Evaluation of Anlotinib Combined with Adriamycin and Ifosfamide as Conversion Therapy for Unresectable Soft Tissue Sarcomas
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Procedures
2.3. Tumor Response and Outcomes
2.4. Safety
2.5. Follow-Up Study
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Tumor Response
3.3. Tumor Size
3.4. Subgroup Analysis
3.5. Long-Term Survival
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; de Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Seddon, B.; Strauss, S.J.; Whelan, J.; Leahy, M.; Woll, P.J.; Cowie, F.; Rothermundt, C.; Wood, Z.; Benson, C.; Ali, N.; et al. Gemcitabine and docetaxel versus doxorubicin as first-line treatment in previously untreated advanced unresectable or metastatic soft-tissue sarcomas (GeDDiS): A randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Kopp, H.G.; Patel, S.; Brücher, B.; Hartmann, J.T. Potential combination chemotherapy approaches for advanced adult-type soft-tissue sarcoma. Am. J. Clin. Dermatol. 2008, 9, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Ferrari, S.; Quagliuolo, V.; Broto, J.M.; Pousa, A.L.; Grignani, G.; Basso, U.; Blay, J.Y.; Tendero, O.; Beveridge, R.D.; et al. Histotype-tailored neoadjuvant chemotherapy versus standard chemotherapy in patients with high-risk soft-tissue sarcomas (ISG-STS 1001): An international, open-label, randomised, controlled, phase 3, multicentre trial. Lancet Oncol. 2017, 18, 812–822. [Google Scholar] [CrossRef]
- Ratan, R.; Patel, S.R. Chemotherapy for soft tissue sarcoma. Cancer 2016, 122, 2952–2960. [Google Scholar] [CrossRef]
- Salah, S.; Lewin, J.; Amir, E.; Razak, A.A. Tumor necrosis and clinical outcomes following neoadjuvant therapy in soft tissue sarcoma: A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 69, 1–10. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar]
- Folkman, J.; Merler, E.; Abernathy, C.; Williams, G. Isolation of a tumor factor responsible for angiogenesis. J. Exp. Med. 1971, 133, 275–288. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zheng, F.; Ren, D.; Du, F.; Dong, Q.; Wang, Z.; Zhao, F.; Ahmad, R.; Zhao, J. Anlotinib: A novel multi-targeting tyrosine kinase inhibitor in clinical development. J. Hematol. Oncol. 2018, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Fang, Z.; Hong, X.; Yao, Y.; Sun, P.; Wang, G.; Du, F.; Sun, Y.; Wu, Q.; Qu, G.; et al. Safety and Efficacy of Anlotinib, a Multikinase Angiogenesis Inhibitor, in Patients with Refractory Metastatic Soft-Tissue Sarcoma. Clin. Cancer. Res. 2018, 24, 5233–5238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Zhang, S.L.; Yang, H.; Zhuang, R.Y.; Guo, X.; Tong, H.X.; Zhang, Y.; Lu, W.Q.; Zhou, Y.H. Efficacy and safety of anlotinib, a multikinase angiogenesis inhibitor, in combination with epirubicin in preclinical models of soft tissue sarcoma. Cancer Med. 2020, 9, 3344–3352. [Google Scholar] [CrossRef]
