Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Dataset
- Age, gender, and race;
- Baseline American Society of Anesthesiologists (ASA) score;
- Tumor side, clinical size, and surgical complexity (defined according to the R.E.N.A.L. score) [11];
- Hemostatic technique and eventual conversions from SL to RR;
- Serum creatinine levels assessed at baseline, at discharge, and 3, 6, and 12 months after surgery. For each timepoint, eGFR was calculated by means of the Chronic Kidney Disease Epidemiology Collaboration formula [12] and the National Kidney Foundation (NKF); chronic kidney disease (CKD) stages were defined accordingly [13]. Based on the NKF recommendations, a >30% reduction in the postoperative eGFR was considered as a “significant renal function deterioration” (sRFD), while any worsening from stages I-II to ≧ IIIa (from IIIa to ≧ IIIb, and from IIIb to ≧ IV) was defined as “significant CKD stage migration” (sCKDsm) [14,15];
- Postoperative complications (stratified according to the Clavien–Dindo (CD) classification system [16]) and length of hospital stay (LOS);
- Perioperative outcomes combined into our previously published trifecta (negative surgical margins, no CD≧3 complications, and no sRFD) to assess surgical quality [14].
- Final histology;
- Functional outcomes at last available follow-up.
2.2. Perioperative Care and Surgical Technique
2.3. Study Objective
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simone, G.; De Nunzio, C.; Ferriero, M.; Cindolo, L.; Brookman-May, S.; Papalia, R.; Sperduti, I.; Collura, D.; Leonardo, C.; Anceschi, U.; et al. Trends in the Use of Pa0rtial Nephrectomy for CT1 Renal Tumors: Analysis of a 10-Yr European Multicenter Dataset. Eur. J. Surg. Oncol. 2016, 42, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Demirjian, S.; Derweesh, I.H.; Takagi, T.; Zhang, Z.; Velet, L.; Ercole, C.E.; Fergany, A.F.; Campbell, S.C. Survival and Functional Stability in Chronic Kidney Disease Due to Surgical Removal of Nephrons: Importance of the New Baseline Glomerular Filtration Rate. Eur. Urol. 2015, 68, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Lane, B.R.; Lohse, C.M.; Leibovich, B.C.; Fergany, A.; Frank, I.; Gill, I.S.; Blute, M.L.; Campbell, S.C. Every Minute Counts When the Renal Hilum Is Clamped during Partial Nephrectomy. Eur. Urol. 2010, 58, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.M.; De Castro Abreu, A.L.; Leslie, S.; Cai, J.; Huang, E.Y.H.; Lewandowski, P.M.; Lee, D.; Dharmaraja, A.; Berger, A.K.; Goh, A.; et al. Robotic Partial Nephrectomy with Superselective versus Main Artery Clamping: A Retrospective Comparison. Eur. Urol. 2014, 66, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Baumert, H.; Mathieu, R.; Masson-Lecomte, A.; Grassano, Y.; Roumiguié, M.; Massoud, W.; Abd El Fattah, V.; Bruyère, F.; Droupy, S.; et al. Early Unclamping Technique during Robot-Assisted Laparoscopic Partial Nephrectomy Can Minimise Warm Ischaemia without Increasing Morbidity. BJU Int. 2014, 114, 741–747. [Google Scholar] [CrossRef]
