SBRT of Spinal Metastases Using a Simultaneous Integrated Boost Concept in Oligometastatic Cancer Patients Is Safe and Effective
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
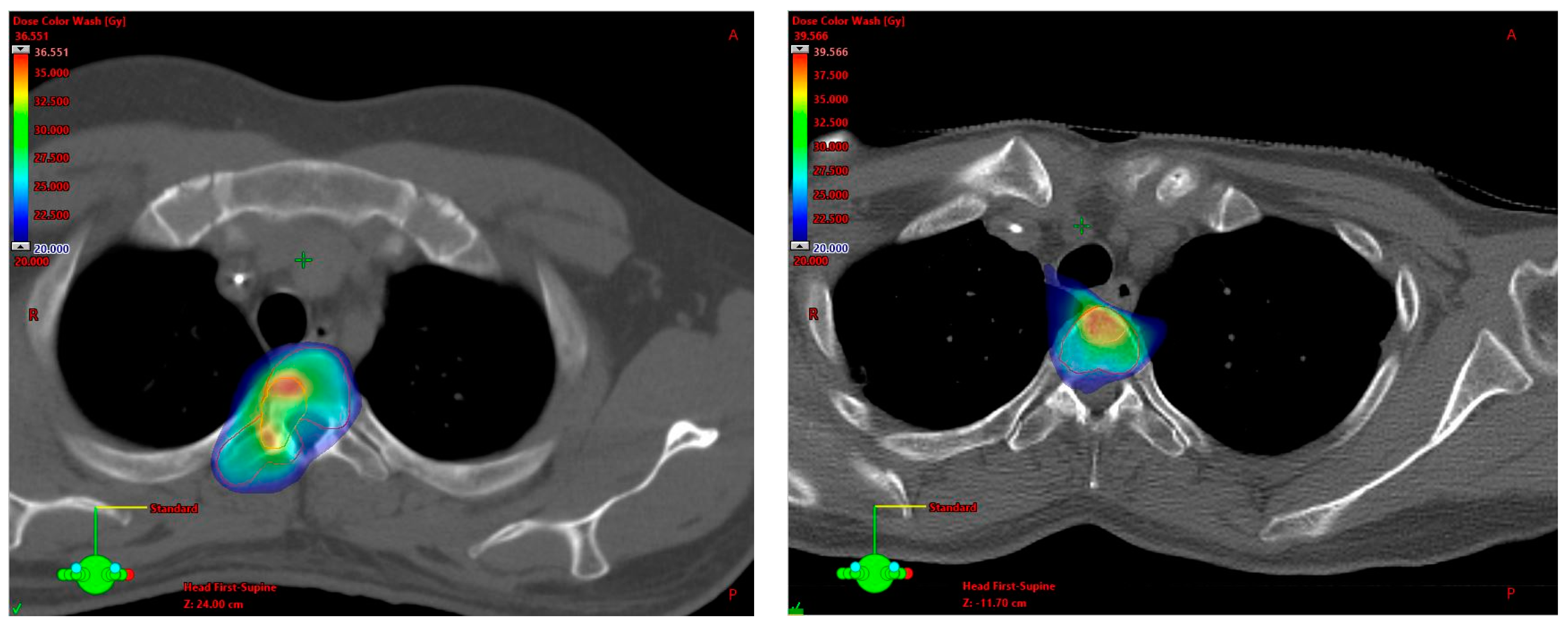
3.2. SBRT Setup, Target Volume Definition and Dose Prescription
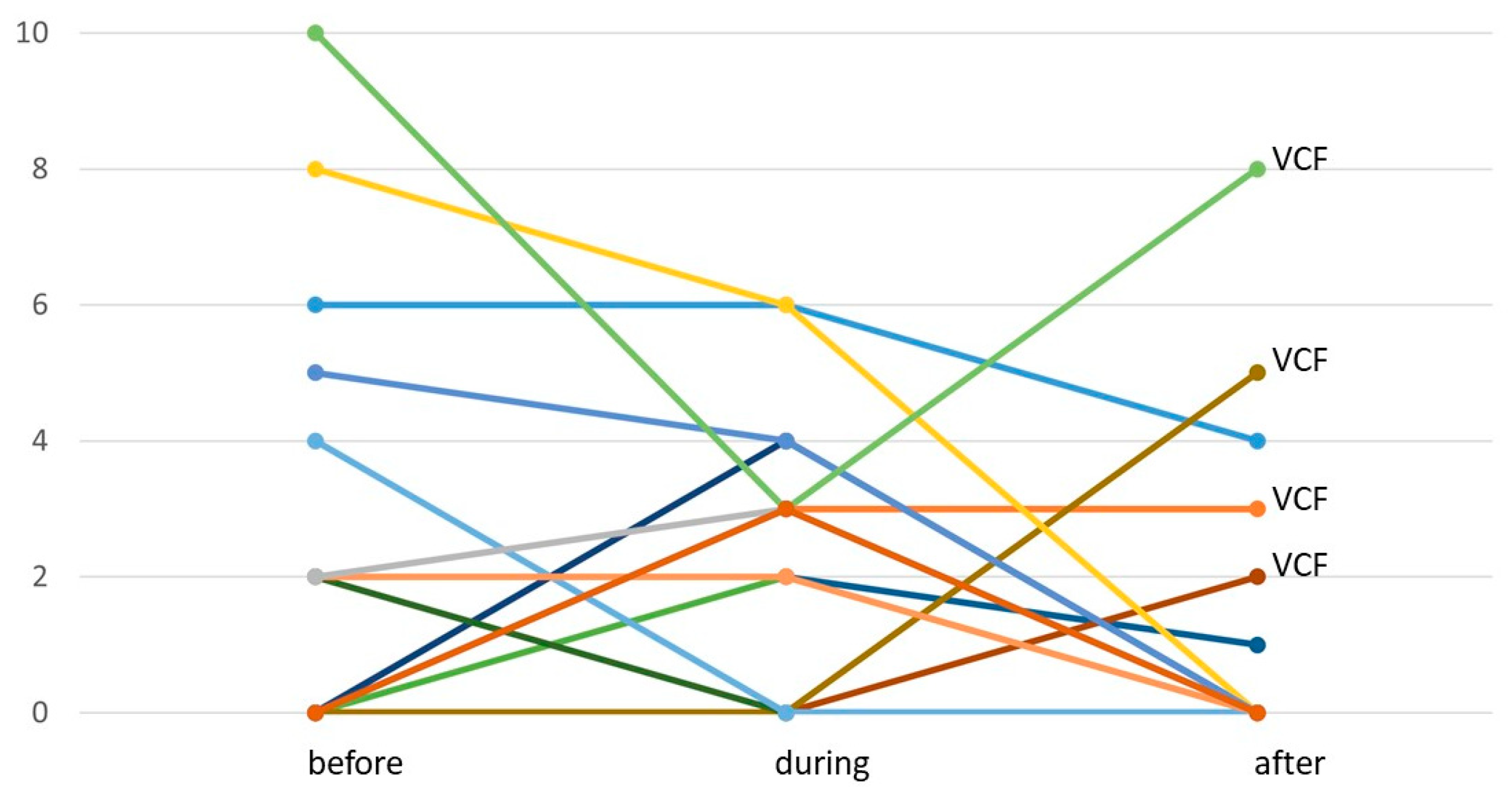
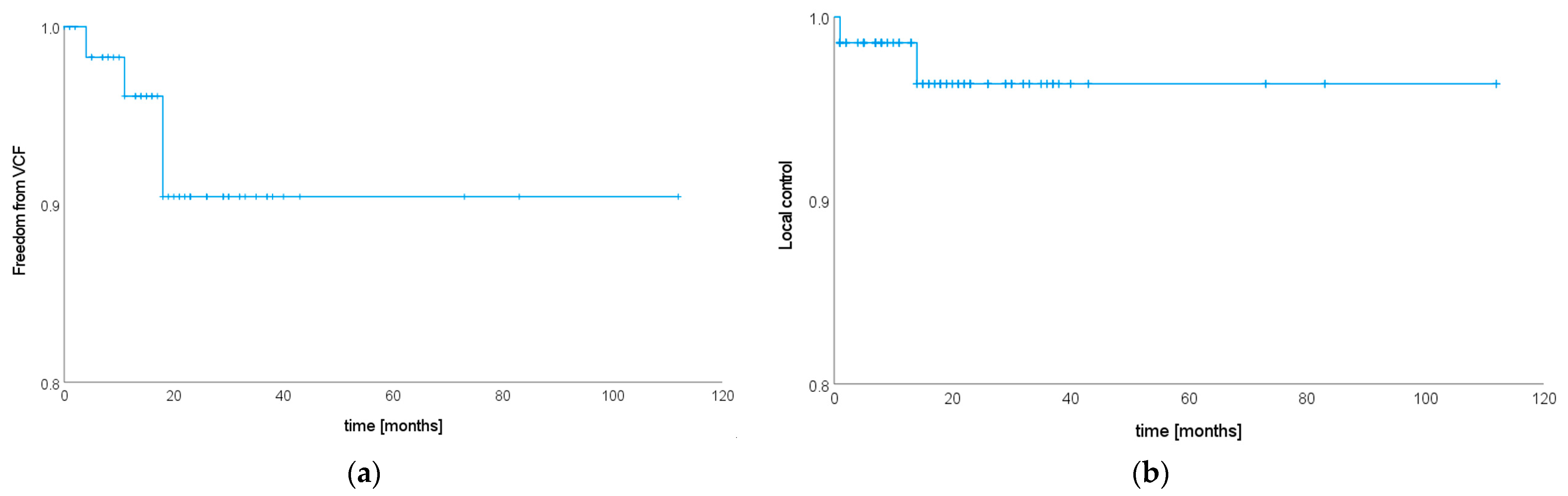
3.3. Treatment Toxicity
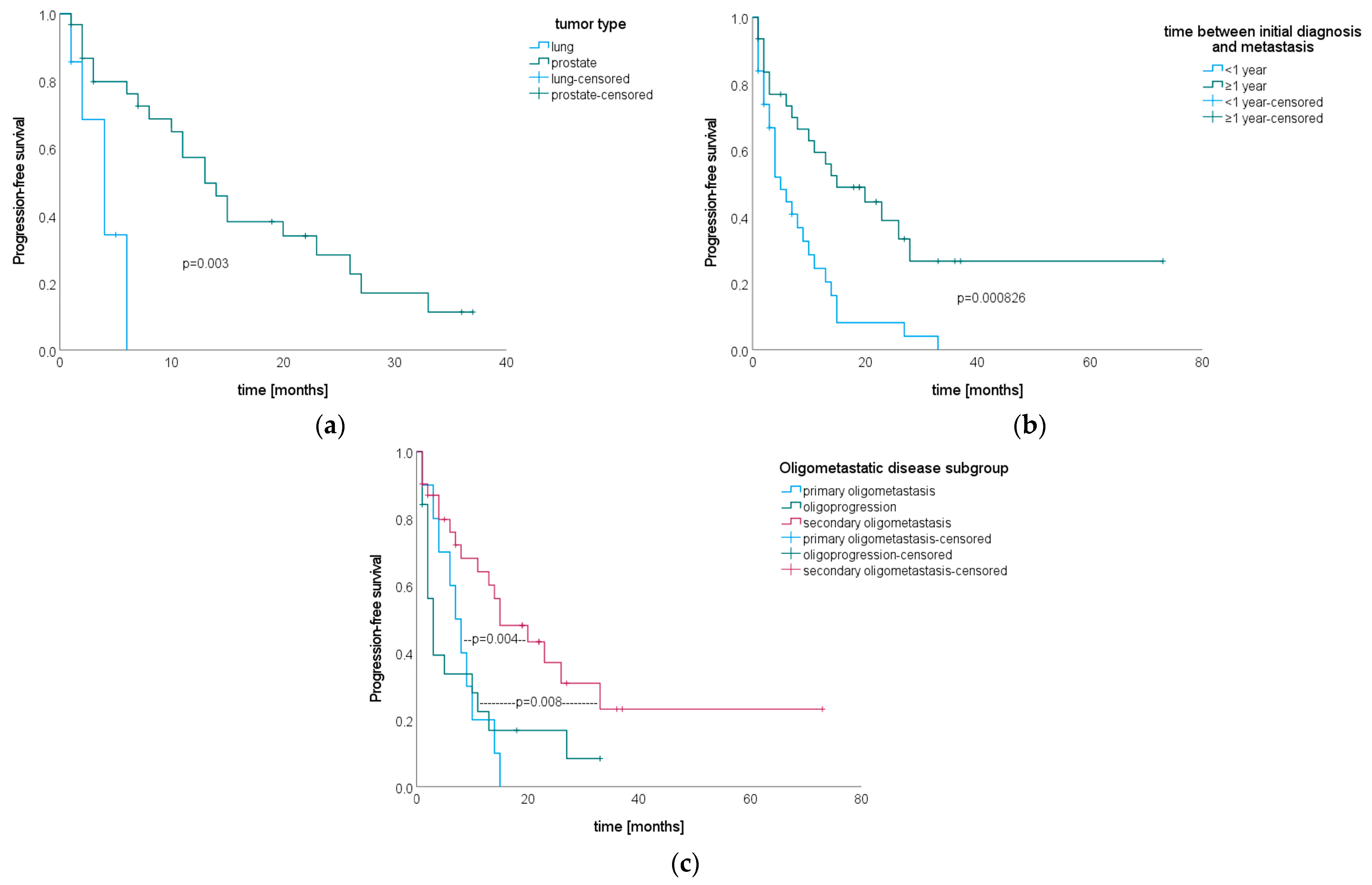
3.4. Local Tumor Control
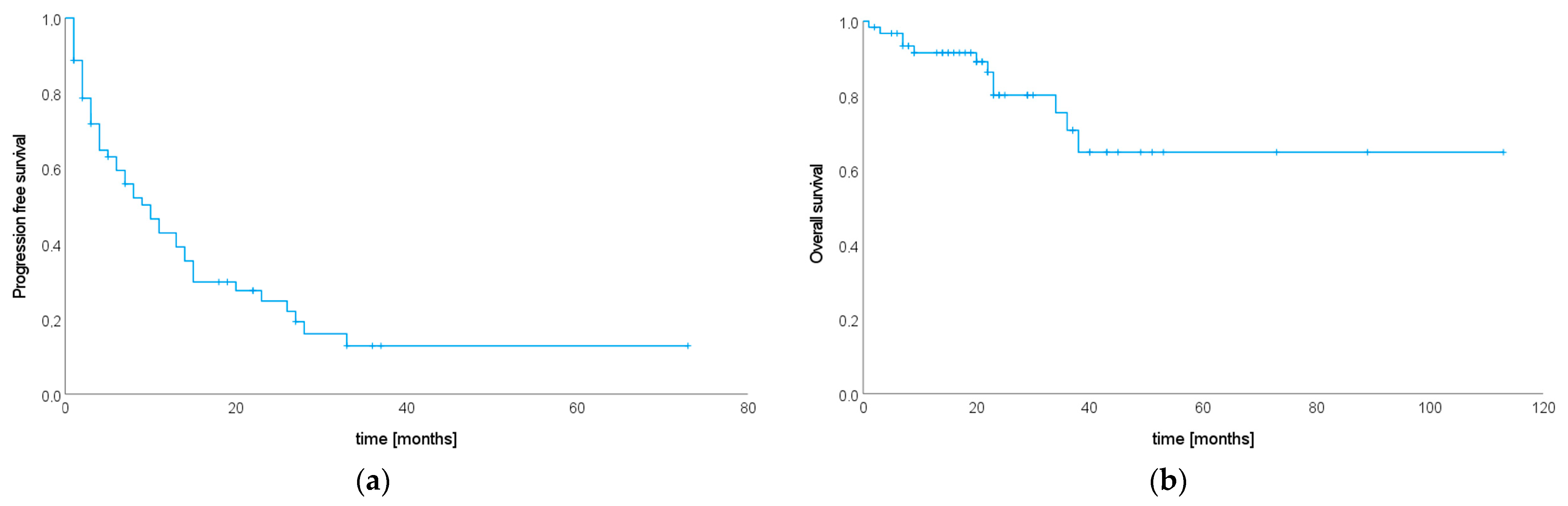
3.5. Progression-Free Survival after SIB–SBRT
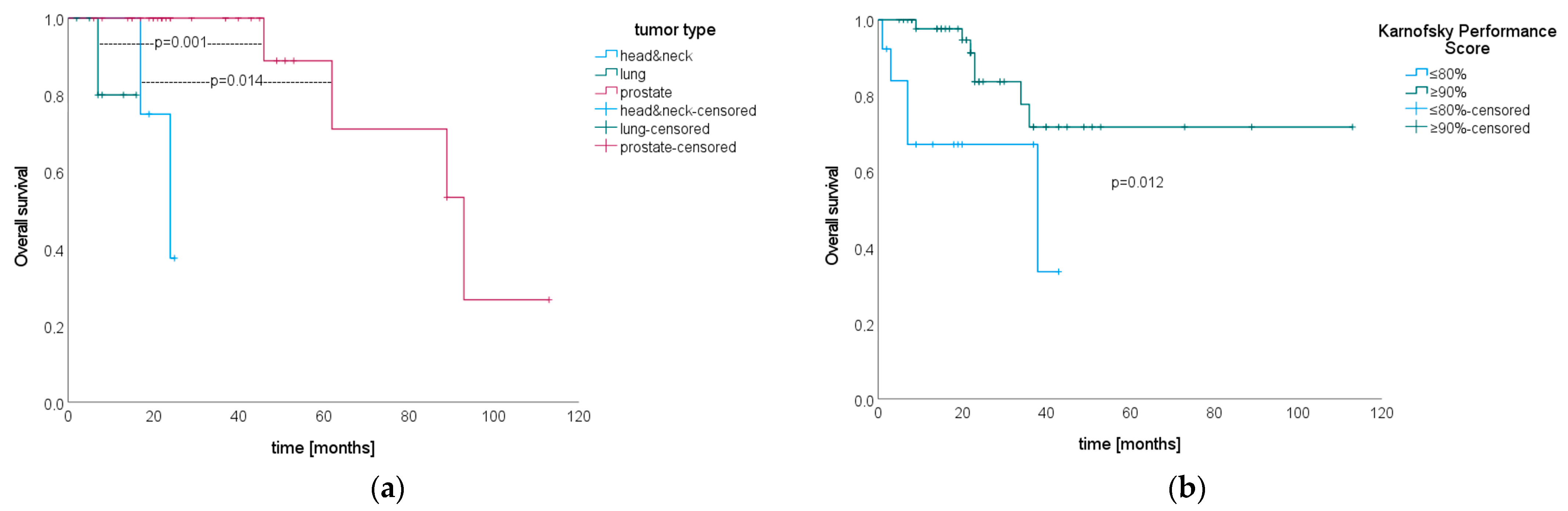
3.6. Overall Survival after SIB–SBRT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Metastasis Is Located in | PTV Definition | n |
|---|---|---|
| proc. spinosus + lamina(e) | proc. spinosus + laminae proc. spinosus + laminae + procc. transversi | 2 1 |
| proc. transversus + lamina unilat. | whole proc. transversus whole vertebra proc. spinosus + laminae + proc. transversus unilat. + pedicle unilat. | 4 1 1 |
| vertebral body central | whole vertebral body whole vertebral body + pedicles circumferential margin around SIB (not whole vertebral body) | 14 2 1 |
