Diffusion Weighted Imaging in Neuro-Oncology: Diagnosis, Post-Treatment Changes, and Advanced Sequences—An Updated Review
Simple Summary
Abstract
1. Introduction
2. Gliomas and Cellularity
3. Gliomas and Molecular Biology
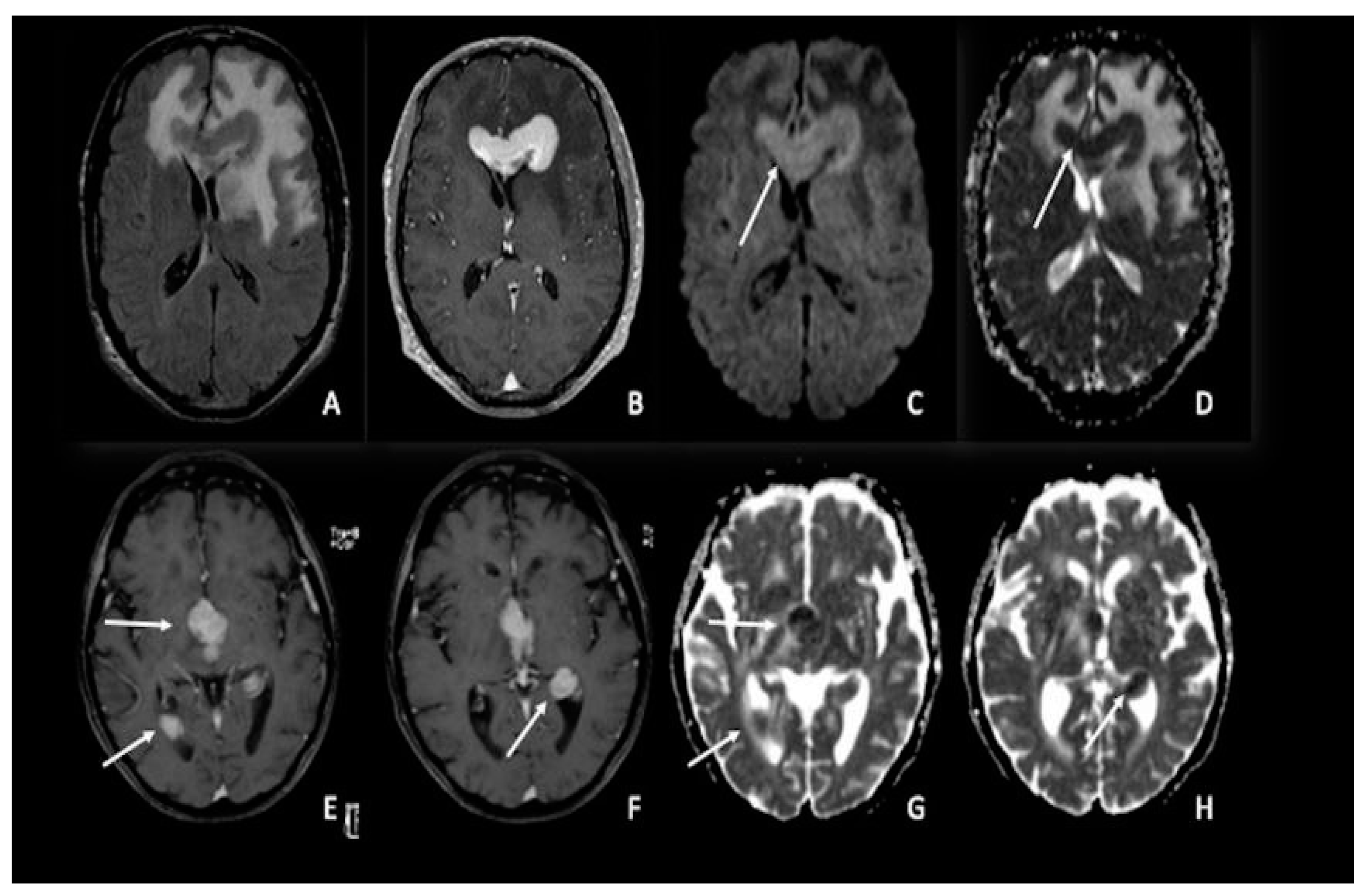
4. Lymphomas
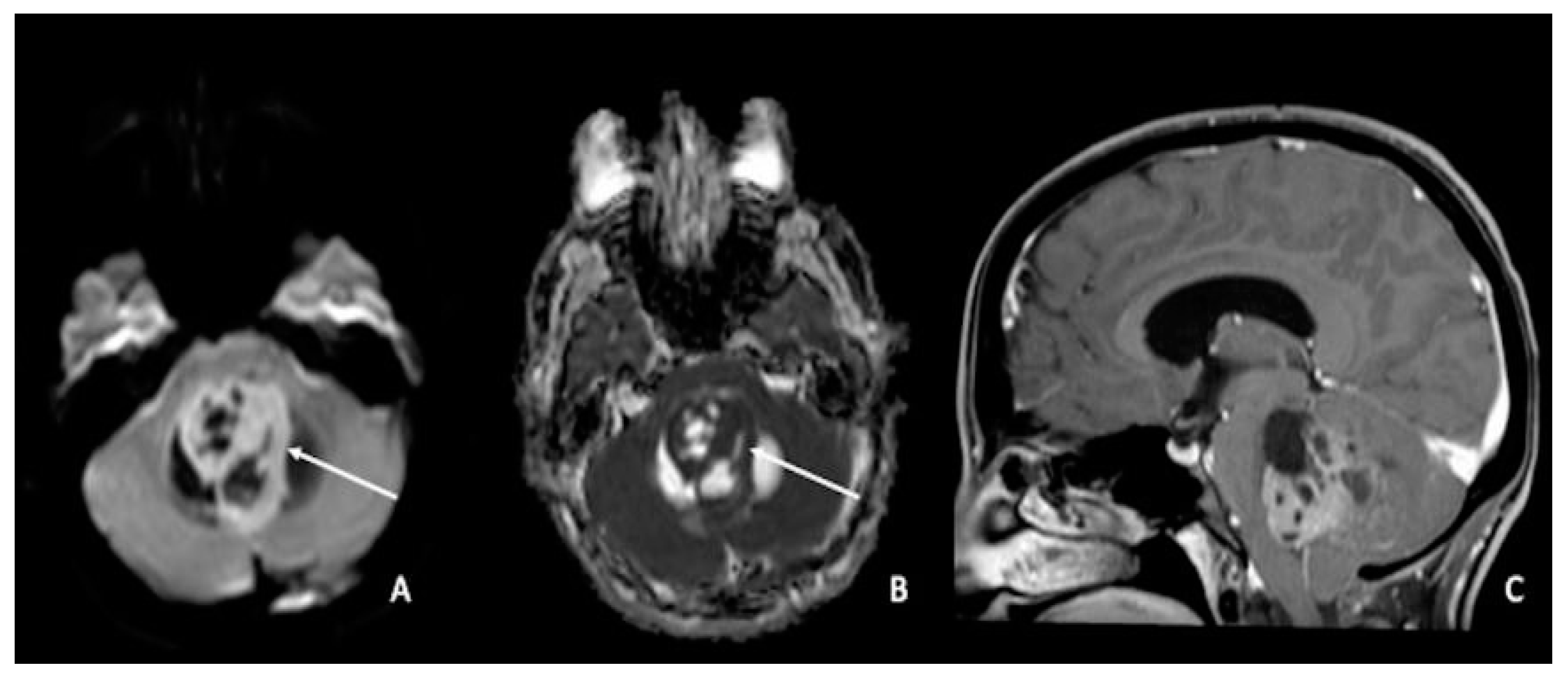
5. Medulloblastomas
6. Meningiomas and Vestibular Schwannomas
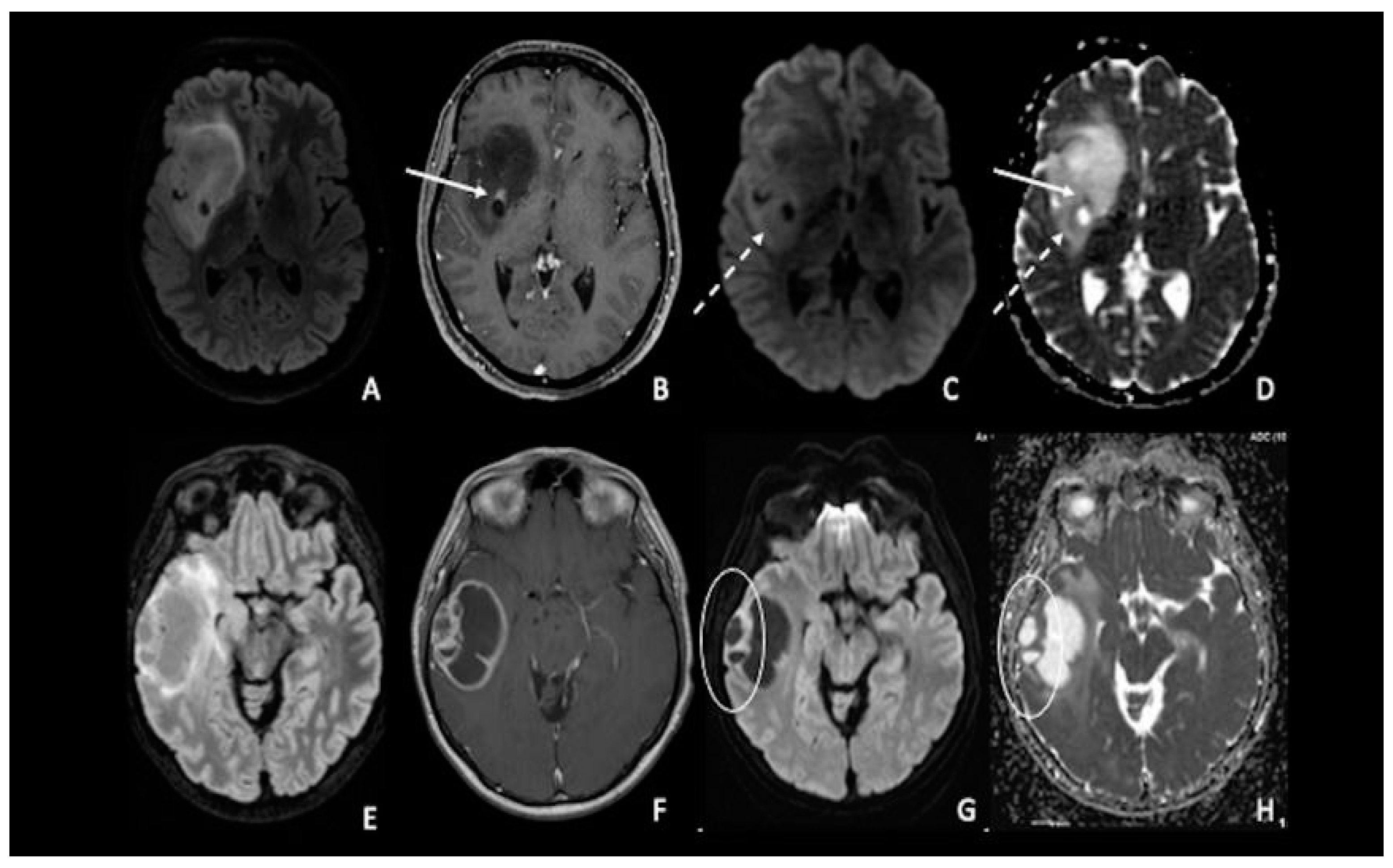
7. Metastasis
8. Gliomas vs. Metastasis
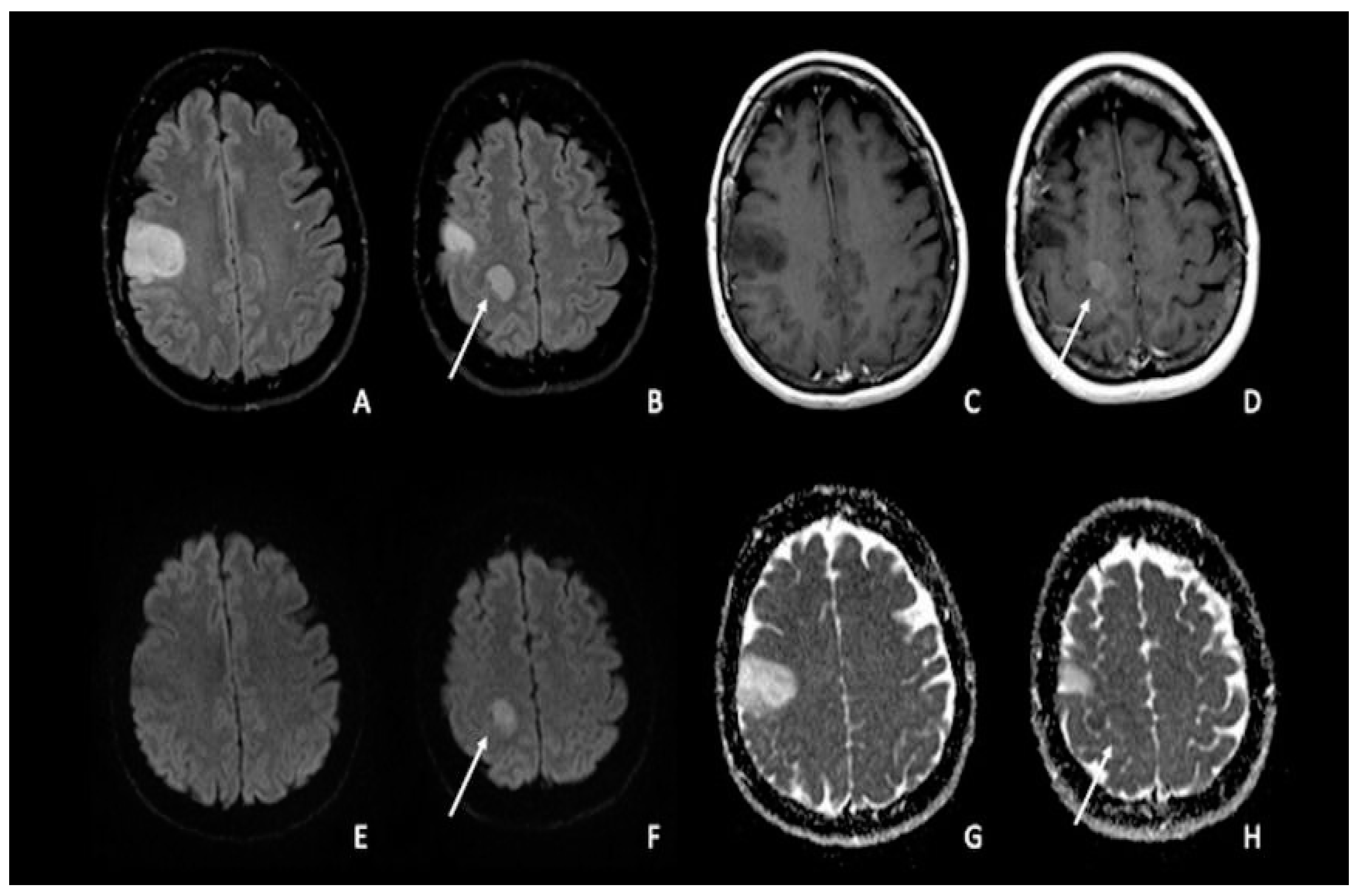
9. Post-Treatment Evaluation
10. Pituitary Adenoma
11. Skull Lesions
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martín-Noguerol, T.; Mohan, S.; Santos-Armentia, E.; Cabrera-Zubizarreta, A.; Luna, A. Advanced MRI assessment of non-enhancing peritumoral signal abnormality in brain lesions. Eur. J. Radiol. 2021, 143, 109900. [Google Scholar] [CrossRef] [PubMed]
- Martín Noguerol, T.; Martínez Barbero, J.P. Advanced diffusion MRI and biomarkers in the central nervous system: A new approach. Radiologia 2017, 59, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Huisman, T.A. Diffusion-weighted imaging: Basic concepts and application in cerebral stroke and head trauma. Eur. Radiol. 2003, 13, 2283–2297. [Google Scholar] [CrossRef] [PubMed]
- Svolos, P.; Kousi, E.; Kapsalaki, E.; Theodorou, K.; Fezoulidis, I.; Kappas, C.; Tsougos, I. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: A review and future perspectives. Cancer Imaging 2014, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Bozzao, A.; Bonamini, M.; Fasoli, F.; Ferrante, M.; Floris, R.; Colonnese, C.; Fantozzi, L.M. Diffusion-weighted MR Imaging: Clinical applications in neuroradiology. Radiol. Med. 2003, 106, 521–548. [Google Scholar]
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010, 9, 906–920. [Google Scholar] [CrossRef] [PubMed]
- Falini, A.; Romano, A.; Bozzao, A. Tumours. Neurol. Sci. 2008, 29 (Suppl. S3), 327–332. [Google Scholar] [CrossRef] [PubMed]
- Gaddamanugu, S.; Shafaat, O.; Sotoudeh, H.; Sarrami, A.H.; Rezaei, A.; Saadatpour, Z.; Singhal, A. Clinical applications of diffusion-weighted sequence in brain imaging: Beyond stroke. Neuroradiology 2022, 64, 15–30. [Google Scholar] [CrossRef]
- Guo, A.C.; Cummings, T.J.; Dash, R.C.; Provenzale, J.M. Lymphomas and high-grade astrocytomas: Comparison of water diffusibility and histologic characteristics. Radiology 2002, 224, 177–183. [Google Scholar] [CrossRef]
- Higano, S.; Yun, X.; Kumabe, T.; Watanabe, M.; Mugikura, S.; Umetsu, A.; Sato, A.; Yamada, T.; Takahashi, S. Malignant astrocytic tumors: Clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 2006, 241, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Moon, W.J.; Choi, J.W.; Roh, H.G.; Lim, S.D.; Koh, Y.C. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: The CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 2012, 54, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, F.; Kurisu, K.; Satoh, K.; Arita, K.; Sugiyama, K.; Ohtaki, M.; Takaba, J.; Tominaga, A.; Hanaya, R.; Yoshioka, H.; et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005, 235, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Murakami, R.; Sugahara, T.; Nakamura, H.; Hirai, T.; Kitajima, M.; Hayashida, Y.; Baba, Y.; Oya, N.; Kuratsu, J.; Yamashita, Y. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: Prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 2007, 243, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Calabria, L.F.; Tavanti, F.; Minniti, G.; Rossi-Espagnet, M.C.; Coppola, V.; Pugliese, S.; Guida, D.; Francione, G.; Colonnese, C.; et al. Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: Correlation with MGMT promoter methylation status. Eur. Radiol. 2013, 23, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.R.; Blank, A.; Bobola, M.S.; Ghatan, S.; Kolstoe, D.D.; Berger, M.S. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: Frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin. Cancer Res. 1999, 5, 807–814. [Google Scholar]
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60. [Google Scholar] [CrossRef]
- Gupta, R.K.; Cloughesy, T.F.; Sinha, U.; Garakian, J.; Lazareff, J.; Rubino, G.; Rubino, L.; Becker, D.P.; Vinters, H.V.; Alger, J.R. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J. Neurooncol. 2000, 50, 215–226. [Google Scholar] [CrossRef]
- Qin, J.B.; Zhang, H.; Wang, X.C.; Tan, Y.; Wu, X.F. Combination value of diffusion-weighted imaging and dynamic susceptibility contrast-enhanced MRI in astrocytoma grading and correlation with GFAP, Topoisomerase IIα and MGMT. Oncol. Lett. 2019, 18, 2763–2770. [Google Scholar] [CrossRef]
- Wang, Q.P.; Lei, D.Q.; Yuan, Y.; Xiong, N.X. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis. Medicine 2020, 99, e19254. [Google Scholar] [CrossRef]
