer Biol. 2017, 46, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Abuduhier, F.M.; Mustafa, S.K.; Usmani, S. Diet-derived small molecules (nutraceuticals) inhibit cellular proliferation by interfering with key oncogenic pathways: An overview of experimental evidence in cancer chemoprevention. Biol. Futur. 2022, 73, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, M.; Kapulnik, Y.; Koltai, H. Plant derived substances with anticancer activity: From folklore to practice. Front. Plant Sci. 2015, 6, 799. [Google Scholar] [CrossRef]
- Wu, C.; Lee, S.-L.; Taylor, C.; Li, J.; Chan, Y.-M.; Agarwal, R.; Temple, R.; Throckmorton, D.; Tyner, K. Scientific and Regulatory Approach to Botanical Drug Development: A U.S. FDA Perspective. J. Nat. Prod. 2020, 83, 552–562. [Google Scholar] [CrossRef]
- Shipkowski, K.A.; Betz, J.M.; Birnbaum, L.S.; Bucher, J.R.; Coates, P.M.; Hopp, D.C.; MacKay, D.; Oketch-Rabah, H.; Walker, N.J.; Welch, C.; et al. Naturally complex: Perspectives and challenges associated with Botanical Dietary Supplement Safety assessment. Food Chem. Toxicol. 2018, 118, 963–971. [Google Scholar] [CrossRef]
- Botanical Drug Development Guidance for Industry. Docket Number: FDA-2000-D-0103. Issued by: Center for Drug Evaluation and Research. 2016. Available online: https://www.FDA.gov/regulatory-information/search-FDA-guidance-documents/botanical-drug-development-guidance-industry (accessed on 13 January 2023).
- Sorkin, B.C.; Kuszak, A.J.; Bloss, G.; Fukagawa, N.K.; Hoffman, F.A.; Jafari, M.; Barrett, B.; Brown, P.N.; Bushman, F.D.; Casper, S.J.; et al. Improving natural product research translation: From source to clinical trial. FASEB J. 2019, 34, 41–65. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Wholegrains, Vegetables and Fruit and the Risk of Cancer. Available online: Dietandcancerreport.org (accessed on 13 January 2023).
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, N.M. Functional foods and their role in cancer prevention and health promotion: A comprehensive review. Am. J. Cancer Res. 2017, 7, 740–769. [Google Scholar] [PubMed]
- Bishayee, A.; Sethi, G. Special Issue: Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin. Cancer Biol. 2016, 40–41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M.; Nabavi, S.M. Special issue: Role of dietary pattern, foods, nutrients and nutraceuticals in supporting cancer prevention and treatment. Semin. Cancer Biol. 2017, 46, iv. [Google Scholar] [CrossRef]
- Farràs, M.; Escolà-Gil, J.C. Special issue: Diet, lipids, and cancer: From Pathogenic mechanisms to potential therapeutic strategies. Semin. Cancer Biol. 2021, 73, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.M.; Nabavi, S.M.; Silva, A.S. Nutraceuticals and Cancer Signaling. In Clinical Aspects and Mode of Action; Springer: Cham, Switzerland, 2021; Volume 9, p. 652. [Google Scholar] [CrossRef]
- Tasaki, K.; Maskarinec, G.; Shumay, D.M.; Tatsumura, Y.; Kakai, H. Communication between physicians and cancer patients about complementary and alternative medicine: Exploring patients’ perspectives. Psychooncology 2002, 11, 212–220. [Google Scholar] [CrossRef]
- Söllner, W.; Maislinger, S.; Kappauf, H. How to improve communication between physicians and cancer patients about use of complementary and alternative medicine? Psychooncology 2003, 12, 203–204. [Google Scholar] [CrossRef]
- Ben-Arye, E.; Frenkel, M.; Margalit, R.S. Approaching complementary and alternative medicine use in patients with cancer: Questions and challenges. J. Ambul. Care Manag. 2004, 27, 53–62. [Google Scholar] [CrossRef]
- PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. “Cancer Therapy Interactions with Foods and Dietary Supplements (PDQ®): Health Professional Version” PDQ Cancer Information Summaries, National Cancer Institute (US), 26 May 2022. Available online: https://www.cancer.gov/about-cancer/treatment/cam/hp/dietary-interactions-pdq (accessed on 13 January 2023).
- Integrative Medicine Service “About Herbs, Botanical & Other Products. Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/cancer-care/diagnosis-treatment/symptom-management/integrative-medicine/herbs/herbs-botanicals-other-products-faqs#dietary-supplements-during-cancer-treatment (accessed on 13 January 2023).
- Foods and Herbs in the Natural Medicines Comprehensive Database. Available online: https://naturalmedicines.therapeuticresearch.com/ (accessed on 13 January 2023).
- Vardell, E. Natural Medicines: A Complementary and Alternative Medicines Tool Combining Natural Standard and the Natural Medicines Comprehensive Database. Med. Ref. Serv. Q. 2015, 34, 461–470. [Google Scholar] [CrossRef]
- Clauson, K.A.; Polen, H.H.; Peak, A.S.; Marsh, W.A.; DiScala, S.L. Clinical decision support tools: Personal digital assistant versus online dietary supplement databases. Ann. Pharmacother. 2008, 42, 1592–1599. [Google Scholar] [CrossRef]
- NCI Drug Dictionary: National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug (accessed on 13 January 2023).
- FoodData Central U.S. Department of Agriculture. Available online: https://fdc.nal.usda.gov/ (accessed on 13 January 2023).
- American Institute for Cancer Research. AICR’s Foods that Fight Cancer and Foods to Steer Clear Of, Explained. Available online: https://www.aicr.org/cancer-prevention/food-facts/ (accessed on 13 January 2023).
- Ng, K.W.; Cao, Z.J.; Chen, H.B.; Zhao, Z.Z.; Zhu, L.; Yi, T. Oolong tea: A critical review of processing methods, chemical composition, health effects, and risk. Crit. Rev. Food Sci. Nutr. 2018, 58, 2957–2980. [Google Scholar] [CrossRef]
- Bai, X.; Li, S.; Liu, X.; An, H.; Kang, X.; Guo, S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022, 70, 8326–8337. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P.; Velazquez, E.G.; Carrera, S.P.P. Spinacia oleracea Linn Considered as One of the Most Perfect Foods: A Pharmacological and Phytochemical Review. Mini Rev. Med. Chem. 2019, 19, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.H.; Liao, Y.L.; Lin, S.C.; Hwang, K.C.; Chou, P. The mushroom Agaricus Blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: A randomized, double-blinded, and placebo-controlled clinical trial. J. Altern. Complement. Med. 2007, 13, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Hanselin, M.R.; Vande Griend, J.P.; Linnebur, S.A. INR elevation with maitake extract in combination with warfarin. Ann. Pharmacother. 2010, 44, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, M.; Okumura, M.; Fujita, K.-I.; Ogikubo, T.; Yamasaki, K.; Iwakiri, T.; Setoguchi, N.; Arimori, K. Effects of pomegranate juice on human cytochrome P450 3A (CYP3A) and tolbutamide pharmacokinetics in rats. Drug Metab. Dispos. 2005, 33, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Farkas, D.; Oleson, L.E.; Zhao, Y.; Harmatz, J.S.; Zinny, M.A.; Court, M.H.; Greenblatt, D.J. Pomegranate Juice Does Not Impair Clearance of Oral or Intravenous Midazolam, a Probe for Cytochrome P450-3A Activity: Comparison With Grapefruit Juice. J. Clin. Pharmacol. 2007, 47, 286–294. [Google Scholar] [CrossRef]
- Yeo, C.; Shon, J.; Liu, K.; Lee, D.; Yoon, Y.; Shin, J. The effects of pomegranate juice on the pharmacokinetics of simvastatin in healthy Korean subjects (PI-63). Clin. Parmacol. Ther. 2006, 79, 23. [Google Scholar] [CrossRef]
- Hanley, M.J.; Masse, G.; Harmatz, J.S.; Court, M.H.; Greenblatt, D.J. Pomegranate juice and pomegranate extract do not impair oral clearance of flurbiprofen in human volunteers: Divergence from in vitro results. Clin. Pharmacol. Ther. 2012, 92, 651–657. [Google Scholar] [CrossRef]
- Nagata, M.; Hidaka, M.; Sekiya, H.; Kawano, Y.; Yamasaki, K.; Okumura, M.; Arimori, K. Effects of Pomegranate Juice on Human Cytochrome P450 2C9 and Tolbutamide Pharmacokinetics in Rats. Drug Metab. Dispos. 2006, 35, 302–305. [Google Scholar] [CrossRef]