| vertebral body lateral + pedicle unilat. + pedicle + proc. transversus + lamina | whole vertebral body whole vertebral body + pedicles whole vertebral body + pedicle unilat. + medial part of proc. transversus unilat. whole vertebral body + pedicles + procc. transversi whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. whole vertebral body + pedicles whole vertebra excluding lateral parts of procc. transversi whole vertebra whole vertebra whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. + proc. spinosus | 8 4 2 1 6 1 1 1 2 1 1 |
| vertebral body central + lateral + pedicle | whole vertebral body + pedicles whole vertebral body whole vertebral body + pedicles + procc. transversi whole vertebral body + pedicles + medial part of proc. transversus unilat. + lamina unilat. whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. whole vertebral body + pedicles | 2 1 1 1 5 1 |
| entire vertebral body + pedicles + medial part of proc. transversus unilat. | whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. | 1 |
| vertebral body lateral + pedicle + medial part of proc. transversus unilat. + lamina unilat. | whole vertebra | 1 |
| vertebral body central + proc. transversus unilat. (bifocal) | whole vertebral body + pedicles + proc. transversus unilat. + lamina unilat. | 1 |
| body of sacrum + lateral extension | whole body of sacrum + lateral part unilat. | 2 |
| SINS Item | p-Value |
|---|---|
| Location | 0.86 |
| Pain | 0.45 |
| Bone lesion | 0.70 |
| Radiographic spinal alignment | 0.75 |