- Bertolo, R.; Simone, G.; Garisto, J.; Nakhoul, G.; Armanyous, S.; Agudelo, J.; Costantini, M.; Tuderti, G.; Gallucci, M.; Kaouk, J. Off-Clamp vs on-Clamp Robotic Partial Nephrectomy: Perioperative, Functional and Oncological Outcomes from a Propensity-Score Matching between Two High-Volume Centers. Eur. J. Surg. Oncol. 2019, 45, 1232–1237. [Google Scholar] [CrossRef]
- Bahler, C.D.; Sundaram, C.P. Effect of Renal Reconstruction on Renal Function after Partial Nephrectomy. J. Endourol. 2016, 30, S37–S41. [Google Scholar] [CrossRef]
- Rouach, Y.; Delongchamps, N.B.; Patey, N.; Fontaine, E.; Timsit, M.O.; Thiounn, N.; Méjean, A. Suture or Hemostatic Agent during Laparoscopic Partial Nephrectomy? A Randomized Study Using a Hypertensive Porcine Model. Urology 2009, 73, 172–177. [Google Scholar] [CrossRef]
- Singh, D.; Gill, I.S. Renal Artery Pseudoaneurysm Following Laparoscopic Partial Nephrectomy. J. Urol. 2005, 174, 2256–2259. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Gallucci, M. “Zero Ischaemia”, Sutureless Laparoscopic Partial Nephrectomy for Renal Tumours with a Low Nephrometry Score. BJU Int. 2012, 110, 124–130. [Google Scholar] [CrossRef]
- Kutikov, A.; Smaldone, M.C.; Egleston, B.L.; Manley, B.J.; Canter, D.J.; Simhan, J.; Boorjian, S.A.; Viterbo, R.; Chen, D.Y.T.; Greenberg, R.E.; et al. Anatomic Features of Enhancing Renal Masses Predict Malignant and High-Grade Pathology: A Preoperative Nomogram Using the RENAL Nephrometry Score. Eur. Urol. 2011, 60, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L.; et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of Chronic Kidney Disease and Decreased Kidney Function in the Adult US Population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brassetti, A.; Anceschi, U.; Bertolo, R.; Ferriero, M.; Tuderti, G.; Capitanio, U.; Larcher, A.; Garisto, J.; Antonelli, A.; Mottire, A.; et al. Surgical Quality, Cancer Control and Functional Preservation: Introducing a Novel Trifecta for Robot-Assisted Partial Nephrectomy. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2019, 72, 82–90. [Google Scholar] [CrossRef]
- Levey, A.S.; Inker, L.A.; Matsushita, K.; Greene, T.; Willis, K.; Lewis, E.; De Zeeuw, D.; Cheung, A.K.; Coresh, J. GFR Decline as an End Point for Clinical Trials in CKD: A Scientific Workshop Sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 821–835. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal with Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Simone, G.; Misuraca, L.; Tuderti, G.; Minisola, F.; Ferriero, M.; Romeo, G.; Costantini, M.; Al-Rawashdah, S.F.; Guaglianone, S.; Gallucci, M. Purely Off-Clamp Robotic Partial Nephrectomy: Preliminary 3-Year Oncological and Functional Outcomes. Urology 2018, 25, 606–614. [Google Scholar] [CrossRef]