- Jo, V.Y.; Fletcher, C.D. WHO classification of soft tissue tumours: An update based on the 2013 (4th) edition. Pathology 2014, 46, 95–104. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Weiss, A.R.; Chen, Y.L.; Scharschmidt, T.J.; Chi, Y.Y.; Tian, J.; Black, J.O.; Davis, J.L.; Fanburg-Smith, J.C.; Zambrano, E.; Anderson, J.; et al. Pathological response in children and adults with large unresected intermediate-grade or high-grade soft tissue sarcoma receiving preoperative chemoradiotherapy with or without pazopanib (ARST1321): A multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 1110–1122. [Google Scholar] [CrossRef]
- Viallard, C.; Larrivée, B. Tumor angiogenesis and vascular normalization: Alternative therapeutic targets. Angiogenesis 2017, 20, 409–426. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Xie, L.; Ji, T.; Guo, W. Anti-angiogenesis target therapy for advanced osteosarcoma. Oncol. Rep. 2017, 38, 625–636. [Google Scholar] [CrossRef]
- Xie, C.; Wan, X.; Quan, H.; Zheng, M.; Fu, L.; Li, Y.; Lou, L. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor-2 inhibitor. Cancer Sci. 2018, 109, 1207–1219. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Song, X.; Yang, D.; Bai, D.; Yao, Y.; Lu, N.A. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRbeta and FGFR1. Gene 2018, 654, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Niu, W.; Du, F.; Du, C.; Li, S.; Wang, J.; Li, L.; Wang, F.; Hao, Y.; Li, C.; et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J. Hematol. Oncol. 2016, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Y.L.; Chen, G.; Liu, X.; Zhu, Y.; Lu, S.; Feng, J.; He, J.; Han, B.; Wang, J.; et al. BEYOND: A Randomized, Double-Blind, Placebo-Controlled, Multicenter, Phase III Study of First-Line Carboplatin/Paclitaxel Plus Bevacizumab or Placebo in Chinese Patients With Advanced or Recurrent Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2015, 33, 2197–2204. [Google Scholar] [CrossRef]
- Verschraegen, C.F.; Arias-Pulido, H.; Lee, S.J.; Movva, S.; Cerilli, L.A.; Eberhardt, S.; Schmit, B.; Quinn, R.; Muller, C.Y.; Rabinowitz, I.; et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: The Axtell regimen. Ann. Oncol. 2012, 23, 785–790. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, X.; Yan, C.; Feng, R.; Sah, B.K.; Yang, Z.; Zhu, Z.; Liu, W.; Xu, W.; Ni, Z.; et al. Effect of apatinib plus neoadjuvant chemotherapy followed by resection on pathologic response in patients with locally advanced gastric adenocarcinoma: A single-arm, open-label, phase II trial. Eur. J. Cancer 2020, 130, 12–19. [Google Scholar] [CrossRef]