- Van Cauter, S.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; De Keyzer, F.; Van Gool, S.W.; Van Calenbergh, F.; De Vleeschouwer, S.; Van Hecke, W.; et al. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology 2012, 263, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Raab, P.; Hattingen, E.; Franz, K.; Zanella, F.E.; Lanfermann, H. Cerebral gliomas: Diffusional kurtosis imaging analysis of microstructural differences. Radiology 2010, 254, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, G.; Dixon, L.; Sanverdi, E.; Machado, P.M.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Veraart, J.; Van Cauter, S.; et al. The diagnostic role of diffusional kurtosis imaging in glioma grading and differentiation of gliomas from other intra-axial brain tumours: A systematic review with critical appraisal and meta-analysis. Neuroradiology 2020, 62, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Kelley, D.A.; Banerjee, S.; Lupo, J.M.; Chang, S.M.; Xu, D.; Hess, C.P.; Nelson, S.J. Clinically feasible NODDI characterization of glioma using multiband EPI at 7 T. Neuroimage Clin. 2015, 9, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Appin, C.L.; Brat, D.J. Molecular genetics of gliomas. Cancer J. 2014, 20, 66–72. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; Deimling, A.V.; Bendszus, M.; Wiestler, B. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015, 5, 16238. [Google Scholar] [CrossRef]
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.J.; Shih, H.A.; Andronesi, O.C.; Cahill, D.P. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer 2017, 123, 4535–4546. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shi, Z.; Lian, Y.; Li, Z.; Liu, T.; Gao, Y.; Wang, Y.; Chen, L.; Mao, Y. Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur. Radiol. 2017, 27, 3509–3522. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, H.S.; Jung, S.C.; Choi, C.G.; Kim, S.J. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: A systemic review and meta-analysis. Eur. Radiol. 2019, 29, 745–758. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Born, D. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. J. Neurooncol. 2015, 125, 249–251. [Google Scholar] [CrossRef]
- Wu, C.C.; Jain, R.; Radmanesh, A.; Poisson, L.M.; Guo, W.Y.; Zagzag, D.; Snuderl, M.; Placantonakis, D.G.; Golfinos, J.; Chi, A.S. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from the Cancer Genome Atlas. AJNR Am. J. Neuroradiol. 2018, 39, 1814–1820. [Google Scholar] [CrossRef]
- Dang, L.; Yen, K.; Attar, E.C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. 2016, 27, 599–608. [Google Scholar] [CrossRef]
- Zhou, H.; Vallières, M.; Bai, H.X.; Su, C.; Tang, H.; Oldridge, D.; Zhang, Z.; Xiao, B.; Liao, W.; Tao, Y.; et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-Oncology 2017, 19, 862–870. [Google Scholar] [CrossRef]
- Zhang, B.; Chang, K.; Ramkissoon, S.; Tanguturi, S.; Bi, W.L.; Reardon, D.A.; Ligon, K.L.; Alexander, B.M.; Wen, P.Y.; Huang, R.Y. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro-Oncology 2017, 19, 109–117. [Google Scholar] [CrossRef]
- Xing, Z.; Yang, X.; She, D.; Lin, Y.; Zhang, Y.; Cao, D. Noninvasive Assessment of IDH Mutational Status in World Health Organization Grade II and III Astrocytomas Using DWI and DSC-PWI Combined with Conventional MR Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Tietze, A.; Choi, C.; Mickey, B.; Maher, E.A.; Parm Ulhøi, B.; Sangill, R.; Lassen-Ramshad, Y.; Lukacova, S.; Østergaard, L.; von Oettingen, G. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J. Neurosurg. 2018, 128, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Xiong, J.; Huang, W.; Wu, J.; Zhan, S.; Geng, D. Noninvasively detecting Isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J. Magn. Reson. Imaging 2017, 45, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Stadlbauer, A.; Zimmermann, M.; Kitzwögerer, M.; Oberndorfer, S.; Rössler, K.; Dörfler, A.; Buchfelder, M.; Heinz, G. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology 2017, 283, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Allinson, K.; Liu, H.; Boonzaier, N.R.; Yan, J.L.; Lupson, V.C.; Larkin, T.J. Less Invasive Phenotype Found in Isocitrate Dehydrogenase-mutated Glioblastomas than in Isocitrate Dehydrogenase Wild-Type Glioblastomas: A Diffusion-Tensor Imaging Study. Radiology 2017, 283, 215–221. [Google Scholar] [CrossRef]
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2-FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-grade Gliomas: A TCGA/TCIA Project. Clin. Cancer Res. 2017, 23, 6078–6085. [Google Scholar] [CrossRef]
- Nakae, S.; Murayama, K.; Sasaki, H.; Kumon, M.; Nishiyama, Y.; Ohba, S.; Adachi, K.; Nagahisa, S.; Hayashi, T.; Inamasu, J.; et al. Prediction of genetic subgroups in adult supra tentorial gliomas by pre- and intraoperative parameters. J. Neurooncol. 2017, 131, 403–412. [Google Scholar] [CrossRef]
- Leu, K.; Ott, G.A.; Lai, A.; Nghiemphu, P.L.; Pope, W.B.; Yong, W.H.; Liau, L.M.; Cloughesy, T.F.; Ellingson, B.M. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J. Neurooncol. 2017, 134, 177–188. [Google Scholar] [CrossRef]
- Lasocki, A.; Tsui, A.; Gaillard, F.; Tacey, M.; Drummond, K.; Stuckey, S. Reliability of noncontrast-enhancing tumor as a biomarker of IDH1 mutation status in glioblastoma. J. Clin. Neurosci. 2017, 39, 170–175. [Google Scholar] [CrossRef]
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109. [Google Scholar] [CrossRef]
- Hsieh, K.L.; Chen, C.Y.; Lo, C.M. Radiomic model for predicting mutations in the isocitrate dehydrogenase gene in glioblastomas. Oncotarget 2017, 8, 45888–45897. [Google Scholar] [CrossRef] [PubMed]