- Brassetti, A.; Cacciamani, G.E.; Mari, A.; Garisto, J.D.; Bertolo, R.; Sundaram, C.P.; Derweesh, I.; Bindayi, A.; Dasgupta, P.; Porter, J.; et al. On-Clamp vs. Off-Clamp Robot-Assisted Partial Nephrectomy for CT2 Renal Tumors: Retrospective Propensity-Score-Matched Multicenter Outcome Analysis. Cancers 2022, 14, 4431. [Google Scholar] [CrossRef]
- Mir, M.C.; Ercole, C.; Takagi, T.; Zhang, Z.; Velet, L.; Remer, E.M.; Demirjian, S.; Campbell, S.C. Decline in Renal Function after Partial Nephrectomy: Etiology and Prevention. J. Urol. 2015, 193, 1889–1898. [Google Scholar] [CrossRef]
- Minervini, A.; Campi, R.; Lane, B.R.; De Cobelli, O.; Sanguedolce, F.; Hatzichristodoulou, G.; Antonelli, A.; Noyes, S.; Mari, A.; Rodriguez-Faba, O.; et al. Impact of Resection Technique on Perioperative Outcomes and Surgical Margins after Partial Nephrectomy for Localized Renal Masses: A Prospective Multicenter Study. J. Urol. 2020, 203, 496–504. [Google Scholar] [CrossRef]
- Simone, G.; Papalia, R.; Guaglianone, S.; Carpanese, L.; Gallucci, M. Zero Ischemia Laparoscopic Partial Nephrectomy After Superselective Transarterial Tumor Embolization for Tumors with Moderate Nephrometry Score: Long-Term Results of a Single-Center Experience. J. Endourol. 2011, 25, 1443–1446. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Bartsch, G.; Finter, F.; Hautmann, R.; De Petriconi, R. Laparoscopic Partial Nephrectomy with Selective Control of the Renal Parenchyma: Initial Experience with a Novel Laparoscopic Clamp. BJU Int. 2009, 103, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Qin, C.; Yin, C.; Meng, X.; Ju, X.; Li, J.; Lv, Q.; Zhang, W.; Xu, Z. Laparoscopic Partial Nephrectomy with Segmental Renal Artery Clamping: Technique and Clinical Outcomes. Eur. Urol. 2011, 59, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.M.; Gill, I.S. Halving Ischemia Time during Laparoscopic Partial Nephrectomy. J. Urol. 2008, 179, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.S.; Eisenberg, M.S.; Aron, M.; Berger, A.; Ukimura, O.; Patil, M.B.; Campese, V.; Thangathurai, D.; Desai, M.M. “Zero Ischemia” Partial Nephrectomy: Novel Laparoscopic and Robotic Technique. Eur. Urol. 2011, 59, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Gill, I.S.; Mottrie, A.; Kutikov, A.; Patard, J.J.; Alcaraz, A.; Rogers, C.G. Indications, Techniques, Outcomes, and Limitations for Minimally Ischemic and off-Clamp Partial Nephrectomy: A Systematic Review of the Literature. Eur. Urol. 2015, 68, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, U.; Brassetti, A.; Bertolo, R.; Tuderti, G.; Ferriero, M.C.; Mastroianni, R.; Flammia, R.S.; Costantini, M.; Kaouk, J.; Leonardo, C.; et al. On-Clamp versus Purely off-Clamp Robot-Assisted Partial Nephrectomy in Solitary Kidneys: Comparison of Perioperative Outcomes and Chronic Kidney Disease Progression at Two High- Volume Centers. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2020, 73, 739–745. [Google Scholar] [CrossRef]