- Wang, H.Y.; Chu, J.F.; Zhang, P.; Wang, J.Q.; Yan, Z.; Yao, S.N.; Yao, Z.H.; Liu, Y.Y. Safety and Efficacy of Chemotherapy Combined with Anlotinib Plus Anlotinib Maintenance in Chinese Patients with Advanced/Metastatic Soft Tissue Sarcoma. Onco Targets Ther. 2020, 13, 1561–1568. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Wang, J.; Zhang, P.; Li, C.; Wang, B.; Liu, G.; Yao, W. Gemcitabine Plus Anlotinib Is Effective and Safe Compared to Gemcitabine Plus Docetaxel in Advanced Soft Tissue Sarcoma. Front. Oncol. 2022, 12, 3156. [Google Scholar] [CrossRef]
- Liu, C.Y.; Yen, C.C.; Chen, W.M.; Chen, T.H.; Chen, P.C.H.; Wu, H.T.H.; Shiau, C.Y.; Wu, Y.C.; Liu, C.L.; Tzeng, C.H. Soft tissue sarcoma of extremities: The prognostic significance of adequate surgical margins in primary operation and reoperation after recurrence. Ann. Surg. Oncol. 2010, 17, 2102–2111. [Google Scholar] [CrossRef]
- Biau, D.J.; Ferguson, P.C.; Chung, P.; Griffin, A.M.; Catton, C.N.; O’Sullivan, B.; Wunder, J.S. Local recurrence of localized soft tissue sarcoma: A new look at old predictors. Cancer 2012, 118, 5867–5877. [Google Scholar] [CrossRef]
- Potter, B.K.; Hwang, P.F.; Forsberg, J.A.; Hampton, C.B.; Graybill, J.C.; Peoples, G.E.; Stojadinovic, A. Impact of margin status and local recurrence on soft-tissue sarcoma outcomes. JBJS 2013, 95, e151. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.Y.; Kim, H.S.; Han, I. Impact of surgical margin on survival in extremity soft tissue sarcoma: A systematic review and meta-analysis. Medicine 2021, 100, e24124. [Google Scholar] [CrossRef] [PubMed]
- Allignet, B.; Meurgey, A.; Bouhamama, A.; Karanian, M.; Meeus, P.; Vaz, G.; Gouin, F.; Moncharmont, C.; Prapant, S.; Waissi, W.; et al. Impact of histological subtype on radiological and pathological response after neoadjuvant radiotherapy in soft tissue sarcoma. Eur. J. Surg. Oncol. 2021, 47, 2995–3003. [Google Scholar] [CrossRef] [PubMed]


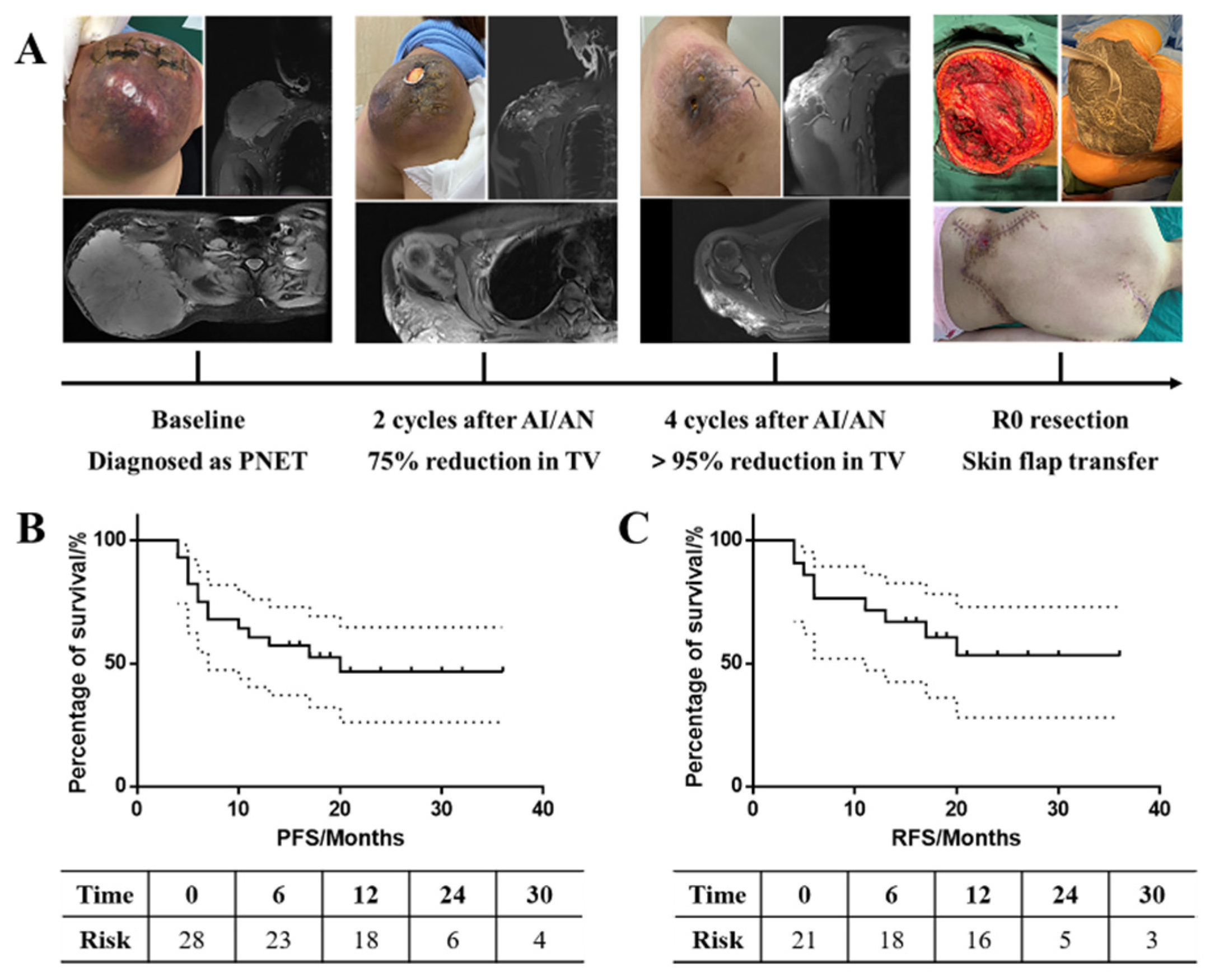
| n | Percentage (%) | |
|---|---|---|
| Gender | ||
| Male | 17 | 60.71% |
| Female | 11 | 39.29% |
| Age (years) | 39.11 ± 13.46 (12–65) | |
| <30 | 10 | 35.71% |
| 31–50 | 11 | 39.29% |
| >50 | 7 | 25.00% |
| Tumor location | ||
| Upper limb | 7 | 25.00% |
| Lower limbs | 17 | 60.71% |
| Trunk | 4 | 14.29% |
| Histology | ||
| Fibrosarcoma | 6 | 21.43% |
| Synovial sarcoma | 6 | 21.43% |
| Liposarcoma | 6 | 21.43% |
| UPS | 4 | 14.28% |
| Others | 6 | 21.43% |
| Surgery | ||
| Yes | 9 | 32.14% |
| No | 19 | 67.86% |
| Radiotherapy | ||
| Yes | 1 | 3.70% |
| No | 27 | 96.30% |
| ECOG PS score | ||
| 0–1 | 27 | 96.30% |
| 2 | 1 | 3.70% |
| Longestdiameter (cm) | ||
| <10 | 10 | 35.71% |
| 10~20 | 14 | 50.00% |
| >20 | 4 | 14.29% |
| Response | n | Percentage (95% CI, %) |