- Hempel, J.M.; Schittenhelm, J.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Tabatabai, G.; Castaneda Vega, S.; Ernemann, U.; Klose, U. Histogram analysis of diffusion kurtosis imaging estimates for in vivo assessment of 2016 WHO glioma grades: A cross-sectional observational study. Eur. J. Radiol. 2017, 95, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.; Kiesel, B.; Wöhrer, A.; Millesi, M.; Wurzer, A.; Göd, S.; Mallouhi, A.; Knosp, E.; Marosi, C.; Trattnig, S.; et al. Local image variance of 7 Tesla SWI is a new technique for preoperative characterization of diffusely infiltrating gliomas: Correlation with tumour grade and IDH1 mutational status. Eur. Radiol. 2017, 27, 1556–1567. [Google Scholar] [CrossRef] [PubMed]
- Delfanti, R.L.; Piccioni, D.E.; Handwerker, J.; Bahrami, N.; Krishnan, A.; Karunamuni, R.; Hattangadi-Gluth, J.A.; Seibert, T.M.; Srikant, A.; Jones, K.A.; et al. Imaging correlates for the 2016 update on WHO classification of grade II/III gliomas: Implications for IDH, 1p/19q and ATRX status. J. Neurooncol. 2017, 135, 601–609. [Google Scholar] [CrossRef]
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Hatae, R.; Yoshimoto, K.; Mizoguchi, M.; Suzuki, S.O.; Yoshiura, T.; Honda, H. MR Imaging-Based Analysis of Glioblastoma Multiforme: Estimation of IDH1 Mutation Status. AJNR Am. J. Neuroradiol. 2016, 37, 58–65. [Google Scholar] [CrossRef]
- Xiong, J.; Tan, W.; Wen, J.; Pan, J.; Wang, Y.; Zhang, J.; Geng, D. Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase 1/2 mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur. Radiol. 2016, 26, 1705–1715. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Fan, X.; Wang, J.; Li, G.; Ma, J.; Ma, J.; Jiang, T.; Dai, J. Radiological features combined with IDH1 status for predicting the survival outcome of glioblastoma patients. Neuro-Oncology 2016, 18, 589–597. [Google Scholar] [CrossRef]
- Choi, C.; Raisanen, J.M.; Ganji, S.K.; Zhang, S.; McNeil, S.S.; An, Z.; Madan, A.; Hatanpaa, K.J.; Vemireddy, V.; Sheppard, C.A.; et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients with IDH-Mutant Glioma. J. Clin. Oncol. 2016, 34, 4030–4039. [Google Scholar] [CrossRef]
- Biller, A.; Badde, S.; Nagel, A.; Neumann, J.O.; Wick, W.; Hertenstein, A.; Bendszus, M.; Sahm, F.; Benkhedah, N.; Kleesiek, J. Improved Brain Tumor Classification by Sodium MR Imaging: Prediction of IDH Mutation Status and Tumor Progression. AJNR Am. J. Neuroradiol. 2016, 37, 66–73. [Google Scholar] [CrossRef]
- Wasserman, J.K.; Nicholas, G.; Yaworski, R.; Wasserman, A.M.; Woulfe, J.M.; Jansen, G.H.; Chakraborty, S.; Nguyen, T.B. Radiological and pathological features associated with IDH1-R132H mutation status and early mortality in newly diagnosed anaplastic astrocytic tumours. PLoS ONE 2015, 10, e0123890. [Google Scholar] [CrossRef]
- Sonoda, Y.; Shibahara, I.; Kawaguchi, T.; Saito, R.; Kanamori, M.; Watanabe, M.; Suzuki, H.; Kumabe, T.; Tominaga, T. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol. 2015, 32, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Choi, S.H.; Ryoo, I.; Yoon, T.J.; Kim, T.M.; Lee, S.H.; Park, C.K.; Kim, J.H.; Sohn, C.H.; Park, S.H.; et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J. Neurooncol. 2015, 121, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Botero, G.; Dehais, C.; Idbaih, A.; Martin-Duverneuil, N.; Lahutte, M.; Carpentier, C.; Letouzé, E.; Chinot, O.; Loiseau, H.; Honnorat, J.; et al. Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro-Oncology 2014, 16, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Yu, L.; Li, H.; Ou, Y.; Qiu, X.; Ding, Y.; Han, H.; Zhang, X. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014, 7, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.A.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.M.; et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am. J. Neuroradiol. 2012, 33, 1349–1355. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, S.K.; Agid, R.; Bae, J.M.; Keller, A.; Terbrugge, K. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: Diagnostic value of minimum apparent diffusion coefficient. AJNR Am. J. Neuroradiol. 2008, 29, 1872–1877. [Google Scholar] [CrossRef]
- Fellah, S.; Caudal, D.; De Paula, A.M.; Dory-Lautrec, P.; Figarella-Branger, D.; Chinot, O.; Metellus, P.; Cozzone, P.J.; Confort-Gouny, S.; Ghattas, B.; et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): Is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am. J. Neuroradiol. 2013, 34, 1326–1333. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Smith, T.S.; Brodbelt, A.R.; Joyce, K.A.; Warnke, P.C.; Walker, C. Apparent diffusion coefficients in oligodendroglial tumors characterized by genotype. J. Magn. Reson. Imaging 2007, 26, 1405–1412. [Google Scholar] [CrossRef]
- Park, Y.W.; Han, K.; Ahn, S.S.; Bae, S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Kang, S.G.; Lee, S.K. Prediction of IDH1-Mutation and 1p/19q-Codeletion Status Using Preoperative MR Imaging Phenotypes in Lower Grade Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 37–42. [Google Scholar] [CrossRef]
- Eichinger, P.; Alberts, E.; Delbridge, C.; Trebeschi, S.; Valentinitsch, A.; Bette, S.; Huber, T.; Gempt, J.; Meyer, B.; Schlegel, J.; et al. Diffusion tensor image features predict IDH genotype in newly diagnosed WHO grade II/III gliomas. Sci. Rep. 2017, 7, 13396. [Google Scholar] [CrossRef]