- Hidas, G.; Lupinsky, L.; Kastin, A.; Moskovitz, B.; Groshar, D.; Nativ, O. Functional Significance of Using Tissue Adhesive Substance in Nephron-Sparing Surgery: Assessment by Quantitative SPECT of 99m Tc-Dimercaptosuccinic Acid Scintigraphy. Eur. Urol. 2007, 52, 785–790. [Google Scholar] [CrossRef]
- Bahler, C.D.; Clint Cary, K.; Garg, S.; DeRoo, E.M.; Tabib, C.H.; Kansal, J.K.; Francesca Monn, M.; Flack, C.K.; Masterson, T.A.; Kumar Sandrasegaran, M.; et al. Differentiating Reconstructive Techniques in Partial Nephrectomy: A Propensity Score Analysis. Can. J. Urol. 2015, 22, 7788–7796. [Google Scholar]
- Tohi, Y.; Murata, S.; Makita, N.; Suzuki, I.; Kubota, M.; Sugino, Y.; Inoue, K.; Ueda, H.; Kawakita, M. Absence of Asymptomatic Unruptured Renal Artery Pseudoaneurysm on Contrast-Enhanced Computed Tomography after Robot-Assisted Partial Nephrectomy without Parenchymal Renorrhaphy. Asian J. Urol. 2020, 7, 24–28. [Google Scholar] [CrossRef]
- Itoh, S.; Fukuzawa, K.; Shitomi, Y.; Okamoto, M.; Kinoshita, T.; Taketomi, A.; Shirabe, K.; Wakasugi, K.; Maehara, Y. Impact of the VIO System in Hepatic Resection for Patients with Hepatocellular Carcinoma. Surg. Today 2012, 42, 1176–1182. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Miyoshi, K.; Nakamura, K. VIO Soft-Coagulation System for Major Pulmonary Resections: Results in 68 Patients with Primary Lung Cancer. Gen. Thorac. Cardiovasc. Surg. 2011, 59, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Nagakawa, Y.; Tsuchida, A.; Saito, H.; Tohyama, Y.; Matsudo, T.; Kawakita, H.; Ikeda, T.; Kasuya, K.; Ozawa, T.; Aoki, T. The VIO Soft-Coagulation System Can Prevent Pancreatic Fistula Following Pancreatectomy. J. Hepato-Biliary-Pancreat. Surg. 2008, 15, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, A.; Takayama, T.; Teratani, T.; Kubo, T.; Kamei, J.; Sugihara, T.; Ando, S.; Morita, T.; Fujimura, T. Histological and Radiological Evaluation of Thermal Denaturation Depth Using Soft Coagulation during Partial Nephrectomy in Living Pigs. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2021, 28, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Komori, H.; Rii, J.; Ochi, A.; Suzuki, K.; Shiga, N.; Nishiyama, H. Soft Coagulation in Partial Nephrectomy without Renorrhaphy: Feasibility of a New Technique and Early Outcomes. Int. J. Urol. 2014, 21, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Hongo, F.; Kawauchi, A.; Ueda, T.; Fujihara-Iwata, A.; Nakamura, T.; Naya, Y.; Kamoi, K.; Okihara, K.; Miki, T.; Fumiya, H.M.D. Laparoscopic Off-Clamp Partial Nephrectomy Using Soft Coagulation. Int. J. Urol. 2015, 22, 731–734. [Google Scholar] [CrossRef]
- Tohi, Y.; Murata, S.; Makita, N.; Suzuki, I.; Kubota, M.; Sugino, Y.; Inoue, K.; Kawakita, M. Comparison of Perioperative Outcomes of Robot-Assisted Partial Nephrectomy without Renorrhaphy: Comparative Outcomes of CT1a versus CT1b Renal Tumors. Int. J. Urol. 2019, 26, 885–889. [Google Scholar] [CrossRef]