- Komperda, K.E. Potential interaction between pomegranate juice and warfarin. Pharmacotherapy 2009, 29, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- de Lange, D.W.; Scholman, W.L.; Kraaijenhagen, R.J.; Akkerman, J.W.; van de Wiel, A. Alcohol and polyphenolic grape extract inhibit platelet adhesion in flowing blood. Eur. J. Clin. Investig. 2004, 34, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Freitas, V.L.; Dalla Costa, T.; Manfro, R.C.; Cruz, L.B.; Schwartsmann, G. Influence of purple grape juice in cyclosporine bioavailability. J. Ren. Nutr. 2010, 20, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.X.; Ping, Z.Z.; Xiao, W.Z.; Shu, C.C.; Fattore, C.; Gatti, G.; D’Urso, S.D.; Perucca, E. Possible enhancement of the first-pass metabolism of phenacetin by ingestion of grape juice in Chinese subjects. Br. J. Clin. Pharmacol. 1999, 48, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; von Moltke, L.L.; Perloff, E.S.; Luo, Y.; Harmatz, J.S.; Zinny, M.A. Interaction of flurbiprofen with cranberry juice, grape juice, tea, and fluconazole: In vitro and clinical studies. Clin. Pharmacol. Ther. 2006, 79, 125–133. [Google Scholar] [CrossRef]
- Nishikawa, M.; Ariyoshi, N.; Kotani, A.; Ishii, I.; Nakamura, H.; Nakasa, H.; Ida, M.; Nakamura, H.; Kimura, N.; Kimura, M.; et al. Effects of Continuous Ingestion of Green Tea or Grape Seed Extracts on the Pharmacokinetics of Midazolam. Drug Metab. Pharmacokinet. 2004, 19, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.D.; Parikh, H.; Hickey, E.; Bagchi, M.; Bagchi, D. Differential effects of IH636 grape seed proanthocyanidin extract and a DNA repair modulator 4-aminobenzamide on liver microsomal cytochrome 4502E1-dependent aniline hydroxylation. Mol. Cell Biochem. 2001, 218, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Crawford, P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef]
- Lu, T.; Sheng, H.; Wu, J.; Cheng, Y.; Zhu, J.; Chen, Y. Cinnamon extract improves fasting blood glucose and glycosylated hemoglobin level in Chinese patients with type 2 diabetes. Nutr. Res. 2012, 32, 408–412. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.T.; Ka, H.; Jung, W.T.; Jung, H.J.; Park, H.J. Constituents of the essential oil of the Cinnamomum cassia stem bark and the biological properties. Arch. Pharm. Res. 2001, 24, 418–423. [Google Scholar] [CrossRef]
- Felter, S.P.; Vassallo, J.D.; Carlton, B.D.; Daston, G.P. A safety assessment of coumarin taking into account species-specificity of toxicokinetics. Food Chem. Toxicol. 2006, 44, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Brancheau, D.; Patel, B.; Zughaib, M. Do cinnamon supplements cause acute hepatitis? Am. J. Case Rep. 2015, 16, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.; Mills, J. An unusual case of HIV virologic failure during treatment with boosted atazanavir. AIDS 2013, 27, 1361–1362. [Google Scholar] [CrossRef]
- Gurley, B.J.; Gardner, S.F.; Hubbard, M.A.; Williams, D.K.; Gentry, W.B.; Cui, Y.; Ang, C.Y.W. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002, 72, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Jalloh, M.A.; Gregory, P.J.; Hein, D.; Risoldi Cochrane, Z.; Rodriguez, A. Dietary supplement interactions with antiretrovirals: A systematic review. Int. J. STD AIDS 2017, 28, 4–15. [Google Scholar] [CrossRef]
- Dhamija, P.; Malhotra, S.; Pandhi, P. Effect of oral administration of crude aqueous extract of garlic on pharmacokinetic parameters of isoniazid and rifampicin in rabbits. Pharmacology 2006, 77, 100–104. [Google Scholar] [CrossRef]
- Gallicano, K.; Foster, B.; Choudhri, S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br. J. Clin. Pharmacol. 2003, 55, 199–202. [Google Scholar] [CrossRef]
- Wasef, A.K.; Wahdan, S.A.; Saeed, N.M.; El-Demerdash, E. Effects of aged garlic and ginkgo biloba extracts on the pharmacokinetics of sofosbuvir in rats. Biopharm. Drug Dispos. 2022, 43, 152–162. [Google Scholar] [CrossRef]
- Gadkari, J.V.; Joshi, V.D. Effect of ingestion of raw garlic on serum cholesterol level, clotting time and fibrinolytic activity in normal subjects. J. Postgrad. Med. 1991, 37, 128–131. [Google Scholar]
- Abdul, M.I.M.; Jiang, X.; Williams, K.M.; Day, R.; Roufogalis, B.; Liauw, W.S.; Xu, H.; McLachlan, A.J. Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br. J. Pharmacol. 2008, 154, 1691–1700. [Google Scholar] [CrossRef]
- Kall, M.A.; Vang, O.; Clausen, J. Effects of dietary broccoli on human drug metabolising activity. Cancer Lett. 1997, 114, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Hakooz, N.; Hamdan, I. Effects of dietary broccoli on human in vivo caffeine metabolism: A pilot study on a group of Jordanian volunteers. Curr. Drug Metab. 2007, 8, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Veeramachaneni, S.; Ausman, L.M.; Choi, S.W.; Russell, R.M.; Wang, X.D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J. Nutr. 2008, 138, 1329–1335. [Google Scholar] [CrossRef] [PubMed]
- Barch, D.H.; Rundhaugen, L.M.; Thomas, P.E.; Kardos, P.; Pillay, N.S. Dietary ellagic acid inhibits the enzymatic activity of CYP1A1 without altering hepatic concentrations of CYP1A1 or CYP1A1 mRNA. Biochem. Biophys. Res. Commun. 1994, 201, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, P.-Z.; Yue, Q.-X.; Li, J.-Q.; Chu, R.-A.; Zhang, W.; Wang, H. Pungent ginger components modulates human cytochrome P450 enzymes in vitro. Acta Pharmacol. Sin. 2013, 34, 1237–1242. [Google Scholar] [CrossRef]
- Marx, W.; McKavanagh, D.; McCarthy, A.L.; Bird, R.; Ried, K.; Chan, A.; Isenring, L. The Effect of Ginger (Zingiber officinale) on Platelet Aggregation: A Systematic Literature Review. PLoS ONE 2015, 10, e0141119. [Google Scholar] [CrossRef]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef]
- Alam, M.A.; Bin Jardan, Y.A.; Alzenaidy, B.; Raish, M.; Al-Mohizea, A.M.; Ahad, A.; Al-Jenoobi, F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on pharmacokinetics and pharmacodynamics of amlodipine. J. Pharm. Pharmacol. 2021, 73, 1151–1160. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chao, P.D.; Hsiu, S.L.; Wen, K.C.; Tsai, S.Y.; Hou, Y.C. Ginger significantly decreased the oral bioavailability of cyclosporine in rats. Am. J. Chin. Med. 2006, 34, 845–855. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Bin Jardan, Y.A.; Alam, M.A.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Effect of Hibiscus sabdariffa and Zingiber officinale on the antihypertensive activity and pharmacokinetic of losartan in hypertensive rats. Xenobiotica 2020, 50, 847–857. [Google Scholar] [CrossRef]
- Okonta, J.M.; Uboh, M.; Obonga, W.O. Herb-Drug Interaction: A Case Study of Effect of Ginger on the Pharmacokinetic of Metronidazole in Rabbit. Indian J. Pharm. Sci. 2008, 70, 232. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Williams, K.M.; Liauw, W.S.; Ammit, A.J.; Roufogalis, B.D.; Duke, C.C.; Day, R.O.; McLachlan, A.J. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 425–432. [Google Scholar] [CrossRef]
- Zhang, Z.; Hamilton, S.M.; Stewart, C.; Strother, A.; Teel, R.W. Inhibition of liver microsomal cytochrome P450 activity and metabolism of the tobacco-specific nitrosamine NNK by capsaicin and ellagic acid. Anticancer Res. 1993, 13, 2341–2346. [Google Scholar] [PubMed]
- Ahn, D.; Putt, D.; Kresty, L.; Stoner, G.D.; Fromm, D.; Hollenberg, P.F. The effects of dietary ellagic acid on rat hepatic and esophageal mucosal cytochromes P450 and phase II enzymes. Carcinogenesis 1996, 17, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.; Martineau, L.C.; Ramassamy, C.; Matar, C.; Haddad, P.S. Fermented Canadian lowbush blueberry juice stimulates glucose uptake and AMP-activated protein kinase in insulin-sensitive cultured muscle cells and adipocytes. Can. J. Physiol. Pharmacol. 2007, 85, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddou-Andaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Le, P.M.; et al. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef]