- Figini, M.; Riva, M.; Graham, M.; Castelli, G.M.; Fernandes, B.; Grimaldi, M.; Baselli, G.; Pessina, F.; Bello, L.; Zhang, H.; et al. Prediction of Isocitrate Dehydrogenase Genotype in Brain Gliomas with MRI: Single-Shell versus Multishell Diffusion Models. Radiology 2018, 289, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Zhang, H.; Yan, X.; Wang, S.; Chen, Q.; Gao, E.; Qi, J.; Bai, J.; Zhang, Y.; Cheng, J. Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology 2022, 302, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Ahn, S.S.; Park, C.J.; Han, K.; Kim, E.H.; Kang, S.G.; Chang, J.H.; Kim, S.H.; Lee, S.K. Diffusion and perfusion MRI may predict EGFR amplification and the TERT promoter mutation status of IDH-wildtype lower-grade gliomas. Eur. Radiol. 2020, 30, 6475–6484. [Google Scholar] [CrossRef] [PubMed]
- Drake-Pérez, M.; Boto, J.; Fitsiori, A.; Lovblad, K.; Vargas, M.I. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018, 9, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Lee, M.; Buck, O.; Woo, K.M.; Zhang, Z.; Hatzoglou, V.; Omuro, A.; Arevalo-Perez, J.; Thomas, A.A.; Huse, J.; et al. Diagnostic Accuracy of T1-Weighted Dynamic Contrast-Enhanced-MRI and DWI-ADC for Differentiation of Glioblastoma and Primary CNS Lymphoma. AJNR Am. J. Neuroradiol. 2017, 38, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Kim, S.; Chawla, S.; Wolf, R.L.; Knipp, D.E.; Vossough, A.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2011, 32, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Toh, C.H.; Castillo, M.; Wong, A.M.; Wei, K.C.; Wong, H.F.; Ng, S.H.; Wan, Y.L. Primary cerebral lymphoma and glioblastoma multiforme: Differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008, 29, 471–475. [Google Scholar] [CrossRef]
- Lu, X.; Xu, W.; Wei, Y.; Li, T.; Gao, L.; Fu, X.; Yao, Y.; Wang, L. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: A systematic review and meta-analysis. Neurol. Sci. 2019, 40, 947–956. [Google Scholar] [CrossRef]
- Ahn, S.J.; Shin, H.J.; Chang, J.H.; Lee, S.K. Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: Comparison of three different ROI methods. PLoS ONE 2014, 9, e112948. [Google Scholar] [CrossRef]
- Horger, M.; Fenchel, M.; Nägele, T.; Moehle, R.; Claussen, C.D.; Beschorner, R.; Ernemann, U. Water diffusivity: Comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am. J. Roentgenol. 2009, 193, 1384–1387. [Google Scholar] [CrossRef]
- Calli, C.; Kitis, O.; Yunten, N.; Yurtseven, T.; Islekel, S.; Akalin, T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur. J. Radiol. 2006, 58, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Server, A.; Kulle, B.; Maehlen, J.; Josefsen, R.; Schellhorn, T.; Kumar, T.; Langberg, C.W.; Nakstad, P.H. Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol. 2009, 50, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Crasto, S.G.; Moruno, P.G.; Cassoni, P.; Rudà, R.; Boccaletti, R.; Brosio, M.; De Lucchi, R.; Fava, C. Role of diffusion- and perfusion-weighted MR imaging for brain tumour characterisation. Radiol. Med. 2009, 114, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Hashimoto, N.; Goto, T.; Kagawa, N.; Kishima, H.; Izumoto, S.; Tanaka, H.; Fujita, N.; Yoshimine, T. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 2008, 43, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Rollin, N.; Guyotat, J.; Streichenberger, N.; Honnorat, J.; Tran Minh, V.A.; Cotton, F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 2006, 48, 150–159. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kumabe, T.; Higano, S.; Watanabe, M.; Tominaga, T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol. Res. 2009, 31, 940–946. [Google Scholar] [CrossRef]
- Poussaint, T.Y.; Panigrahy, A.; Huisman, T.A. Pediatric brain tumors. Pediatr. Radiol. 2015, 45 (Suppl. S3), S443–S453. [Google Scholar] [CrossRef]
- Rasalkar, D.D.; Chu, W.C.; Paunipagar, B.K.; Cheng, F.W.; Li, C.K. Paediatric intra-axial posterior fossa tumours: Pictorial review. Postgrad. Med. J. 2013, 89, 39–46. [Google Scholar] [CrossRef]
- Fruehwald-Pallamar, J.; Puchner, S.B.; Rossi, A.; Garre, M.L.; Cama, A.; Koelblinger, C.; Osborn, A.G.; Thurnher, M.M. Magnetic resonance imaging spectrum of medulloblastoma. Neuroradiology 2011, 53, 387–396. [Google Scholar] [CrossRef]
- Plaza, M.J.; Borja, M.J.; Altman, N.; Saigal, G. Conventional and advanced MRI features of pediatric intracranial tumors: Posterior fossa and suprasellar tumors. AJR Am. J. Roentgenol. 2013, 200, 1115–1124. [Google Scholar] [CrossRef]
- Eran, A.; Ozturk, A.; Aygun, N.; Izbudak, I. Medulloblastoma: Atypical CT and MRI findings in children. Pediatr. Radiol. 2010, 40, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, J.L. Tumeurs de la fosse postérieure [Infra tentorial tumors]. J. Radiol. 2006, 87 Pt 2, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Emmanuel, J.V.; Seow, W.T.; Lou, J.; Teo, H.E.; Lim, C.C. Paediatric PNET: Pre-surgical MRI features. Clin. Radiol. 2007, 62, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.F.; Confort-Gouny, S.; Viola, A.; Le Fur, Y.; Viout, P.; Bennathan, M.; Chapon, F.; Figarella-Branger, D.; Cozzone, P.; Girard, N. Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J. Magn. Reson. Imaging 2007, 26, 1390–1398. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Pang, H.; Ghimire, P.; Liu, G. (1)H magnetic resonance spectroscopy and diffusion weighted imaging findings of medulloblastoma in 3.0T MRI: A retrospective analysis of 17 cases. Neural. Regen. Res. 2012, 7, 2554–2559. [Google Scholar] [CrossRef]