- Yoshida, T.; Okinaka, Y.; Tomita, K.; Tsuru, T.; Kageyama, S.; Narita, M.; Kawauchi, A. Off-Clamp Tumor Excision Using Soft Coagulation in Laparoscopic and Robotic Partial Nephrectomy. Asian J. Endosc. Surg. 2020, 13, 519–525. [Google Scholar] [CrossRef]
- Naito, S.; Nakashima, M.; Kimoto, Y.; Nakamura, M.; Kotoh, S.; Tanaka, M.; Kumazawa, J. Application of Microwave Tissue Coagulator in Partial Nephrectomy for Renal Cell Carcinoma. J. Urol. 1998, 159, 960–962. [Google Scholar] [CrossRef]
- Ishiyama, Y.; Kondo, T.; Ishihara, H.; Yoshida, K.; Iizuka, J.; Tanabe, K.; Takagi, T. Association between Ureteral Clamping Time and Acute Kidney Injury during Robot-Assisted Radical Cystectomy. Curr. Oncol. 2021, 28, 4986–4997. [Google Scholar] [CrossRef]
- Mancini, M.; Righetto, M.; Baggio, G. Gender-Related Approach to Kidney Cancer Management: Moving Forward. Int. J. Mol. Sci. 2020, 21, 3378. [Google Scholar] [CrossRef] [PubMed]
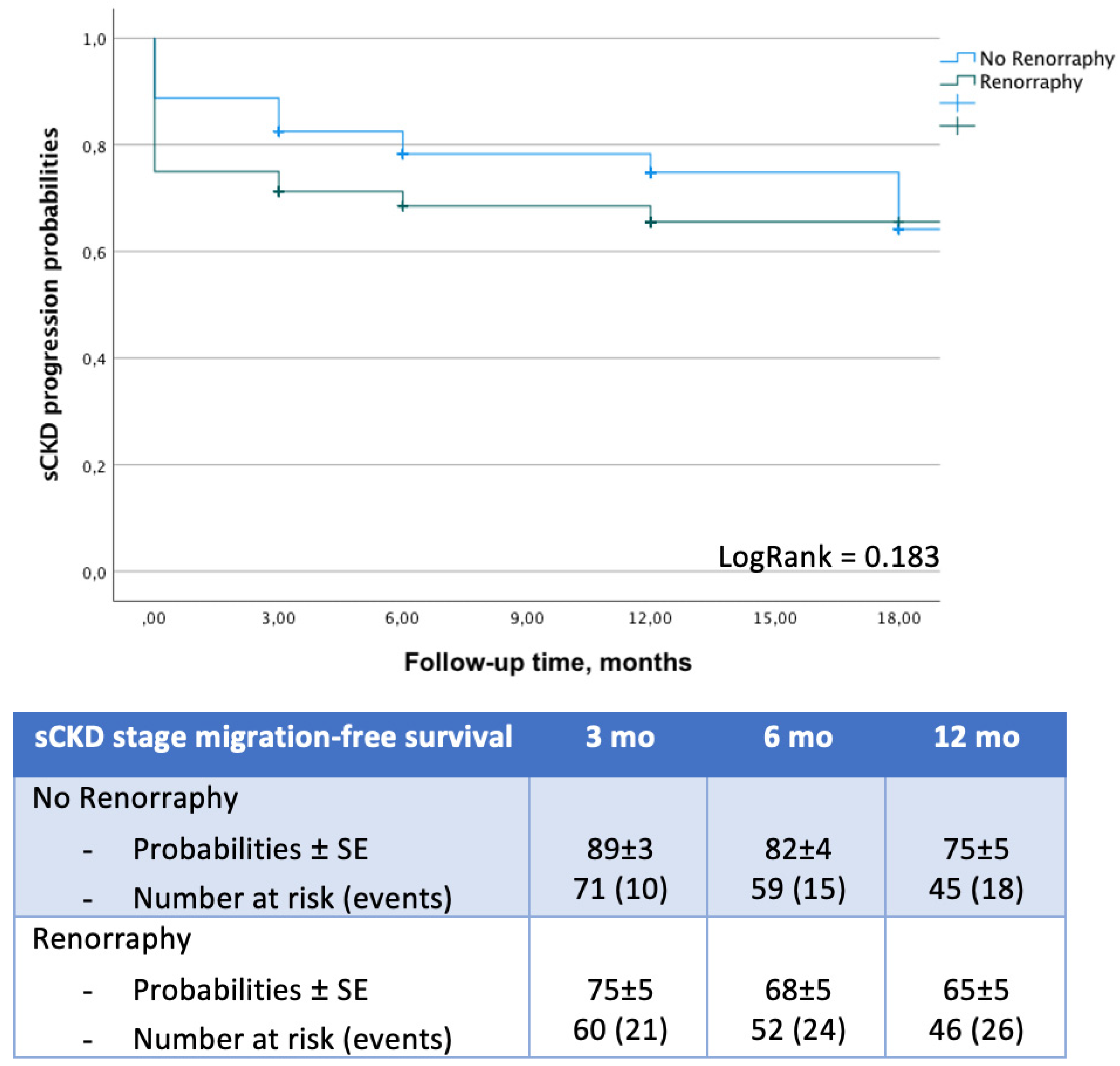
| Overall n = 533 | SL n = 175 (33%) | RR n = 358 (67%) | p | PSM SL n = 80 | PSM RR n = 80 | p | |
|---|---|---|---|---|---|---|---|
| Age, years | 62 (54–71) | 64 (55–72) | 61 (52–70) | 0.04 | 64 (56–73) | 64 (55–73) | 0.84 |
| Male gender, n (%) | 333 (62%) | 108 (62%) | 225 (63%) | 0.79 | 51 (64%) | 53 (66%) | 0.74 |
| ASA ≧ 3, n (%) | 124 (23%) | 32 (18%) | 92 (26%) | 0.06 | 13 (16%) | 21 (26%) | 0.12 |
| Baseline eGFR, ml/min | 81.8 (69.4–95.7) | 82.1 (68.3–97.2) | 81.7 (69.7–94.2) | 0.59 | 79 (66.9–95.1) | 78.1 (69.5–93.5) | 0.86 |
| Baseline CKD stage | 0.76 | 0.74 | |||||
| 1–2 | 466 (87%) | 155 (88%) | 311 (87%) | 71 (89%) | 70 (88%) | ||
| 3A–3B | 59 (11%) | 17 (10%) | 42 (12%) | 7 (9%) | 9 (11%) | ||
| 4–5 | 8 (2%) | 3 (2%) | 5 (1%) | 2 (2%) | 1 (1%) | ||
| Tumor size, cm | 3.5 (2.5–5) | 3 (2.5–5) | 4 (3–5) | 0.16 | 3 (2.5–4.5) | 3.5 (2.5–5) | 0.70 |
| cT2, n (%) | 70 (13%) | 20 (11%) | 50 (14%) | 0.41 | 6 (7%) | 6 (7%) | 1.000 |
| RENAL score | 0.005 | 1.000 | |||||
| ≤6 | 192 (36%) | 80 (46%) | 112 (31%) | 32 (40%) | 32 (40%) | ||
| 7–9 | 228 (43%) | 65 (37%) | 163 (46%) | 37 (46%) | 37 (46%) | ||
| ≥10 | 113 (21%) | 30 (17%) | 83 (23%) | 11 (14%) | 11 (14%) | ||
| LOS, d | 3 (2–5) | 2 (2–5) | 3 (3–5) | <0.001 | 2 (2–3) | 3 (3–4) | <0.001 |
| Blood transfusions, n (%) | 24 (4%) | 6 (3%) | 18 (5%) | 0.40 | 2 (2%) | 5 (6%) | 0.25 |
| Postoperative eGFR, ml/min | 73.6 (58.9–87.7) | 75.7 (60.9–90.9) | 72.7 (58.1–86.7) | 0.13 | 76.1 (62.7–89.5) | 72.9 (56.3–84.1) | 0.15 |
| Postoperative CKD stage | 0.61 | 0.12 | |||||
| 1–2 | 397 (75%) | 135 (77%) | 262 (73%) | 65 (81%) | 54 (68%) | ||
| 3A–3B | 120 (22%) | 35 (20%) | 85 (24%) | 13 (17%) | 24 (30%) | ||
| 4–5 | 16 (3%) | 5 (3%) | 11 (3%) | 2 (2%) | 2 (2%) | ||
| Trifecta, n (%) | 458 (86%) | 163 (93%) | 295 (83%) | <0.001 | 77 (96%) | 67 (84%) | 0.008 |
| pSM, n (%) | 8 (1.5%) | 3 (1.7%) | 5 (1.4%) | 0.78 | 1 (1%) | 1 (1%) | 0.99 |