- Hanley, M.J.; Masse, G.; Harmatz, J.S.; Cancalon, P.F.; Dolnikowski, G.; Court, M.H.; Greenblatt, D.J. Effect of blueberry juice on clearance of buspirone and flurbiprofen in human volunteers. Br. J. Clin. Pharmacol. 2013, 75, 1041–1052. [Google Scholar] [CrossRef]
- Fujita, K. Food-drug interactions via human cytochrome P450 3A (CYP3A). Drug Metabol. Drug Interact. 2004, 20, 195–217. [Google Scholar] [CrossRef]
- Sasaki, T.; Sato, Y.; Kumagai, T.; Yoshinari, K.; Nagata, K. Effect of health foods on cytochrome P450-mediated drug metabolism. J. Pharm. Health Care Sci. 2017, 3, 14. [Google Scholar] [CrossRef]
- Lynch, T.; Price, A. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar]
- Kawauchi, S.; Nakamura, T.; Yasui, H.; Nishikawa, C.; Miki, I.; Inoue, J.; Horibe, S.; Hamaguchi, T.; Tanahashi, T.; Mizuno, S. Intestinal and hepatic expression of cytochrome P450s and mdr1a in rats with indomethacin-induced small intestinal ulcers. Int. J. Med. Sci. 2014, 11, 1208–1217. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Liu, M.L.; Iyanagi, T.; Legesse, K.; Lee, T.D.; Chen, S.A. Inhibition of rat liver NAD(P)H:quinone acceptor oxidoreductase (DT-diaphorase) by flavonoids isolated from the Chinese herb scutellariae radix (Huang Qin). Mol. Pharmacol. 1990, 37. [Google Scholar]
- Vinarov, Z.; Abdallah, M.; Agundez, J.A.; Allegaert, K.; Basit, A.W.; Braeckmans, M.; Ceulemans, J.; Corsetti, M.; Griffin, B.T.; Grimm, M.; et al. Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: An UNGAP review. Eur. J. Pharm. Sci. 2021, 162, 105812. [Google Scholar] [CrossRef] [PubMed]
- Selby-Pham, S.N.B.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Sci. Rep. 2017, 7, 1931. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 28, 133–141. [Google Scholar] [CrossRef]
- Nemati, M.; Singh, B.; Mir, R.A.; Nemati, M.; Babaei, A.; Ahmadi, M.; Rasmi, Y.; Golezani, A.G.; Rezaie, J. Plant-derived extracellular vesicles: A novel nanomedicine approach with advantages and challenges. Cell Commun. Signal. 2022, 20, 1–16. [Google Scholar] [CrossRef]
- Malloci, M.; Perdomo, L.; Veerasamy, M.; Andriantsitohaina, R.; Simard, G.; Martínez, M.C. Extracellular Vesicles: Mechanisms in Human Health and Disease. Antioxid. Redox Signal 2019, 30, 813–856. [Google Scholar] [CrossRef]
- Urzì, O.; Raimondo, S.; Alessandro, R. Extracellular Vesicles from Plants: Current Knowledge and Open Questions. Int. J. Mol. Sci. 2021, 22, 5366. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; Hazas, M.-C.L.D.L.; Tomé-Carneiro, J.; del Saz-Lara, A.; Gil-Zamorano, J.; Balaguer, L.; Chapado, L.A.; Busto, R.; Visioli, F.; Dávalos, A. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol. Res. 2022, 185. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef]
- Mörbe, U.M.; Jørgensen, P.B.; Fenton, T.M.; von Burg, N.; Riis, L.B.; Spencer, J.; Agace, W.W. Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunol. 2021, 14, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Spahn, T.W. Modulating the intestinal immune system: The role of lymphotoxin and GALT organs. Gut 2004, 53, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Zgair, A.; Wong, J.C.M.; Gershkovich, P. Targeting Immunomodulatory Agents to the Gut-Associated Lymphoid Tissue. Neuro-Immuno-Gastroenterol. 2016, 25, 237–261. [Google Scholar] [CrossRef]
- Cohen-Kedar, S.; Baram, L.; Elad, H.; Brazowski, E.; Guzner-Gur, H.; Dotan, I. Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk. Eur. J. Immunol. 2014, 44, 3729–3740. [Google Scholar] [CrossRef]
- Batbayar, S.; Lee, D.-H.; Kim, H.-W. Immunomodulation of Fungal β-Glucan in Host Defense Signaling by Dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef]
- De Graaff, P.; Govers, C.; Wichers, H.; Debets, R. Consumption of β-glucans to spice up T cell treatment of tumors: A review. Expert Opin. Biol. Ther. 2018, 18, 1023–1040. [Google Scholar] [CrossRef]
- Chan, G.C.-F.; Chan, W.K.; Sze, D.M.-Y. The effects of β-glucan on human immune and cancer cells. J. Hematol. Oncol. 2009, 2, 25. [Google Scholar] [CrossRef]
- Enright, E.F.; Gahan, C.G.; Joyce, S.A.; Griffin, B.T. The Impact of the Gut Microbiota on Drug Metabolism and Clinical Out-come. Yale J. Biol. Med. 2016, 89, 375–382. [Google Scholar]
- Santhiravel, S.; Bekhit, A.E.-D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The Impact of Plant Phytochemicals on the Gut Microbiota of Humans for a Balanced Life. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef]
- Song, E.-J. Personalized Diets based on the Gut Microbiome as a Target for Health Maintenance: From Current Evidence to Future Possibilities. J. Microbiol. Biotechnol. 2022, 32, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T.; International Natural Product Sciences Taskforce. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Upton, R.; David, B.; Gafner, S.; Glasl, S. Botanical ingredient identification and quality assessment: Strengths and limitations of analytical techniques. Phytochem. Rev. 2020, 19, 1157–1177. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol Biol. 2013, 1055, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Bahadar, H.; Abdollahi, M. Inappropriate use of the term "cytotoxicity" in scientific literature. DARU J. Pharm. Sci. 2015, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.; Cohen, I.; Campbell, M. Reduction of MTT by aqueous herbal extracts in the absence of cells. J. Ethnopharmacol. 2004, 93, 381–384. [Google Scholar] [CrossRef]
- Dhingra, K. Oncology 2020: A drug development and approval paradigm. Ann. Oncol. 2015, 26, 2347–2350. [Google Scholar] [CrossRef]
- Langhof, H.; Chin, W.W.L.; Wieschowski, S.; Federico, C.; Kimmelman, J.; Strech, D. Preclinical efficacy in therapeutic area guidelines from the U.S. Food and Drug Administration and the European Medicines Agency: A cross-sectional study. Br. J. Pharmacol. 2018, 175, 4229–4238. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide–identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]
- Zitter, R.; Chugh, R.M.; Saha, S. Patient Derived Ex-Vivo Cancer Models in Drug Development, Personalized Medicine, and Radiotherapy. Cancers 2022, 14, 3006. [Google Scholar] [CrossRef]
- Gertsch, J. Botanical Drugs, Synergy, and Network Pharmacology: Forth and Back to Intelligent Mixtures. Planta Med. 2011, 77, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.L.; Bodmer, W.F. Cancer Cell Lines for Drug Discovery and Development. Cancer Res. 2014, 74, 2377–2384. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, L.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Vellonen, K.-S.; Malinen, M.; Mannermaa, E.; Subrizi, A.; Toropainen, E.; Lou, Y.-R.; Kidron, H.; Yliperttula, M.; Urtti, A. A critical assessment of in vitro tissue models for ADME and drug delivery. J. Control. Release 2014, 190, 94–114. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Williams, S.; McDermott, U. The Pursuit of Therapeutic Biomarkers with High-Throughput Cancer Cell Drug Screens. Cell Chem. Biol. 2017, 24, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Krushkal, J.; Negi, S.; Yee, L.M.; Evans, J.R.; Grkovic, T.; Palmisano, A.; Fang, J.; Sankaran, H.; McShane, L.M.; Zhao, Y.; et al. Molecular genomic features associated within vitroresponse of the NCI-60 cancer cell line panel to natural products. Mol. Oncol. 2021, 15, 381–406. [Google Scholar] [CrossRef]