- Bull, J.G.; Saunders, D.E.; Clark, C.A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 2012, 22, 447–457. [Google Scholar] [CrossRef]
- Gimi, B.; Cederberg, K.; Derinkuyu, B.; Gargan, L.; Koral, K.M.; Bowers, D.C.; Koral, K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad. Radiol. 2012, 19, 794–800. [Google Scholar] [CrossRef]
- Poussaint, T.Y.; Rodriguez, D. Advanced neuroimaging of pediatric brain tumors: MR diffusion, MR perfusion, and MR spectroscopy. Neuroimaging Clin. N. Am. 2006, 16, 169–192, ix. [Google Scholar] [CrossRef] [PubMed]
- Rodallec, M.; Colombat, M.; Krainik, A.; Kalamaridès, M.; Redondo, A.; Feydy, A. Diffusion-weighted MR imaging and pathologic findings in adult cerebellar medulloblastoma. J. Neuroradiol. 2004, 31, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Quadery, F.A.; Okamoto, K. Diffusion-weighted MRI of haemangioblastomas and other cerebellar tumours. Neuroradiology 2003, 45, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Wilke, M.; Eidenschink, A.; Müller-Weihrich, S.; Auer, D.P. MR diffusion imaging and 1H spectroscopy in a child with medulloblastoma. A case report. Acta Radiol. 2001, 42, 39–42. [Google Scholar] [PubMed]
- Kotsenas, A.L.; Roth, T.C.; Manness, W.K.; Faerber, E.N. Abnormal diffusion-weighted MRI in medulloblastoma: Does it reflect small cell histology? Pediatr. Radiol. 1999, 29, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Zitouni, S.; Koc, G.; Doganay, S.; Saracoglu, S.; Gumus, K.Z.; Ciraci, S.; Coskun, A.; Unal, E.; Per, H.; Kurtsoy, A.; et al. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn. J. Radiol. 2017, 35, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Pillai, S.; Singhal, A.; Byrne, A.T.; Dunham, C.; Cochrane, D.D.; Steinbok, P. Diffusion-weighted imaging and pathological correlation in pediatric medulloblastomas-“They are not always restricted!”. Childs Nerv. Syst. 2011, 27, 1407–1411. [Google Scholar] [CrossRef]
- Douglas-Akinwande, A.C.; Payner, T.D.; Hattab, E.M. Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin. Neurol. Neurosurg. 2009, 111, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Jaremko, J.L.; Jans, L.B.; Coleman, L.T.; Ditchfield, M.R. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am. J. Neuroradiol. 2010, 31, 1613–1616. [Google Scholar] [CrossRef]
- Forbes, J.A.; Reig, A.S.; Smith, J.G.; Jermakowicz, W.; Tomycz, L.; Shay, S.D.; Sun, D.A.; Wushensky, C.A.; Pearson, M.M. Findings on preoperative brain MRI predict histopathology in children with cerebellar neoplasms. Pediatr. Neurosurg. 2011, 47, 51–59. [Google Scholar] [CrossRef]
- Orman, G.; Bosemani, T.; Higgins, L.; Carson, K.A.; Huisman, T.A.; Poretti, A. Pediatric Cerebellar Tumors: Does ADC Analysis of Solid, Contrast-Enhancing Tumor Components Correlate Better with Tumor Grade than ADC Analysis of the Entire Tumor? J. Neuroimaging 2015, 25, 785–791. [Google Scholar] [CrossRef]
- Porto, L.; Jurcoane, A.; Schwabe, D.; Kieslich, M.; Hattingen, E. Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur. J. Paediatr. Neurol. 2013, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Pierce, T.; Kranz, P.G.; Roth, C.; Leong, D.; Wei, P.; Provenzale, J.M. Use of apparent diffusion coefficient values for diagnosis of pediatric posterior fossa tumors. Neuroradiol. J. 2014, 27, 233–244. [Google Scholar] [CrossRef]
- Pierce, T.T.; Provenzale, J.M. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol. J. 2014, 27, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Koral, K.; Mathis, D.; Gimi, B.; Gargan, L.; Weprin, B.; Bowers, D.C.; Margraf, L. Common pediatric cerebellar tumors: Correlation between cell densities and apparent diffusion coefficient metrics. Radiology 2013, 268, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Koral, K.; Alford, R.; Choudhury, N.; Mossa-Basha, M.; Gargan, L.; Gimi, B.; Gao, A.; Zhang, S.; Bowers, D.C.; Koral, K.M.; et al. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology 2014, 56, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Pinilla, N.; Martínez de Aragón, A.; Diéguez Tapias, S.; Toldos, O.; Hinojosa Bernal, J.; Rigal Andrés, M.; González-Granado, L.I. Evaluating the apparent diffusion coefficient in MRI studies as a means of determining paediatric brain tumour stages. Neurologia 2016, 31, 459–465. [Google Scholar] [CrossRef]
- Wagner, M.W.; Narayan, A.K.; Bosemani, T.; Huisman, T.A.; Poretti, A. Histogram Analysis of Diffusion Tensor Imaging Parameters in Pediatric Cerebellar Tumors. J. Neuroimaging 2016, 26, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Assis, Z.A.; Saini, J.; Ranjan, M.; Gupta, A.K.; Sabharwal, P.; Naidu, P.R. Diffusion tensor imaging in evaluation of posterior fossa tumors in children on a 3T MRI scanner. Indian J. Radiol. Imaging 2015, 25, 445–452. [Google Scholar] [CrossRef]
- Dangouloff-Ros, V.; Varlet, P.; Levy, R.; Beccaria, K.; Puget, S.; Dufour, C.; Boddaert, N. Imaging features of medulloblastoma: Conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy: From general features to subtypes and characteristics. Neurochirurgie 2021, 67, 6–13. [Google Scholar] [CrossRef]
- Gauvain, K.M.; McKinstry, R.C.; Mukherjee, P.; Perry, A.; Neil, J.J.; Kaufman, B.A.; Hayashi, R.J. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am. J. Roentgenol. 2001, 177, 449–454. [Google Scholar] [CrossRef]