| CD ≧ 3 complications, n (%) | 21 (4%) | 3 (2%) | 18 (5%) | 0.06 | 1 (1%) | 5 (6%) | 0.09 |
| sRFD, n (%) | 54 (10%) | 10 (6%) | 44 (12%) | 0.02 | 2 (2%) | 9 (11%) | 0.03 |
| Univariable Logistic Regression Analysis to Identify Predictors of Trifecta Achievement | Multivariable Logistic Regression Analysis to Identify Predictors of Trifecta Achievement | Univariable Cox Analysis to Identify Predictors of Significant CDK Stage Migration | Multivariable Cox Analysis to Identify Predictors of Significant CDK Stage Migration | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |||||
| Lower | Higher | Lower | Higher | Lower | Higher | Lower | Higher | |||||||||
| Age | 0.98 | 0.96 | 0.99 | 0.03 | 0.97 | 0.95 | 0.99 | 0.01 | 1.04 | 1.01 | 1.07 | 0.003 | 1.03 | 1.003 | 1.07 | 0.03 |
| Male gender | 0.85 | 0.51 | 1.41 | 0.53 | - | - | - | - | 0.72 | 0.39 | 1.34 | 0.30 | - | - | - | - |
| ASA ≧ 3 | 0.68 | 0.39 | 1.15 | 0.15 | - | - | - | - | 1.54 | 0.79 | 2.99 | 0.21 | - | - | - | - |
| Pre-eGFR | 1.02 | 1.02 | 1.02 | <0.001 | 0.99 | 0.99 | 1.01 | 0.92 | 0.98 | 0.97 | 0.99 | 0.006 | 0.99 | 0.97 | 0.99 | 0.05 |
| RENAL score | <0.001 | <0.001 | 0.65 | - | - | - | - | |||||||||
| ≤6 | ref | - | - | ref | - | - | ref | - | - | |||||||
| 7–9 | 0.68 | 0.37 | 1.26 | 0.22 | 0.67 | 0.35 | 1.27 | 0.22 | 0.82 | 0.40 | 1.68 | 0.63 | ||||
| ≥10 | 0.29 | 0.15 | 0.56 | <0.001 | 0.29 | 0.15 | 0.57 | <0.001 | 0.40 | 0.53 | 2.66 | 0.39 | ||||
| Renorrhaphy | 0.31 | 0.16 | 0.60 | <0.001 | 0.34 | 0.17 | 0.67 | 0.002 | 0.87 | 0.43 | 1.75 | 0.69 | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brassetti, A.; Misuraca, L.; Anceschi, U.; Bove, A.M.; Costantini, M.; Ferriero, M.C.; Guaglianone, S.; Mastroianni, R.; Torregiani, G.; Covotta, M.; et al. Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes. Cancers 2023, 15, 698. https://doi.org/10.3390/cancers15030698
Brassetti A, Misuraca L, Anceschi U, Bove AM, Costantini M, Ferriero MC, Guaglianone S, Mastroianni R, Torregiani G, Covotta M, et al. Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes. Cancers. 2023; 15(3):698. https://doi.org/10.3390/cancers15030698
Chicago/Turabian StyleBrassetti, Aldo, Leonardo Misuraca, Umberto Anceschi, Alfredo Maria Bove, Manuela Costantini, Maria Consiglia Ferriero, Salvatore Guaglianone, Riccardo Mastroianni, Giulia Torregiani, Marco Covotta, and et al. 2023. "Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes" Cancers 15, no. 3: 698. https://doi.org/10.3390/cancers15030698
APA StyleBrassetti, A., Misuraca, L., Anceschi, U., Bove, A. M., Costantini, M., Ferriero, M. C., Guaglianone, S., Mastroianni, R., Torregiani, G., Covotta, M., Tuderti, G., & Simone, G. (2023). Sutureless Purely Off-Clamp Robot-Assisted Partial Nephrectomy: Avoiding Renorrhaphy Does Not Jeopardize Surgical and Functional Outcomes. Cancers, 15(3), 698. https://doi.org/10.3390/cancers15030698