- Sankaran, H.; Negi, S.; McShane, L.M.; Zhao, Y.; Krushkal, J. Pharmacogenomics of in vitro response of the NCI-60 cancer cell line panel to Indian natural products. BMC Cancer 2022, 22, 512. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.-Y.; Jia, J.; Fang, M.; Zhao, L.; Jiang, Y.; Shi, Y.; Tu, P.-F.; Guo, X.-Y. A Novel System for Evaluating the Inhibition Effect of Drugs on Cytochrome P450 Enzymes in vitro Based on Human-Induced Hepatocytes (hiHeps). Front. Pharmacol. 2021, 12, 748658. [Google Scholar] [CrossRef]
- Ji, H.Y.; Kim, S.Y.; Kim, D.K.; Jeong, J.H.; Lee, H.S. Effects of Eupatilin and Jaceosidin on Cytochrome P450 Enzyme Activities in Human Liver Microsomes. Molecules 2010, 15, 6466–6475. [Google Scholar] [CrossRef]
- Milani, N.; Parrott, N.; Franyuti, D.O.; Godoy, P.; Galetin, A.; Gertz, M.; Fowler, S. Application of a gut–liver-on-a-chip device and mechanistic modelling to the quantitative in vitro pharmacokinetic study of mycophenolate mofetil. Lab a Chip 2022, 22, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Cong, L.; Cong, X. Patient-Derived Organoids in Precision Medicine: Drug Screening, Organoid-on-a-Chip and Living Organoid Biobank. Front. Oncol. 2021, 11, 762184. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, L.; Li, Z.; Li, W.; Huang, W. Patient-derived organoid (PDO) platforms to facilitate clinical decision making. J. Transl. Med. 2021, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, L.; Liu, M.; Mao, Y. Patient-derived organoids: A promising model for personalized cancer treatment. Gastroenterol. Rep. 2018, 6, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; Van De Haar, J.; Fanchi, L.F.; Slagter, M.; Van Der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598e12. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, N.J.; Nicholls, C.; Templeton, A.R.; Perera, M.P.; Jeffery, P.L.; Zimmermann, K.; Kulasinghe, A.; Kenna, T.J.; Vela, I.; Williams, E.D. Modelling the tumor immune microenvironment for precision immunotherapy. Clin. Transl. Immunol. 2022, 11, e1400. [Google Scholar] [CrossRef]
- Patwardhan, B.; Gautam, M. Botanical immunodrugs: Scope and opportunities. Drug Discov. Today 2005, 10, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.S.; Veening-Griffioen, D.H.; Boon, W.P.C.; Moors, E.H.M.; Van Meer, P.J.K. Levelling the Translational Gap for Animal to Human Efficacy Data. Animals 2020, 10, 1199. [Google Scholar] [CrossRef]
- Ireson, C.R.; Alavijeh, M.; Palmer, A.M.; Fowler, E.R.; Jones, H.J. The role of mouse tumour models in the discovery and development of anticancer drugs. Br. J. Cancer 2019, 121, 101–108. [Google Scholar] [CrossRef]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Aghdaei, H.A.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J. Transl. Med. 2022, 20, 1–15. [Google Scholar] [CrossRef]
- Chulpanova, D.S.; Kitaeva, K.V.; Rutland, C.S.; Rizvanov, A.A.; Solovyeva, V.V. Mouse Tumor Models for Advanced Cancer Immunotherapy. Int. J. Mol. Sci. 2020, 21, 4118. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Du, W.; Lan, H.; Liu, Y.; Mao, C.; Du, J.; Mou, X. Development of humanized mouse with patient-derived xenografts for cancer immunotherapy studies: A comprehensive review. Cancer Sci. 2021, 112, 2592–2606. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.J. Diet, nutrition, and cancer: Development of hypotheses and their evaluation in animal studies. Cancer Res. 1993, 53 (Suppl. 10), 2442S–2445S. [Google Scholar]
- Giles, K.; Guan, C.; Jagoe, T.R.; Mazurak, V. Diet composition as a source of variation in experimental animal models of cancer cachexia. J. Cachex Sarcopenia Muscle 2016, 7, 110–125. [Google Scholar] [CrossRef]
- Jensen, M.N.; Ritskes-Hoitinga, M. How isoflavone levels in common rodent diets can interfere with the value of animal models and with experimental results. Lab. Anim. 2007, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yajima, Y.; Okuno, A.; Nakamura, I.; Miyazaki, T.; Honda, A.; Toyoda, A. Differential Effect of Non-Purified and Semi-Purified Standard Diets on Kynurenine and Peripheral Metabolites in Male C57BL/6J Mice. Int. J. Tryptophan Res. 2022, 15, 11786469211066285. [Google Scholar] [CrossRef]
- Zhang, J.; Onakpoya, I.J.; Posadzki, P.; Eddouks, M. The Safety of Herbal Medicine: From Prejudice to Evidence. Evidence-Based Complement. Altern. Med. 2015, 2015, 316706. [Google Scholar] [CrossRef]
- Bailey, J.; Thew, M.; Balls, M. An Analysis of the Use of Animal Models in Predicting Human Toxicology and Drug Safety. Altern. Lab. Anim. 2014, 42, 181–199. [Google Scholar] [CrossRef]
- Kranse, R.; Barbet, N.; Hop, W.C.; Kandra, A.; Lassus, M. Prostate-specific antigen: A surrogate endpoint for screening new agents against prostate cancer? Prostate 2000, 42, 107–115. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Zhang, R.; Zhu, X.; Bai, H.; Ning, K. Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Skinner, M.A.; Laing, W.A. Pharmacogenomics and nutrigenomics: Synergies and differences. Eur. J. Clin. Nutr. 2007, 61, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kaput, J.; Perlina, A.; Hatipoglu, B.; Bartholomew, A.; Nikolsky, Y. Nutrigenomics: Concepts and applications to pharmacogenomics and clinical medicine. Pharmacogenomics 2007, 8, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Miteva-Marcheva, N.N.; Ivanov, H.Y.; Dimitrov, D.K.; Stoyanova, V.K. Application of pharmacogenetics in oncology. Biomark. Res. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Braicu, C.; Mehterov, N.; Vladimirov, B.; Sarafian, V.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. Nutrigenomics in cancer: Revisiting the effects of natural compounds. Semin. Cancer Biol. 2017, 46, 84–106. [Google Scholar] [CrossRef]
- Grimstein, M.; Huang, S.-M. A regulatory science viewpoint on botanical–drug interactions. J. Food Drug Anal. 2018, 26, S12–S25. [Google Scholar] [CrossRef]
- Rahman, M.; Vadrev, S.M.; Magana-Mora, A.; Levman, J.; Soufan, O. A novel graph mining approach to predict and evaluate food-drug interactions. Sci. Rep. 2022, 12, 1–16. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep learning improves prediction of drug–drug and drug–food interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E4304–E4311. [Google Scholar] [CrossRef]
- Haymart, M.R.; Miller, D.C.; Hawley, S.T. Active Surveillance for Low-Risk Cancers—A Viable Solution to Overtreatment? N. Engl. J. Med. 2017, 377, 203–206. [Google Scholar] [CrossRef]
- Cook, N.; Hansen, A.R.; Siu, L.L.; Razak, A.R.A. Early phase clinical trials to identify optimal dosing and safety. Mol. Oncol. 2015, 9, 997–1007. [Google Scholar] [CrossRef]
- Katz, R. Biomarkers and Surrogate Markers: An FDA Perspective. Neurorx 2004, 1, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Izzo, A.A.; Teixeira, M.; Alexander, S.P.; Cirino, G.; Docherty, J.R.; George, C.H.; Insel, P.A.; Ji, Y.; Kendall, D.A.; Panettieri, R.A.; et al. A practical guide for transparent reporting of research on natural products in the British Journal of Pharmacology: Reproducibility of natural product research. Br. J. Pharmacol. 2020, 177, 2169–2178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ha, D.; Yoshitake, R.; Chan, Y.S.; Sadava, D.; Chen, S. Exploring the Biological Activity and Mechanism of Xenoestrogens and Phytoestrogens in Cancers: Emerging Methods and Concepts. Int. J. Mol. Sci. 2021, 22, 8798. [Google Scholar] [CrossRef] [PubMed]
- Fogel, D.B. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: A review. Contemp. Clin. Trials Commun. 2018, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.R. Fundamentals of clinical trial design. J. Exp. Stroke Transl. Med. 2010, 3, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry. 2022. Available online: https://www.FDA.gov/regulatory-information/search-FDA-guidance-documents/diversity-plans-improve-enrollment-participants-underrepresented-racial-and-ethnic-populations (accessed on 13 January 2023).