- Burger, P.C.; Yu, I.T.; Tihan, T.; Friedman, H.S.; Strother, D.R.; Kepner, J.L.; Duffner, P.K.; Kun, L.E.; Perlman, E.J. Atypical teratoid/rhabdoid tumor of the central nervous system: A highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: A Pediatric Oncology Group study. Am. J. Surg. Pathol. 1998, 22, 1083–1092. [Google Scholar] [CrossRef]
- Phuttharak, W.; Wannasarnmetha, M.; Wara-Asawapati, S.; Yuthawong, S. Diffusion MRI in Evaluation of Pediatric Posterior Fossa Tumors. Asian Pac. J. Cancer Prev. 2021, 22, 1129–1136. [Google Scholar] [CrossRef]
- Filippi, C.G.; Edgar, M.A.; Uluğ, A.M.; Prowda, J.C.; Heier, L.A.; Zimmerman, R.D. Appearance of meningiomas on diffusion-weighted images: Correlating diffusion constants with histopathologic findings. AJNR Am. J. Neuroradiol. 2001, 22, 65–72. [Google Scholar] [PubMed]
- Toh, C.H.; Castillo, M.; Wong, A.M.; Wei, K.C.; Wong, H.F.; Ng, S.H.; Wan, Y.L. Differentiation between classic and atypical meningiomas with use of diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008, 29, 1630–1635. [Google Scholar] [CrossRef] [PubMed]
- Tamrazi, B.; Shiroishi, M.S.; Liu, C.S. Advanced Imaging of Intracranial Meningiomas. Neurosurg. Clin. N. Am. 2016, 27, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Babu, S.; Uppin, S.G.; Uppin, M.S.; Panigrahi, M.K.; Saradhi, V.; Bhattacharjee, S.; Sahu, B.P.; Purohit, A.K.; Challa, S. Meningiomas: Correlation of Ki67 with histological grade. Neurol. India 2011, 59, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Abry, E.; Thomassen, I.Ø.; Salvesen, Ø.O.; Torp, S.H. The significance of Ki-67/MIB-1 labeling index in human meningiomas: A literature study. Pathol. Res. Pract. 2010, 206, 810–815. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.T.; Hung, K.C.; Shih, Y.J.; Lim, S.W.; Yang, C.C.; Kuo, Y.T.; Chen, J.H.; Ko, C.C. Preoperative Apparent Diffusion Coefficient Values for Differentiation between Low and High Grade Meningiomas: An Updated Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 630. [Google Scholar] [CrossRef]
- Meyer, H.J.; Wienke, A.; Surov, A. ADC values of benign and high grade meningiomas and associations with tumor cellularity and proliferation—A systematic review and meta-analysis. J. Neurol. Sci. 2020, 415, 116975. [Google Scholar] [CrossRef]
- Watanabe, K.; Kakeda, S.; Yamamoto, J.; Ide, S.; Ohnari, N.; Nishizawa, S.; Korogi, Y. Prediction of hard meningiomas: Quantitative evaluation based on the magnetic resonance signal intensity. Acta Radiol. 2016, 57, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Hoover, J.M.; Morris, J.M.; Meyer, F.B. Use of preoperative magnetic resonance imaging T1 and T2 sequences to determine intraoperative meningioma consistency. Surg. Neurol. Int. 2011, 2, 142. [Google Scholar] [CrossRef]
- Yogi, A.; Koga, T.; Azama, K.; Higa, D.; Ogawa, K.; Watanabe, T.; Ishiuchi, S.; Murayama, S. Usefulness of the apparent diffusion coefficient (ADC) for predicting the consistency of intracranial meningiomas. Clin. Imaging 2014, 38, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Kashimura, H.; Inoue, T.; Ogasawara, K.; Arai, H.; Otawara, Y.; Kanbara, Y.; Ogawa, A. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J. Neurosurg. 2007, 107, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Shiroishi, M.S.; Cen, S.Y.; Tamrazi, B.; D’Amore, F.; Lerner, A.; King, K.S.; Kim, P.E.; Law, M.; Hwang, D.H.; Boyko, O.B.; et al. Predicting Meningioma Consistency on Preoperative Neuroimaging Studies. Neurosurg. Clin. N. Am. 2016, 27, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Sharma, B.S. Cystic acoustic neuromas: Surgical outcome in a series of 58 patients. J. Clin. Neurosci. 2008, 15, 511–515. [Google Scholar] [CrossRef]
- Sawamura, Y.; Shirato, H.; Sakamoto, T.; Aoyama, H.; Suzuki, K.; Onimaru, R.; Isu, T.; Fukuda, S.; Miyasaka, K. Management of vestibular schwannoma by fractionated stereotactic radiotherapy and associated cerebrospinal fluid malabsorption. J. Neurosurg. 2003, 99, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Myrseth, E.; Møller, P.; Pedersen, P.H.; Lund-Johansen, M. Vestibular schwannoma: Surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery 2009, 64, 654–661; Discussion, 661–663. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wang, C.C.; Wai, Y.Y.; Wan, Y.L.; Ng, S.H.; Chen, Y.L.; Liu, H.L.; Wang, J.J. Significant temporal evolution of diffusion anisotropy for evaluating early response to radiosurgery in patients with vestibular schwannoma: Findings from functional diffusion maps. AJNR Am. J. Neuroradiol. 2010, 31, 269–274. [Google Scholar] [CrossRef]
- Camargo, A.; Schneider, T.; Liu, L.; Pakpoor, J.; Kleinberg, L.; Yousem, D.M. Pretreatment ADC Values Predict Response to Radiosurgery in Vestibular Schwannomas. AJNR Am. J. Neuroradiol. 2017, 38, 1200–1205. [Google Scholar] [CrossRef]
- Barajas, R.F., Jr.; Cha, S. Metastasis in Adult Brain Tumors. Neuroimaging Clin. N. Am. 2016, 26, 601–620. [Google Scholar] [CrossRef]
- Svokos, K.A.; Salhia, B.; Toms, S.A. Molecular biology of brain metastasis. Int. J. Mol. Sci. 2014, 15, 9519–9530. [Google Scholar] [CrossRef]