| Vertebral body collapse | NA (all cases censored for vertebral body collapse) |
| Posterolateral involvement | 0.76 |
References
- Harel, R.; Angelov, L. Spine Metastases: Current Treatments and Future Directions. Eur. J. Cancer 2010, 46, 2696–2707. [Google Scholar] [CrossRef]
- von Moos, R.; Body, J.J.; Egerdie, B.; Stopeck, A.; Brown, J.; Fallowfield, L.; Patrick, D.L.; Cleeland, C.; Damyanov, D.; Palazzo, F.S.; et al. Pain and Analgesic Use Associated with Skeletal-Related Events in Patients with Advanced Cancer and Bone Metastases. Support. Care Cancer 2016, 24, 1327–1337. [Google Scholar] [CrossRef]
- Westhoff, P.G.; De Graeff, A.; Monninkhof, E.M.; Pomp, J.; Van Vulpen, M.; Leer, J.W.H.; Marijnen, C.A.M.; Van Der Linden, Y.M. Quality of Life in Relation to Pain Response to Radiation Therapy for Painful Bone Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Schild, S.E.; Abrahm, J.L. Treatment of Painful Bone Metastases. Nat. Rev. Clin. Oncol. 2010, 7, 220–229. [Google Scholar] [CrossRef]
- Sprave, T.; Verma, V.; Förster, R.; Schlampp, I.; Bruckner, T.; Bostel, T.; Welte, S.E.; Tonndorf-Martini, E.; Nicolay, N.H.; Debus, J.; et al. Randomized Phase II Trial Evaluating Pain Response in Patients with Spinal Metastases Following Stereotactic Body Radiotherapy versus Three-Dimensional Conformal Radiotherapy. Radiother. Oncol. 