- Borrello, K.; Lim, U.; Park, S.-Y.; Monroe, K.R.; Maskarinec, G.; Boushey, C.J.; Wilkens, L.R.; Randolph, T.W.; Le Marchand, L.; Hullar, M.A.; et al. Dietary Intake Mediates Ethnic Differences in Gut Microbial Composition. Nutrients 2022, 14, 660. [Google Scholar] [CrossRef]
- Swanson, H.I. Drug Metabolism by the Host and Gut Microbiota: A Partnership or Rivalry? Drug Metab. Dispos. 2015, 43, 1499–1504. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, R.; Zhao, C.; Tang, X.; Lu, A.; Bian, Z. Placebo design in WHO-registered trials of Chinese herbal medicine need improvements. BMC Complement. Altern. Med. 2019, 19, 299. [Google Scholar] [CrossRef]
- Kardum, N.; Ristic, A.K.; Zec, M.; Kojadinovic, M.; Petrovic-Oggiano, G.; Zekovic, M.; Kroon, P.A.; Glibetić, M. Design, formulation and sensory evaluation of a polyphenol-rich food placebo: An example of aronia juice for food intervention studies. Int. J. Food Sci. Nutr. 2017, 68, 742–749. [Google Scholar] [CrossRef]
- Chen, S.; Oh, S.-R.; Phung, S.; Hur, G.; Ye, J.J.; Kwok, S.L.; Shrode, G.E.; Belury, M.; Adams, L.S.; Williams, D. Anti-Aromatase Activity of Phytochemicals in White Button Mushrooms (Agaricus bisporus). Cancer Res. 2006, 66, 12026–12034. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Wu, X.; Ki, L.; Chen, S. White Button Mushroom (Agaricus Bisporus) Exhibits Antiproliferative and Proapoptotic Properties and Inhibits Prostate Tumor Growth in Athymic Mice. Nutr. Cancer 2008, 60, 744–756. [Google Scholar] [CrossRef]
- Kanaya, N.; Kubo, M.; Liu, Z.; Chu, P.; Wang, C.; Yuan, S.C.Y.-C. Protective Effects of White Button Mushroom (Agaricus bisporus) against Hepatic Steatosis in Ovariectomized Mice as a Model of Postmenopausal Women. PLoS ONE 2011, 6, e26654. [Google Scholar] [CrossRef]
- Twardowski, P.; Kanaya, N.; Frankel, P.; Synold, T.; Ruel, C.; Pal, S.K.; Junqueira, M.; Prajapati, M.; Moore, T.; Tryon, P.; et al. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus -induced prostate-specific antigen responses. Cancer 2015, 121, 2942–2950. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Mori, H.; Chen, S. White button mushroom (Agaricus bisporus) disrupts androgen receptor signaling in human prostate cancer cells and patient-derived xenograft. J. Nutr. Biochem. 2021, 89, 108580. [Google Scholar] [CrossRef]
- Wang, X.; Ha, D.; Yoshitake, R.; Chen, S. White button mushroom interrupts tissue AR-mediated TMPRSS2 expression and attenuates pro-inflammatory cytokines in C57BL/6 mice. NPJ Sci. Food 2021, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Kijima, I.; Phung, S.; Hur, G.; Kwok, S.-L.; Chen, S. Grape Seed Extract Is an Aromatase Inhibitor and a Suppressor of Aromatase Expression. Cancer Res. 2006, 66, 5960–5967. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, K.; Chen, S.; Wen, W. 20 Grape seed extract inhibits VEGF expression via reducing HIF-1 protein expression. Carcinogenesis 2009, 30, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Lu, J.; Zhang, K.; Chen, S. Grape Seed Extract Inhibits Angiogenesis via Suppression of the Vascular Endothelial Growth Factor Receptor Signaling Pathway. Cancer Prev. Res. 2008, 1, 554–561. [Google Scholar] [CrossRef]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate Ellagitannin–Derived Compounds Exhibit Antiproliferative and Antiaromatase Activity in Breast Cancer Cells In vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L.; Chen, S. Blueberry Phytochemicals Inhibit Growth and Metastatic Potential of MDA-MB-231 Breast Cancer Cells through Modulation of the Phosphatidylinositol 3-Kinase Pathway. Cancer Res. 2010, 70, 3594–3605. [Google Scholar] [CrossRef]
- Adams, L.S.; Kanaya, N.; Phung, S.; Liu, Z.; Chen, S. Whole Blueberry Powder Modulates the Growth and Metastasis of MDA-MB-231 Triple Negative Breast Tumors in Nude Mice. J. Nutr. 2011, 141, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Adams, L.S.; Chen, S.; Killian, C.; Ahmed, A.; Seeram, N.P. Eugenia jambolana Lam. Berry Extract Inhibits Growth and Induces Apoptosis of Human Breast Cancer but Not Non-Tumorigenic Breast Cells. J. Agric. Food Chem. 2009, 57, 826–831. [Google Scholar] [CrossRef]
- Lee, J.-W.; Lee, W.B.; Kim, W.; Min, B.-I.; Lee, H.; Cho, S.-H. Traditional herbal medicine for cancer pain: A systematic review and meta-analysis. Complement. Ther. Med. 2015, 23, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sugawara, Y.; Chen, S.; Beelman, R.B.; Tsuduki, T.; Tomata, Y.; Matsuyama, S.; Tsuji, I. Mushroom consumption and incident risk of prostate cancer in Japan: A pooled analysis of the Miyagi Cohort Study and the Ohsaki Cohort Study. Int. J. Cancer 2019, 146, 2712–2720. [Google Scholar] [CrossRef] [PubMed]
- Ba, D.M.; Ssentongo, P.; Beelman, R.B.; Muscat, J.; Gao, X.; Richie, J.P. Higher Mushroom Consumption Is Associated with Lower Risk of Cancer: A Systematic Review and Meta-Analysis of Observational Studies. Adv. Nutr. 2021, 12, 1691–1704. [Google Scholar] [CrossRef]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant Endocrine Therapy for Women with Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef]
- Lui, K.; Tamura, T.; Mori, T.; Zhou, D.; Chen, S. MCF-7aro/ERE, a novel cell line for rapid screening of aromatase inhibitors, ERα ligands and ERRα ligands. Biochem. Pharmacol. 2008, 76, 208–215. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, D.; Hsin, L.-Y.; Kanaya, N.; Wong, C.; Yip, R.; Sakamuru, S.; Xia, M.; Yuan, Y.-C.; Witt, K.; et al. AroER Tri-Screen Is a Biologically Relevant Assay for Endocrine Disrupting Chemicals Modulating the Activity of Aromatase and/or the Estrogen Receptor. Toxicol. Sci. 2014, 139, 198–209. [Google Scholar] [CrossRef]
- Chen, S.; Hsieh, J.-H.; Huang, R.; Sakamuru, S.; Hsin, L.-Y.; Xia, M.; Shockley, K.R.; Auerbach, S.; Kanaya, N.; Lu, H.; et al. Cell-Based High-Throughput Screening for Aromatase Inhibitors in the Tox21 10K Library. Toxicol. Sci. 2015, 147, 446–457. [Google Scholar] [CrossRef]
- Kanaya, N.; Nguyen, D.M.; Lu, H.; Wang, Y.-Z.; Hsin, L.-Y.; Petreas, M.; Nelson, D.; Guo, W.; Reynolds, P.; Synold, T.; et al. AroER tri-screen™ is a novel functional assay to estimate both estrogenic and estrogen precursor activity of chemicals or biological specimens. Breast Cancer Res. Treat. 2015, 151, 335–345. [Google Scholar] [CrossRef]
- Jones, J.O.; An, W.F.; Diamond, M.I. AR Inhibitors Identified by High-Throughput Microscopy Detection of Conformational Change and Subcellular Localization. ACS Chem. Biol. 2009, 4, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Roszczyk, A.; Turło, J.; Zagożdżon, R.; Kaleta, B. Immunomodulatory Properties of Polysaccharides from Lentinula edodes. Int. J. Mol. Sci. 2022, 23, 8980. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Y.; Zhang, L.; Tian, Q. Mushroom polysaccharide lentinan for treating different types of cancers: A review of 12 years clinical studies in China. Prog. Mol. Biol. Transl. Sci. 2019, 163, 297–328. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.L.; Smith, B.D. Radiation Treatment in Older Patients: A Framework for Clinical Decision Making. J. Clin. Oncol. 2014, 32, 2669–2678. [Google Scholar] [CrossRef]
- Cooperberg, M.R.; Zheng, Y.; Faino, A.V.; Newcomb, L.F.; Zhu, K.; Cowan, J.E.; Brooks, J.D.; Dash, A.; Gleave, M.E.; Martin, F.; et al. Tailoring Intensity of Active Surveillance for Low-Risk Prostate Cancer Based on Individualized Prediction of Risk Stability. JAMA Oncol. 2020, 6, e203187. [Google Scholar] [CrossRef]
- Paller, C.J.; Antonarakis, E.S. Management of biochemically recurrent prostate cancer after local therapy: Evolving standards of care and new directions. Clin. Adv. Hematol. Oncol. 2013, 11, 14–23. [Google Scholar]