- Ulu, E.; Ozturk, B.; Atalay, K.; Okumus, I.B.; Erdem, D.; Gul, M.K.; Terzi, O. Diffusion-Weighted Imaging of Brain Metastasis: Correlation of MRI Parameters with Histologic Type. Turk. Neurosurg. 2022, 32, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Duygulu, G.; Ovali, G.Y.; Calli, C.; Kitis, O.; Yünten, N.; Akalin, T.; Islekel, S. Intracerebral metastasis showing restricted diffusion: Correlation with histopathologic findings. Eur. J. Radiol. 2010, 74, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, Y.; Hirai, T.; Morishita, S.; Kitajima, M.; Murakami, R.; Korogi, Y.; Makino, K.; Nakamura, H.; Ikushima, I.; Yamura, M.; et al. Diffusion-weighted imaging of metastatic brain tumors: Comparison with histologic type and tumor cellularity. AJNR Am. J. Neuroradiol. 2006, 27, 1419–1425. [Google Scholar] [PubMed]
- Koyama, H.; Ohno, Y.; Nishio, M.; Takenaka, D.; Yoshikawa, T.; Matsumoto, S.; Seki, S.; Maniwa, Y.; Ito, T.; Nishimura, Y.; et al. Diffusion-weighted imaging vs. STIR turbo SE imaging: Capability for quantitative differentiation of small-cell lung cancer from non-small-cell lung cancer. Br. J. Radiol. 2014, 87, 20130307. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Yu, T.; Ye, N. Usefulness of diffusion-weighted MR imaging in the evaluation of pulmonary lesions. Eur. Radiol. 2010, 20, 807–815. [Google Scholar] [CrossRef]
- Meyer, H.J.; Fiedler, E.; Kornhuber, M.; Spielmann, R.P.; Surov, A. Comparison of diffusion-weighted imaging findings in brain metastases of different origin. Clin. Imaging 2015, 39, 965–969. [Google Scholar] [CrossRef]
- Zakaria, R.; Das, K.; Radon, M.; Bhojak, M.; Rudland, P.R.; Sluming, V.; Jenkinson, M.D. Diffusion-weighted MRI characteristics of the cerebral metastasis to brain boundary predicts patient outcomes. BMC Med. Imaging 2014, 14, 26. [Google Scholar] [CrossRef]
- Jung, W.S.; Park, C.H.; Hong, C.K.; Suh, S.H.; Ahn, S.J. Diffusion-Weighted Imaging of Brain Metastasis from Lung Cancer: Correlation of MRI Parameters with the Histologic Type and Gene Mutation Status. AJNR Am. J. Neuroradiol. 2018, 39, 273–279. [Google Scholar] [CrossRef]
- Mahendru, G.; Chong, V. Meninges in cancer imaging. Cancer Imaging 2009, 9, S14–S21. [Google Scholar] [CrossRef]
- Bulakbasi, N.; Guvenc, I.; Onguru, O.; Erdogan, E.; Tayfun, C.; Ucoz, T. The added value of the apparent diffusion coefficient calculation to magnetic resonance imaging in the differentiation and grading of malignant brain tumors. J. Comput. Assist. Tomogr. 2004, 28, 735–746. [Google Scholar] [CrossRef]
- Pope, W.B. Brain metastases: Neuroimaging. Handb. Clin. Neurol. 2018, 149, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Moltoni, G.; Guarnera, A.; Pasquini, L.; Di Napoli, A.; Napolitano, A.; Espagnet, M.C.R.; Bozzao, A. Single brain metastasis versus glioblastoma multiforme: A VOI-based multiparametric analysis for differential diagnosis. Radiol. Med. 2022, 127, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Pavlisa, G.; Rados, M.; Pavlisa, G.; Pavic, L.; Potocki, K.; Mayer, D. The differences of water diffusion between brain tissue infiltrated by tumor and peritumoral vasogenic edema. Clin. Imaging 2009, 33, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Huang, S.; Guo, J.; Zhuang, X.; Han, H. Use of a high b-value for diffusion weighted imaging of peritumoral regions to differentiate high-grade gliomas and solitary metastases. J. Magn. Reson. Imaging 2015, 42, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Miquelini, L.A.; Pérez Akly, M.S.; Funes, J.A.; Besada, C.H. Usefulness of the apparent diffusion coefficient for the evaluation of the white matter to differentiate between glioblastoma and brain metastases. Radiologia 2016, 58, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Kalpathy-Cramer, J.; Gerstner, E.R.; Emblem, K.E.; Andronesi, O.; Rosen, B. Advanced magnetic resonance imaging of the physical processes in human glioblastoma. Cancer Res. 2014, 74, 4622–4637. [Google Scholar] [CrossRef] [PubMed]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro-Oncology 2009, 11, 842–852. [Google Scholar] [CrossRef]
- Oberheim Bush, N.A.; Hervey-Jumper, S.L.; Berger, M.S. Management of Glioblastoma, Present and Future. World Neurosurg. 2019, 131, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Anami, D.; Arioka, T.; Inoue, S.; Kariya, Y.; Fujimoto, M.; Ida, K.; Sasai, N.; Kaji, M.; Kanazawa, S.; et al. Basic study of susceptibility-weighted imaging at 1.5T. Acta Med. Okayama 2008, 62, 159–168. [Google Scholar] [CrossRef]
- Li, H.; Duan, Y.; Liu, N.; Dong, J.; Liang, Y.; Ju, R. Value of DWI Combined with Magnetic Resonance Spectroscopy in the Differential Diagnosis between Recurrent Glioma and Radiation Injury: A Meta-Analysis. Int. J. Clin. Pract. 2022, 2022, 1629570. [Google Scholar] [CrossRef]
- Hegi, M.E.; Liu, L.; Herman, J.G.; Stupp, R.; Wick, W.; Weller, M.; Mehta, M.P.; Gilbert, M.R. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J. Clin. Oncol. 2008, 26, 4189–4199. [Google Scholar] [CrossRef] [PubMed]
- Kondo, M.; Uchiyama, Y. Apparent diffusion coefficient histogram analysis for prediction of prognosis in glioblastoma. J. Neuroradiol. 2018, 45, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F., Jr.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro-Oncology 2012, 14, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Akyurek, S.; Avalos, T.; Rebueno, N.; Spicer, C.; Garcia, J.; Famiglietti, R.; Allen, P.K.; Chao, K.S.; Mahajan, A.; et al. Evaluation of peritumoral edema in the delineation of radiotherapy clinical target volumes for glioblastoma. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 144–150. [Google Scholar] [CrossRef]