|---|---|---|
| CR | 0 | 0 |
| PR | 8 | 28.57 (10.7, 46.4) |
| SD | 20 | 71.43 (53.6, 89.3) |
| PD | 0 | 0 |
| ORR | 28.57 (10.7, 46.4) | |
| DCR | 100 | |
| Surgical margins | 24 | |
| R0 | 21 | 87.50 |
| R2 | 3 | 12.50 |
| Surgical type | ||
| Limb-salvage | 22 | 91.67 |
| Amputation | 2 | 8.33 |
| Mean PFS/months | 21.70 (16.38, 27.02) | |
| 6 months PFR | 75.00 (54.60, 87.22) | |
| 12 months PFR | 60.71 (40.38, 75.99) | |
| Mean RFS/months | 23.97 (18.00, 29.94) | |
| 6 months RFR | 76.19 (51.93, 89.33) | |
| 12 months RFR | 71.43 (47.15, 86.02) | |
| Overall AEs | Grade ≥ 3 AEs | |||
|---|---|---|---|---|
| n | % | n | % | |
| Leukocytopenia | 22 | 78.57 | 16 | 57.14 |
| Anorexia | 17 | 60.71 | 0 | 0 |
| Fatigue | 16 | 57.14 | 0 | 0 |
| Hypertension | 12 | 42.86 | 4 | 14.29 |
| Oral mucositis | 12 | 42.86 | 0 | 0 |
| Hand-foot syndrome | 11 | 39.29 | 2 | 7.14 |
| Anemia | 8 | 28.57 | 0 | 0 |
| Abnormal liver function | 7 | 25.00 | 0 | 0 |
| Nausea and vomiting | 7 | 25.00 | 1 | 3.57 |
| Weight loss | 5 | 17.86 | 0 | 0 |
| Thrombocytopenia | 5 | 17.86 | 0 | 0 |
| Decrease in total protein | 5 | 17.86 | 0 | 0 |
| Diarrhea | 5 | 17.86 | 0 | 0 |
| Gingival bleeding | 5 | 17.86 | 1 | 3.57 |
| Hypothyroidism | 4 | 14.29 | 0 | 0 |
| Proteinuria | 4 | 14.29 | 1 | 3.57 |
| Skin pigmentation | 2 | 7.14 | 0 | 0 |
| Alopecia | 2 | 7.14 | 0 | 0 |
| Hematuresis | 2 | 7.14 | 0 | 0 |
| Delayed wound healing | 2 | 7.14 | 0 | 0 |
| Throat pain | 1 | 3.57 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, Z.; Lu, Y.; Li, M.; Fu, Z.; Akbar, Y.; Li, J.; Chen, G.; Zhang, H.-M.; Wang, Q.; Xiang, L.; et al. Evaluation of Anlotinib Combined with Adriamycin and Ifosfamide as Conversion Therapy for Unresectable Soft Tissue Sarcomas. Cancers 2023, 15, 700. https://doi.org/10.3390/cancers15030700
Long Z, Lu Y, Li M, Fu Z, Akbar Y, Li J, Chen G, Zhang H-M, Wang Q, Xiang L, et al. Evaluation of Anlotinib Combined with Adriamycin and Ifosfamide as Conversion Therapy for Unresectable Soft Tissue Sarcomas. Cancers. 2023; 15(3):700. https://doi.org/10.3390/cancers15030700
Chicago/Turabian StyleLong, Zuoyao, Yajie Lu, Minghui Li, Zhanli Fu, Yunus Akbar, Jing Li, Guojing Chen, Hong-Mei Zhang, Qi Wang, Liangbi Xiang, and et al. 2023. "Evaluation of Anlotinib Combined with Adriamycin and Ifosfamide as Conversion Therapy for Unresectable Soft Tissue Sarcomas" Cancers 15, no. 3: 700. https://doi.org/10.3390/cancers15030700
APA StyleLong, Z., Lu, Y., Li, M., Fu, Z., Akbar, Y., Li, J., Chen, G., Zhang, H.-M., Wang, Q., Xiang, L., & Wang, Z. (2023). Evaluation of Anlotinib Combined with Adriamycin and Ifosfamide as Conversion Therapy for Unresectable Soft Tissue Sarcomas. Cancers, 15(3), 700. https://doi.org/10.3390/cancers15030700