- Roydhouse, J.; Menapace, L.; Xia, H.; Song, P.; Berman, T.; Agarwal, R.; Suzman, D.; Wright, K.; Beaver, J.; Kluetz, P. Concomitant botanical medicine use among patients participating in commercial prostate cancer trials. Complement. Ther. Med. 2020, 54, 102549. [Google Scholar] [CrossRef]
- Qato, D.M.; Wilder, J.; Schumm, P.; Gillet, V.; Alexander, G.C. Changes in Prescription and Over-the-Counter Medication and Dietary Supplement Use Among Older Adults in the United States, 2005 vs. 2011. JAMA Intern. Med. 2016, 176, 473–482. [Google Scholar] [CrossRef]
- Gurley, B.J.; Yates, C.R.; Markowitz, J.S. “…Not Intended to Diagnose, Treat, Cure or Prevent Any Disease.” 25 Years of Botanical Dietary Supplement Research and the Lessons Learned. Clin. Pharmacol. Ther. 2018, 104, 470–483. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, N.; Wang, Z.; Hao, Y.; Wang, Z.; Yang, Z.; Liu, J.; Wang, J. Analysis of the multi-physiological and functional mechanism of wheat alkylresorcinols based on reverse molecular docking and network pharmacology. Food Funct. 2022, 13, 9091–9107. [Google Scholar] [CrossRef]
- Kazybay, B.; Sun, Q.; Dukenbayev, K.; Nurkesh, A.A.; Xu, N.; Kutzhanova, A.; Razbekova, M.; Kabylda, A.; Yang, Q.; Wang, Q.; et al. Network Pharmacology with Experimental Investigation of the Mechanisms of Rhizoma Polygonati against Prostate Cancer with Additional Herbzymatic Activity. ACS Omega 2022, 7, 14465–14477. [Google Scholar] [CrossRef] [PubMed]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic Dose-Response Induced by Phytochemicals: Experimental Evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Kumar, A.P.; Ghosh, R. Food-based natural products for cancer management: Is the whole greater than the sum of the parts? Semin. Cancer Biol. 2016, 40–41, 233–246. [Google Scholar] [CrossRef]
- Sauter, E.R. Cancer prevention and treatment using combination therapy with natural compounds. Expert Rev. Clin. Pharmacol. 2020, 13, 265–285. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.-Y.; Wei, W.-C.; Jian, F.-Y.; Yang, N.-S. Therapeutic Applications of Herbal Medicines for Cancer Patients. Evid.-Based Complement. Alternat. Med. 2013, 2013, 302426. [Google Scholar] [CrossRef] [PubMed]
- Block, K.I.; Koch, A.C.; Mead, M.N.; Tothy, P.K.; Newman, R.A.; Gyllenhaal, C. Impact of antioxidant supplementation on chemotherapeutic toxicity: A systematic review of the evidence from randomized controlled trials. Int. J. Cancer 2008, 123, 1227–1239. [Google Scholar] [CrossRef]
- Talib, W.H.; Alsayed, A.R.; Barakat, M.; Abu-Taha, M.I.; Mahmod, A.I. Targeting Drug Chemo-Resistance in Cancer Using Natural Products. Biomedicines 2021, 9, 1353. [Google Scholar] [CrossRef] [PubMed]
- Cognigni, V.; Ranallo, N.; Tronconi, F.; Morgese, F.; Berardi, R. Potential benefit of β-glucans as adjuvant therapy in immuno-oncology: A review. Explor. Target. Explor. Target. Antitumor Ther. 2021, 2, 122–138. [Google Scholar] [CrossRef]
- Del Cornò, M.; Gessani, S.; Conti, L. Shaping the Innate Immune Response by Dietary Glucans: Any Role in the Control of Cancer? Cancers 2020, 12, 155. [Google Scholar] [CrossRef]
- Wang, M.; Bai, Y.; Pei, J.; Li, D.; Pu, X.; Zhu, W.; Xia, L.; Qi, C.; Jiang, H.; Ning, Y. β-Glucan Combined With PD-1/PD-L1 Checkpoint Blockade for Immunotherapy in Patients With Advanced Cancer. Front. Pharmacol. 2022, 13. [Google Scholar] [CrossRef]
- Steimbach, L.; Borgmann, A.V.; Gomar, G.G.; Hoffmann, L.V.; Rutckeviski, R.; de Andrade, D.P.; Smiderle, F.R. Fungal beta-glucans as adjuvants for treating cancer patients – A systematic review of clinical trials. Clin. Nutr. 2020, 40, 3104–3113. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef]
- You, S. Mushrooms as Functional and Nutritious Food Ingredients for Multiple Applications. ACS Food Sci. Technol. 2022, 2, 1184–1195. [Google Scholar] [CrossRef]
- Shiitake Mushroom. Memorial Sloan Kettering Cancer Center. 2020. Available online: www.mskcc.org/cancer-care/integrative-medicine/herbs/shiitake-mushroom (accessed on 13 November 2022).
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2015, 376387. [Google Scholar] [CrossRef]
- Vamanu, E. Antioxidant properties of mushroom mycelia obtained by batch cultivation and tocopherol content affected by extraction procedures. Biomed. Res. Int. 2014, 974804. [Google Scholar] [CrossRef]
- Albeituni, S.H.; Ding, C.; Liu, M.; Hu, X.; Luo, F.; Kloecker, G.; Bousamra, M.; Zhang, H.; Yan, J. Yeast-Derived Particulate β-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer. J. Immunol. 2016, 196, 2167–2180. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Xie, Y.; Ding, Y.; Du, W.W.; Sdiri, M.; Yang, B.B. Ergosterol purified from medicinal mushroom Amauroderma rude inhibits cancer growth in vitro and in vivo by up-regulating multiple tumor suppressors. Oncotarget 2015, 6, 17832–17846. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ali, F.; Ahmad, A.; Aatif, M.; Malik, A.; Alam, M.W.; Muteeb, G.; Ahmad, S.; Noor, A.; et al. Pomegranate juice anthocyanidins induce cell death in human cancer cells by mobilizing intracellular copper ions and producing reactive oxygen species. Front. Oncol. 2022, 12, 998346. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef]
- Banerjee, N.; Talcott, S.; Safe, S.; Mertens-Talcott, S.U. Cytotoxicity of pomegranate polyphenolics in breast cancer cells in vitro and vivo: Potential role of miRNA-27a and miRNA-155 in cell survival and inflammation. Breast Cancer Res. Treat. 2012, 136, 21–34. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, S.; Choudhury, S.; Gupta, P.; Adhikary, A.; Chattopadhyay, S. Pomegranate Polyphenols Attenuate Inflammation and Hepatic Damage in Tumor-Bearing Mice: Crucial Role of NF-κB and the Nrf2/GSH Axis. J. Nutr. Biochem. 2021, 97, 108812. [Google Scholar] [CrossRef] [PubMed]
- Nallanthighal, S.; Elmaliki, K.M.; Reliene, R. Pomegranate extract alters breast cancer stem cell properties in association with inhibition of epithelial-to-mesenchymal transition. Nutr Cancer. 2017, 69, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in cancer chemoprevention and treatment. Cancer Lett. 2018, 28, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Gao, C.; Wang, W.; Yao, L.P.; Zhang, J.; Zhang, S.D.; Li, J.; Fang, S.H.; Fu, Y.J. Juglone induces apoptosis and autophagy via modulation of mitogen-activated protein kinase pathways in human hepatocellular carcinoma cells. Food Chem. Toxicol. 2018, 116, 40–50. [Google Scholar] [CrossRef]
- Wang, Y.; Lee, K.W.; Chan, F.L.; Chen, S.; Leung, L.K. The Red Wine Polyphenol Resveratrol Displays Bilevel Inhibition on Aromatase in Breast Cancer Cells. Toxicol. Sci. 2006, 92, 71–77. [Google Scholar] [CrossRef]
- Kaur, M.; Agarwal, C.; Agarwal, R. Anticancer and cancer chemopreventive potential of grape seed extract and other grape-based products. J. Nutr. 2009, 139, 1806S–1812S. [Google Scholar] [CrossRef]
- Dutta, A.; Chakraborty, A. Cinnamon in Anticancer Armamentarium: A Molecular Approach. J. Toxicol. 2018, 29, 8978731. [Google Scholar] [CrossRef]
- Sadeghi, S.; Davoodvandi, A.; Pourhanifeh, M.H.; Sharifi, N.; Arefi-Nezhad, R.; Sahebnasagh, R.; Moghadam, S.A.; Sahebkar, A.; Mirzaei, H. Anti-cancer effects of cinnamon: Insights into its apoptosis effects. Eur. J. Med. Chem. 2019, 15, 131–140. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Ismail, A. Aromatic monophenols from cinnamon bark act as proteasome inhibitors by upregulating ER stress, suppressing FoxM1 expression, and inducing apoptosis in prostate cancer cells. Phytother. Res. 2021, 35, 5781–5794. [Google Scholar] [CrossRef]