- Rathore, S.; Akbari, H.; Doshi, J.; Shukla, G.; Rozycki, M.; Bilello, M.; Lustig, R.; Davatzikos, C. Radiomic signature of infiltration in peritumoral edema predicts subsequent recurrence in glioblastoma: Implications for personalized radiotherapy planning. J. Med. Imaging 2018, 5, 021219. [Google Scholar] [CrossRef]
- Pasquini, L.; Di Napoli, A.; Napolitano, A.; Lucignani, M.; Dellepiane, F.; Vidiri, A.; Villani, V.; Romano, A.; Bozzao, A. Glioblastoma radiomics to predict survival: Diffusion characteristics of surrounding nonenhancing tissue to select patients for extensive resection. J. Neuroimaging 2021, 31, 1192–1200. [Google Scholar] [CrossRef]
- Fathalla, H.; Cusimano, M.D.; Di Ieva, A.; Lee, J.; Alsharif, O.; Goguen, J.; Zhang, S.; Smyth, H. Endoscopic versus microscopic approach for surgical treatment of acromegaly. Neurosurg. Rev. 2015, 38, 541–548; Discussion, 548–549. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Iwata, T.; Kawamura, N.; Izumiyama, H.; Ikeda, H.; Matsumoto, K. Staged transsphenoidal surgery for fibrous nonfunctioning pituitary adenomas with suprasellar extension. Neurol. Med. Chir. 1997, 37, 830–835; Discussion, 835–837. [Google Scholar] [CrossRef]
- Mohamed, F.F.; Safwat, A. Diagnostic value of apparent diffusion coefficient (ADC) in assessment of pituitary macroadenoma consistency. Egypt. J. Radiol. Nucl. Med. 2013, 44, 617–624. [Google Scholar] [CrossRef]
- Pierallini, A.; Caramia, F.; Falcone, C.; Tinelli, E.; Paonessa, A.; Ciddio, A.B.; Fiorelli, M.; Bianco, F.; Natalizi, S.; Ferrante, L.; et al. Pituitary macroadenomas: Preoperative evaluation of consistency with diffusion-weighted MR imaging—Initial experience. Radiology 2006, 239, 223–231. [Google Scholar] [CrossRef]
- Suzuki, C.; Maeda, M.; Hori, K.; Kozuka, Y.; Sakuma, H.; Taki, W.; Takeda, K. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J. Neuroradiol. 2007, 34, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Rutland, J.W.; Loewenstern, J.; Ranti, D.; Tsankova, N.M.; Bellaire, C.P.; Bederson, J.B.; Delman, B.N.; Shrivastava, R.K.; Balchandani, P. Analysis of 7-tesla diffusion-weighted imaging in the prediction of pituitary macroadenoma consistency. J. Neurosurg. 2020, 134, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Huang, Z.; Zhou, G.; Li, L.; Zhang, M.; Li, Z. Diffusion-weighted imaging for predicting tumor consistency and extent of resection in patients with pituitary adenoma. Neurosurg. Rev. 2021, 44, 2933–2941. [Google Scholar] [CrossRef] [PubMed]
- Sanei Taheri, M.; Kimia, F.; Mehrnahad, M.; Saligheh Rad, H.; Haghighatkhah, H.; Moradi, A.; Kazerooni, A.F.; Alviri, M.; Absalan, A. Accuracy of diffusion-weighted imaging-magnetic resonance in differentiating functional from non-functional pituitary macro-adenoma and classification of tumor consistency. Neuroradiol. J. 2019, 32, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Doai, M.; Tonami, H.; Matoba, M.; Tachibana, O.; Iizuka, H.; Nakada, S.; Yamada, S. Pituitary macroadenoma: Accuracy of apparent diffusion coefficient magnetic resonance imaging in grading tumor aggressiveness. Neuroradiol. J. 2019, 32, 86–91. [Google Scholar] [CrossRef]
- Gomez, C.K.; Schiffman, S.R.; Bhatt, A.A. Radiological review of skull lesions. Insights Imaging 2018, 9, 857–882. [Google Scholar] [CrossRef]
- Tu, Z.; Xiao, Z.; Zheng, Y.; Huang, H.; Yang, L.; Cao, D. Benign and malignant skull-involved lesions: Discriminative value of conventional CT and MRI combined with diffusion-weighted MRI. Acta Radiol. 2019, 60, 880–886. [Google Scholar] [CrossRef]
- Soni, N.; Gupta, N.; Kumar, Y.; Mangla, M.; Mangla, R. Role of diffusion-weighted imaging in skull base lesions: A pictorial review. Neuroradiol. J. 2017, 30, 370–384. [Google Scholar] [CrossRef]
- Mahendrakar, A.K.; Kumaran, S.P.; Reddy, B.N.; Viswamitra, S. Utility of apparent diffusion coefficient (ADC) values in differentiating benign and malignant skull lesions with histopathological (HPE) correlation. J. Clin. Neurosci. 2022, 98, 21–28. [Google Scholar] [CrossRef]
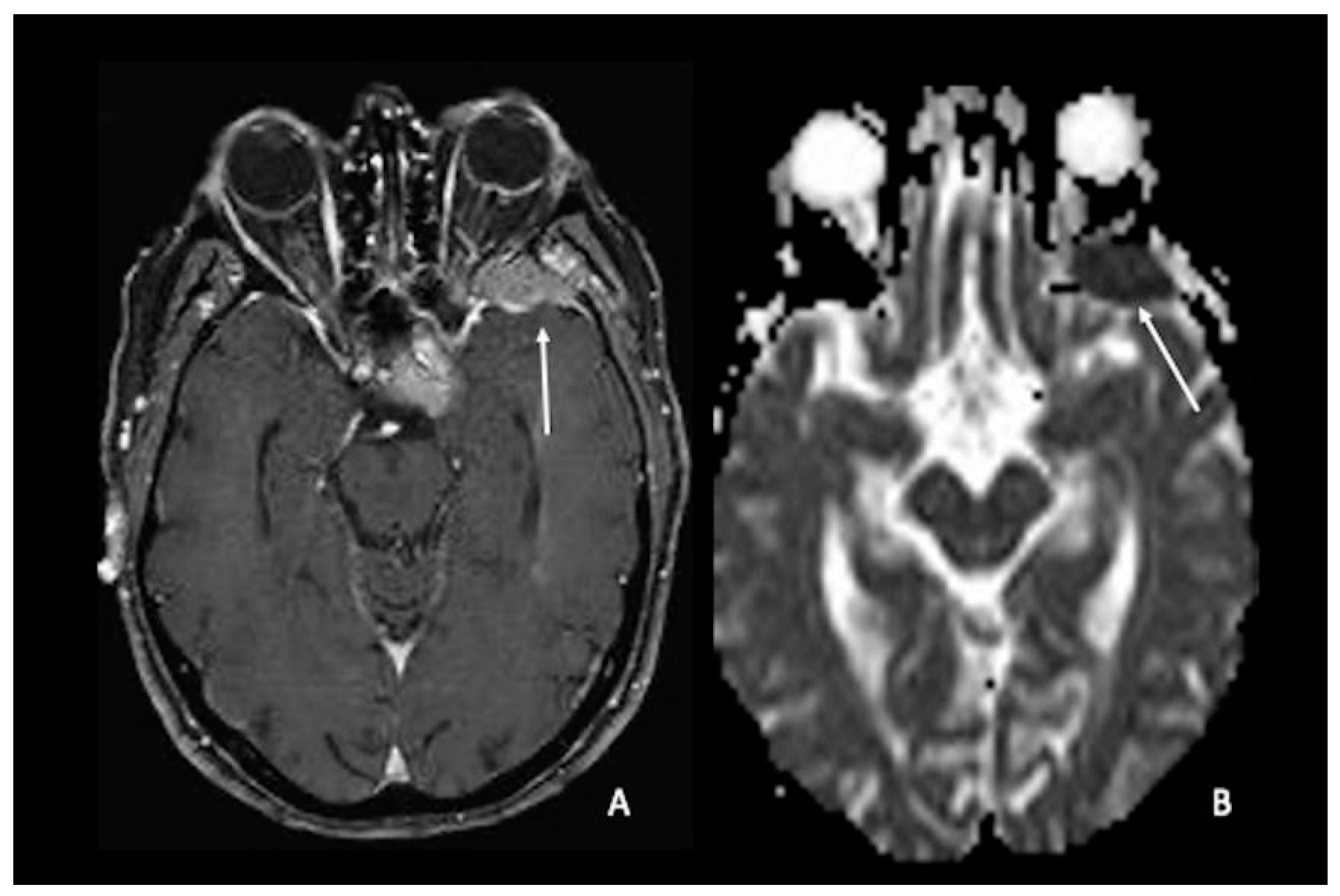
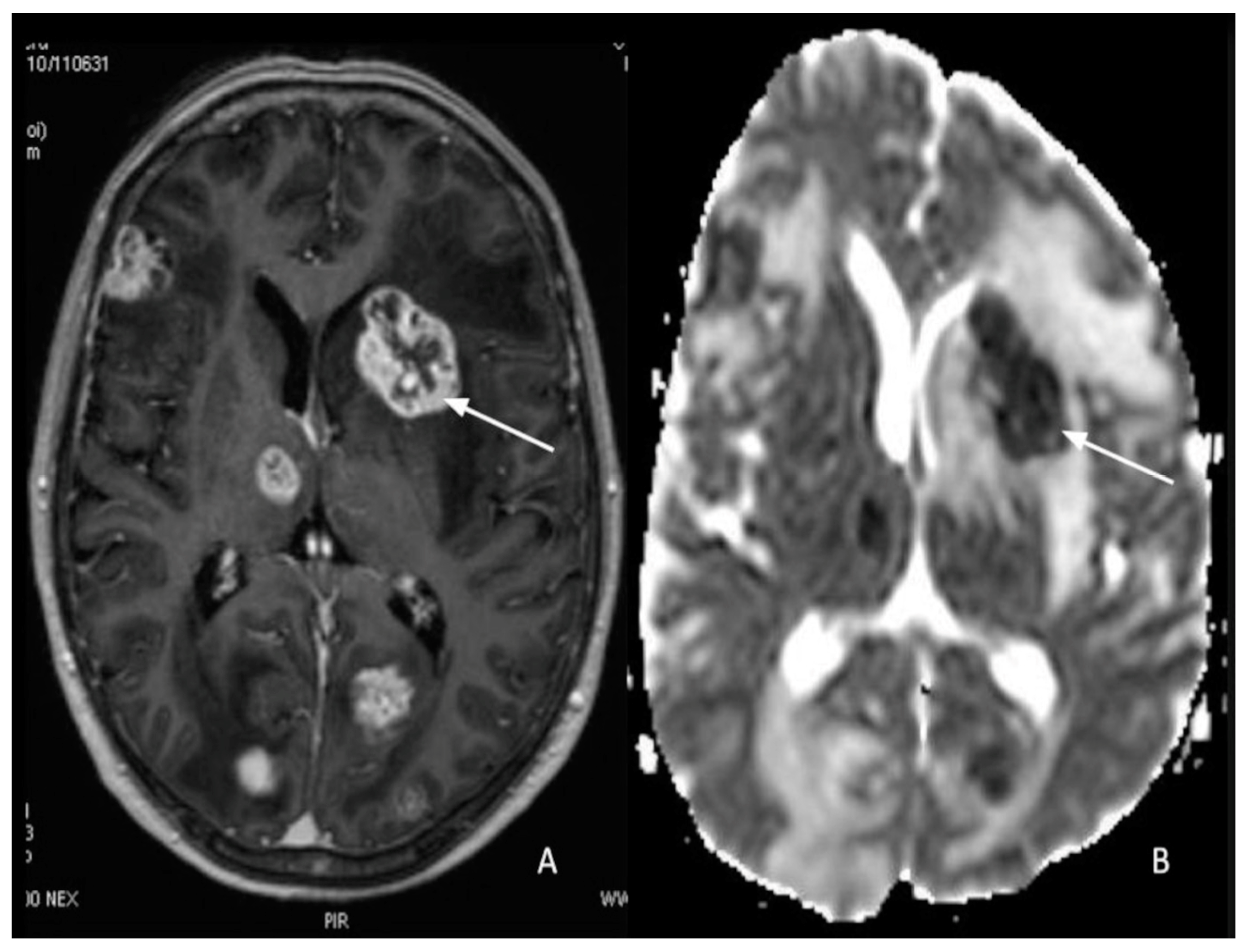

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, A.; Palizzi, S.; Romano, A.; Moltoni, G.; Di Napoli, A.; Maccioni, F.; Bozzao, A. Diffusion Weighted Imaging in Neuro-Oncology: Diagnosis, Post-Treatment Changes, and Advanced Sequences—An Updated Review. Cancers 2023, 15, 618. https://doi.org/10.3390/cancers15030618
Romano A, Palizzi S, Romano A, Moltoni G, Di Napoli A, Maccioni F, Bozzao A. Diffusion Weighted Imaging in Neuro-Oncology: Diagnosis, Post-Treatment Changes, and Advanced Sequences—An Updated Review. Cancers. 2023; 15(3):618. https://doi.org/10.3390/cancers15030618
Chicago/Turabian StyleRomano, Andrea, Serena Palizzi, Allegra Romano, Giulia Moltoni, Alberto Di Napoli, Francesca Maccioni, and Alessandro Bozzao. 2023. "Diffusion Weighted Imaging in Neuro-Oncology: Diagnosis, Post-Treatment Changes, and Advanced Sequences—An Updated Review" Cancers 15, no. 3: 618. https://doi.org/10.3390/cancers15030618
APA StyleRomano, A., Palizzi, S., Romano, A., Moltoni, G., Di Napoli, A., Maccioni, F., & Bozzao, A. (2023). Diffusion Weighted Imaging in Neuro-Oncology: Diagnosis, Post-Treatment Changes, and Advanced Sequences—An Updated Review. Cancers, 15(3), 618. https://doi.org/10.3390/cancers15030618