2018, 128, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic Body Radiotherapy versus Conventional External Beam Radiotherapy in Patients with Painful Spinal Metastases: An Open-Label, Multicentre, Randomised, Controlled, Phase 2/3 Trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef]
- Pielkenrood, B.J.; van der Velden, J.M.; van der Linden, Y.M.; Bartels, M.M.T.; Kasperts, N.; Verhoeff, J.J.C.; Eppinga, W.S.C.; Gal, R.; Verlaan, J.J.; Verkooijen, H.M. Pain Response After Stereotactic Body Radiation Therapy Versus Conventional Radiation Therapy in Patients with Bone Metastases—A Phase 2 Randomized Controlled Trial within a Prospective Cohort. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 358–367. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, J.; Willmann, J.; Spałek, M.; Oldenburger, E.; Brown, S.; Kazmierska, J.; Andratschke, N.; Menten, J.; van der Linden, Y.; Hoskin, P. ESTRO ACROP Guidelines for External Beam Radiotherapy of Patients with Uncomplicated Bone Metastases. Radiother. Oncol. 2022, 173, 197–206. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Gomez, D.R.; Blumenschein, G.R.; Lee, J.J.; Hernandez, M.; Ye, R.; Camidge, D.R.; Doebele, R.C.; Skoulidis, F.; Gaspar, L.E.; Gibbons, D.L.; et al. Local Consolidative Therapy versus Maintenance Therapy or Observation for Patients with Oligometastatic Non-Small-Cell Lung Cancer without Progression after First-Line Systemic Therapy: A Multicentre, Randomised, Controlled, Phase 2 Study. Lancet Oncol. 