- Sarvizadeh, M.; Hasanpour, O.; Naderi Ghale-Noie, Z.; Mollazadeh, S.; Rezaei, M.; Pourghadamyari, H.; Masoud Khooy, M.; Aschner, M.; Khan, H.; Rezaei, N.; et al. Allicin and Digestive System Cancers: From Chemical Structure to Its Therapeutic Opportunities. Front. Oncol. 2021, 11, 650256. [Google Scholar] [CrossRef]
- Hosono, T.; Fukao, T.; Ogihara, J.; Ito, Y.; Shiba, H.; Seki, T.; Ariga, T. Diallyl trisulfide suppresses the proliferation and induces apoptosis of human colon cancer cells through oxidative modification of beta-tubulin. J. Biol. Chem. 2005, 280, 41487–41493. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, S.O.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Chang, Y.C.; Kim, W.J.; Kim, C.M.; Yoo, Y.H.; Choi, Y.H. An exploration of the antioxidant effects of garlic saponins in mouse-derived C2C12 myoblasts. Int. J. Mol. Med. 2016, 37, 149–156. [Google Scholar] [CrossRef]
- Imran, S.B.; Estevinho, L.M. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 19, 2277. [Google Scholar] [CrossRef]
- Lee, G.A.; Choi, K.C.; Hwang, K.A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef]
- Su, X.; Shen, Y.; Xin, Y. Anticancer Activity of Sulforaphane: The Epigenetic Mechanisms and the Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Peng, X.; Pumera, M. Self-propelled magnetic dendrite-shaped microrobots for photodynamic prostate cancer therapy. Angew. Chem. Int. Ed. Engl. 2022, 61, 30. [Google Scholar] [CrossRef]
- Melnikov, F.; Botta, D.; White, C.C.; Schmuck, S.C.; Winfough, M.; Schaupp, C.M.; Gallagher, E.P.; Brooks, B.W.; Williams, E.S.; Coish, P.; et al. Kinetics of Glutathione Depletion and Antioxidant Gene Expression as Indicators of Chemical Modes of Action Assessed in Vitro in Mouse Hepatocytes with Enhanced Glutathione Synthesis. Chem. Res. Toxicol. 2019, 32, 421–436. [Google Scholar] [CrossRef]
- Chang, W.J.; Chen, B.H.; Inbaraj, B.S.; Chien, J.T. Preparation of allyl isothiocyanate nanoparticles, their anti-inflammatory activity towards RAW 264.7 macrophage cells and anti-proliferative effect on HT1376 bladder cancer cells. J. Sci. Food Agric. 2019, 99, 3106–3116. [Google Scholar] [CrossRef]
- Campbell, J.K.; Canene-Adams, K.; Lindshield, B.L.; Boileau, T.W.; Clinton, S.K.; Erdman, J.W. Tomato phytochemicals and prostate cancer risk. J. Nutr. 2004, 134, 3486S–3492S. [Google Scholar] [CrossRef]
- Applegate, C.C.; Lowerison, M.R.; Hambley, E.; Song, P.; Wallig, M.A.; Erdman, J.W. Dietary tomato inhibits angiogenesis in TRAMP prostate cancer but is not protective with a Western-style diet in this pilot study. Sci. Rep. 2021, 17, 18548. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, H. Anticancer Effect of Lycopene in Gastric Carcinogenesis. J. Cancer Prev. 2015, 20, 92–96. [Google Scholar] [CrossRef]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amosi, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Le, V.; Esposito, D.; Grace, M.H.; Ha, D.; Pham, A.; Bortolazzo, A.; Bevens, Z.; Kim, J.; Okuda, R.; Komarnytsky, S.; et al. Cytotoxic effects of ellagitannins isolated from walnuts in human cancer cells. Nutr. Cancer. 2014, 66, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef]
- Catanzaro, E.; Greco, G.; Potenza, L.; Calcabrini, C.; Fimognari, C. Natural Products to Fight Cancer: A Focus on Juglans regia. Toxins 2018, 14, 469. [Google Scholar] [CrossRef]
- Bae, H.; Song, G.; Lim, W. Stigmasterol Causes Ovarian Cancer Cell Apoptosis by Inducing Endoplasmic Reticulum and Mitochondrial Dysfunction. Pharmaceutics 2020, 12, 488. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Yazdanparast, R.; Roshanzamir, F. Augmentation of oxidative stress-induced apoptosis in MCF7 cells by ascorbate-tamoxifen and/or ascorbate-juglone treatments. In Vitro Cell. Dev. Biol. Anim. 2016, 52, 193–203. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 142979. [Google Scholar] [CrossRef]
- Parham, S.; Berto, F. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants 2020, 21, 1309. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Chen, P.; He, Z.; Xu, J.; Chen, Y.; Liu, X.; Jiang, J. [6]-Paradol suppresses proliferation and metastases of pancreatic cancer by decreasing EGFR and inactivating PI3K/AKT signaling. Cancer Cell Int. 2021, 21, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Wang, D.; Lu, F.; Zhao, R.; Ye, X.; He, L.; Ai, L.; Wu, C.J. Identification of the active substances and mechanisms of ginger for the treatment of colon cancer based on network pharmacology and molecular docking. BioData Min. 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.J. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [Google Scholar] [CrossRef]
- Kanaya, N.; Adams, L.; Takasaki, A.; Chen, S. Whole blueberry powder inhibits metastasis of triple negative breast cancer in a xenograft mouse model through modulation of inflammatory cytokines. Nutr. Cancer. 2014, 66, 242–248. [Google Scholar] [CrossRef]
- Oba, K.; Kobayashi, M.; Matsui, T.; Kodera, Y.; Sakamoto, J. Individual patient based meta-analysis of lentinan for unresectable/recurrent gastric cancer. Anticancer Res. 2009, 29, 2739–2745. [Google Scholar] [PubMed]
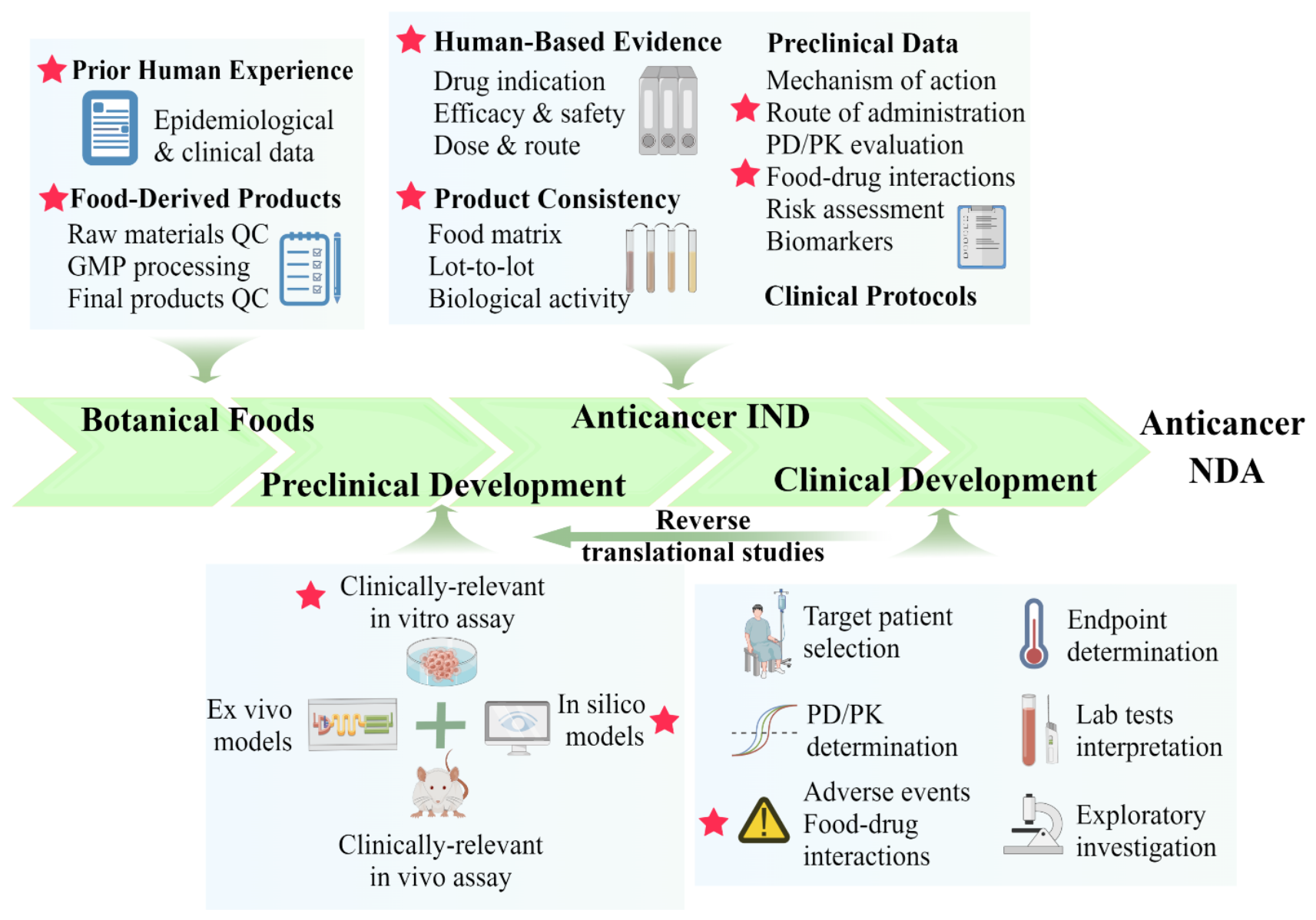

| No. | Checkpoints | Explanations |
|---|---|---|
| 1 | Propose hypothesis based on “prior human experience” | The documented history of human data would allow the researchers and regulatory agents to proceed directly into a clinical evaluation of efficacy and safety. This could potentially shorten expensive preclinical development efforts, as well as reduce the likelihood of development failure. |
| 2 | Establish mechanism-based and clinically relevant in vitro bioassays | A bioassay that reflects the drug’s presumed mechanisms of action or measures the drug-responsive cancer marker should be developed if the active constituents of foods cannot be quantified chemically. This would help assess product batch quality and activity. |
| 3 | Choose clinically representative animal models for testing | Selecting a model that reflects the pathobiology of the cancer (carcinogenesis, progression, and metastasis) as well as the clinically relevant biomarkers and endpoints that can provide supportive evidence for future clinical development. |
| 4 | Perform rigorous quality control measurements and assays to ensure the therapeutic consistency | Therapeutic consistency is maintained, including the source and quality of botanical raw materials, assuring manufacturing processes are GMP compliant, and performing extensive chemical and biological characterization. |
| 5 | Design clinical trials rationally by selecting the target population with evidence-based indication and clinical need | For both ethical and safety concerns, lower-risk cancer patients are recommended. For the botanical food product with clinical indications and an expected mechanism of action, first-in-human studies should focus on toxicity determination and proof-of-concept target engagement PD studies, while randomized control trials (RCTs) may be preferred in Phase II/III study. |
| 6 | Conduct reverse translational studies to discover mechanisms underlying patient outcomes and clinical observations. | Reverse translational studies, also known as bedside-to-bench research, allow for seamless and cyclical research, where observing patient responses can stimulate new hypotheses that may help refine and guide bench research that can lead to future clinical trials. |