2016, 17, 1672–1682. [Google Scholar] [CrossRef]
- Ost, P.; Reynders, D.; Decaestecker, K.; Fonteyne, V.; Lumen, N.; DeBruycker, A.; Lambert, B.; Delrue, L.; Bultijnck, R.; Claeys, T.; et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J. Clin. Oncol. 2018, 36, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, A.M.C.; Hendriks, L.E.L.; Berghmans, T.; Levy, A.; Hasan, B.; Faivre-Finn, C.; Giaj-Levra, M.; Giaj-Levra, N.; Girard, N.; Greillier, L.; et al. Definition of Synchronous Oligometastatic Non–Small Cell Lung Cancer—A Consensus Report. J. Thorac. Oncol. 2019, 14, 2109–2119. [Google Scholar] [CrossRef]
- Phillips, R.; Shi, W.Y.; Deek, M.; Radwan, N.; Lim, S.J.; Antonarakis, E.S.; Rowe, S.P.; Ross, A.E.; Gorin, M.A.; Deville, C.; et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 650–659. [Google Scholar] [CrossRef]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.M.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and Classification of Oligometastatic Disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer Consensus Recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Pugh, S.L.; Gerszten, P.C.; Yin, F.-F.; Timmerman, R.D.; Hitchcock, Y.J.; Movsas, B.; Kanner, A.A.; Berk, L.B.; Followill, D.S.; et al. RTOG 0631 Phase 2/3 Study of Image Guided Stereotactic Radiosurgery for Localized (1-3) Spine Metastases: Phase 2 Results. Pract. Radiat. Oncol. 2014, 4, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Hawkins, M.; Flentje, M.; Sweeney, R.A. Fractionated Radiosurgery for Painful Spinal Metastases: DOSIS—A Phase II Trial. BMC Cancer 2012, 12, 530. [Google Scholar] [CrossRef]
- Waltenberger, M.; Vogel, M.M.E.; Bernhardt, D.; Münch, S.; Dobiasch, S.; Redmond, K.J.; Lo, S.S.; Acker, G.; Fehlings, M.G.; Ringel, F.; et al. Radiotherapy Concepts for Spinal Metastases—Results from an Online Survey among Radiation Oncologists of the German Society for Radiation Oncology. Strahlenther. Onkol. 2023, 1–16. [Google Scholar] [CrossRef]
- Cox, B.W.; Spratt, D.E.; Lovelock, M.; Bilsky, M.H.; Lis, E.; Ryu, S.; Sheehan, J.; Gerszten, P.C.; Chang, E.; Gibbs, I.; et al. International Spine Radiosurgery Consortium Consensus Guidelines for Target Volume Definition in Spinal Stereotactic Radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e597–e605. [Google Scholar] [CrossRef]
- Dunne, E.M.; Sahgal, A.; Lo, S.S.; Bergman, A.; Kosztyla, R.; Dea, N.; Chang, E.L.; Chang, U.K.; Chao, S.T.; Faruqi, S.; et al. International Consensus Recommendations for Target Volume Delineation Specific to Sacral Metastases and Spinal Stereotactic Body Radiation Therapy (SBRT). Radiother. Oncol. 2020, 145, 21–29. [Google Scholar] [CrossRef]