| 7 | Introduce advanced multitarget and computational characterization techniques | Botanical foods can target multiple pathways simultaneously acting on a large response network. Using a single biochemical assay or bulk analysis of genes/proteins has insurmountable limitations. The development of computer-based network pharmacology along with state-of-the-art single-cell and spatial multi-omics analysis would help to define the “network-targets, multiple-component-therapeutics” concept. |
| Name of Database | Food/Supplement | Information Provided | Reference |
|---|---|---|---|
| National Cancer Institute (NCI) Complementary and Alternative Medicine Cancer Therapy Interactions with Food and Dietary Supplements (Physician Data Query, PDQ®) | Over 200 | General information on foods/supplements List of antioxidants, herbs, and foods Foods, dietary supplements, and cancer drug interactions Interactions of foods with cancer therapies Food and dietary supplement summary | [41] |
| Memorial Sloan Kettering Cancer Center Integrative Medicine About Herbs, Botanicals, & Other Products | Over 290 | Clinical summary Food source Purposed uses and benefits of food and herbs Mechanism of action, herb–drug, and herb–lab interactions Contraindications and adverse reactions | [42] |
| The Natural Medicines Comprehensive Database for Foods and Herbs | Over 1200 | Overview of foods and herbs Warnings, safety, adverse effects, dosing, administration, and effectiveness of foods and herbs Drug/Supplement interactions Condition/lab interactions Pharmacokinetics and overdose Mechanism of action | [43,44,45] |
| NCI Drug Dictionary | Over 200 | Scientific names of food and herbs General studies and the description of food/substance Potential benefits and adverse effects Active clinical trials using the agent | [46] |
| US Department of Agriculture Food Data Central Foundation & Experimental Foods | Over 700 | The chemical composition of food Analytical methodology Food procedures research purpose Study design, results, and supplemental information | [47] |
| American Institute for Cancer Research AICR’s Foods that Fight Cancer™ | 26 types of plant-based foods | Food ingredients Ongoing area of investigation in labs and humans “Convincing” or “probable” evidence “Limited suggestive evidence” | [48] |
| Foods | Food–Drug Interaction | Level of Evidence | References |
|---|---|---|---|
| Edible Mushroom | Cyclophosphamide | In vitro or animal study | [39,40,41,42,43,44,45,46,47,48,52,53] |
| Tamoxifen | In vitro or animal study | ||
| Cytochrome P450 2C9 and 3A4 | In vitro or animal study | ||
| Immunosuppressants | Theoretical based on pharmacology | ||
| Antidiabetic drugs | In vitro or animal study Lower-quality randomized controlled trial Theoretical based on pharmacology | ||
| Warfarin | Anecdotal evidence | ||
| Antihypertensive drugs | Theoretical based on pharmacology | ||
| Pomegranate | Cytochrome P450 2C9 and 3A4 | Nonrandomized clinical trial | [39,40,41,42,43,44,45,46,47,48,54,55,56,57,58,59,60] |
| Cytochrome P450 1B1 and 2D6 | In vitro or animal study | ||
| ACE inhibitors | Nonrandomized clinical trial | ||
| Antihypertensive drugs | Nonrandomized clinical trial | ||
| Rosuvastatin (Crestor) | Anecdotal evidence | ||
| Warfarin (Coumadin) | Anecdotal evidence | ||
| Carbamazepine (Tegretol) | In vitro or animal study | ||
| Tolbutamide (Orinase) | In vitro or animal study | ||
| Grape | Cytochrome P450 1A2, 2C19, 2D6, 2E1, and 3A4 | Lower-quality randomized controlled trial In vitro or animal study | [39,40,41,42,43,44,45,46,47,48,61,62,63,64,65,66] |
| Cytochrome P450 2C9 | In vitro or animal study | ||
| Cyclosporine (Neoral, Sandimmume) | Lower-quality randomized controlled trial | ||
| Phenacetin | Lower-quality randomized controlled trial | ||
| Anticoagulant/Antiplatelet drugs | In vitro or animal study | ||
| Midazolam (versed) | In vitro or animal study | ||
| Cinnamon | Cytochrome P450 2C9, 3A4, 2A6, and 2D | In vitro or animal study | [39,40,41,42,43,44,45,46,47,48,67,68,69,70,71] |
| Antidiabetic drugs | Lower quality randomized controlled trial | ||
| Hepatotoxic drugs | Theoretical based on pharmacology | ||
| Statins | Case–control study | ||
| Pioglitazone | In vitro or animal study | ||
| Garlic | Cytochrome P450 2E1 and 3A4 | Nonrandomized clinical trial, lower quality randomized controlled trial | [39,40,41,42,43,44,45,46,47,48,72,73,74,75,76,77,78,79] |
| Cytochrome P450 2C9 and 2C19 | In vitro or animal study | ||
| Tacrolimus (Prograf) | Case–control study | ||
| Antidiabetic drugs | Lower-quality randomized controlled trial | ||
| Protease Inhibitors (Darunavir, Saquinavir) | Nonrandomized clinical trial | ||
| Anticoagulant/Antiplatelet drugs | Theoretical based on pharmacology | ||
| Antihypertensive drugs | Theoretical based on pharmacology | ||
| Atazanavir (Reyataz) | Anecdotal evidence | ||
| Isoniazid | In vitro or animal study | ||
| Warfarin (Coumadin) | Anecdotal evidence | ||
| Insulin | In vitro or animal study | ||
| P-Glycoprotein substrates | Clinical cohort study | ||
| Broccoli | Cytochrome P450 1A2 and 2A6 | Lower-quality randomized controlled trial | [39,40,41,42,43,44,45,46,47,48,80,81] |
| Tomato | Cytochrome P450 1A2, 2C9, 2D6, 2E1, and 3A4 | Clinical cohort study In vitro or animal study | [39,40,41,42,82] |
| Walnut | Cytochrome P450 2A2, 2B1, 2B2, 2C6, 2C11, and 3A1 | In vitro or animal study | [39,40,41,42,83] |
| Ginger | Cytochrome P450 2C9, 2C19, 2D6 and 3A4 | In vitro or animal study | [39,40,41,42,43,84,85,86,87,88,89,90,91] |
| Anticoagulant/Antiplatelet drugs | Nonrandomized clinical trial | ||
| Nifedipine (Procardia) | Nonrandomized clinical trial | ||
| Warfarin (Coumadin) | Lower-quality randomized controlled trial | ||
| Losartan (Cozaar) | In vitro or animal study | ||
| Phenprocoumon (Marcoumar) | Anecdotal evidence | ||
| Antidiabetic drugs | In vitro or animal study | ||
| Calcium channel blockers | In vitro or animal study | ||
| Cyclosporine (Neoral, Sandimmune) | In vitro or animal study | ||
| Metronidazole (Flagyl) | In vitro or animal study | ||
| NSAIDS (diclofenac or ibuprofen) | Clinical cohort study | ||
| Tacrolimus | In vitro or animal study | ||
| Cyclosporine | In vitro or animal study Clinical cohort study | ||
| Berries | Cytochrome P450 1A1, 2A2, 3A1, 2B1, 2B2, 2C6 and 2C11 | In vitro or animal study | [39,40,41,42,43,92,93,94,95,96] |
| Buspirone (BuSpar) | Nonrandomized clinical trial | ||
| Flurbiprofen (NSAID)) | Nonrandomized clinical trial | ||
| Antidiabetic drugs | In vitro or animal study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Chan, Y.S.; Wong, K.; Yoshitake, R.; Sadava, D.; Synold, T.W.; Frankel, P.; Twardowski, P.W.; Lau, C.; Chen, S. Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials. Cancers 2023, 15, 701. https://doi.org/10.3390/cancers15030701
Wang X, Chan YS, Wong K, Yoshitake R, Sadava D, Synold TW, Frankel P, Twardowski PW, Lau C, Chen S. Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials. Cancers. 2023; 15(3):701. https://doi.org/10.3390/cancers15030701
Chicago/Turabian StyleWang, Xiaoqiang, Yin S. Chan, Kelly Wong, Ryohei Yoshitake, David Sadava, Timothy W. Synold, Paul Frankel, Przemyslaw W. Twardowski, Clayton Lau, and Shiuan Chen. 2023. "Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials" Cancers 15, no. 3: 701. https://doi.org/10.3390/cancers15030701
APA StyleWang, X., Chan, Y. S., Wong, K., Yoshitake, R., Sadava, D., Synold, T. W., Frankel, P., Twardowski, P. W., Lau, C., & Chen, S. (2023). Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials. Cancers, 15(3), 701. https://doi.org/10.3390/cancers15030701