- Chen, X.; LeCompte, M.C.; Gui, C.; Huang, E.; Khan, M.A.; Hu, C.; Sciubba, D.M.; Kleinberg, L.R.; Lo, S.; Fu, L.; et al. Deviation from Consensus Contouring Guidelines Predicts Inferior Local Control after Spine Stereotactic Body Radiotherapy. Radiother. Oncol. 2022, 173, 215–222. [Google Scholar] [CrossRef]
- Guckenberger, M.; Mantel, F.; Sweeney, R.A.; Hawkins, M.; Belderbos, J.; Ahmed, M.; Andratschke, N.; Madani, I.; Flentje, M. Long-Term Results of Dose-Intensified Fractionated Stereotactic Body Radiation Therapy (SBRT) for Painful Spinal Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 348–357. [Google Scholar] [CrossRef]
- Ning, M.S.; Deegan, B.J.; Ho, J.C.; Chapman, B.V.; Bishop, A.J.; Allen, P.K.; Tannir, N.M.; Amini, B.; Briere, T.M.; Wang, X.A.; et al. Low Incidence of Late Failure and Toxicity after Spine Stereotactic Radiosurgery: Secondary Analysis of Phase I/II Trials with Long-Term Follow-Up. Radiother. Oncol. 2019, 138, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Whyne, C.M.; Ma, L.; Larson, D.A.; Fehlings, M.G. Vertebral Compression Fracture after Stereotactic Body Radiotherapy for Spinal Metastases. Lancet Oncol. 2013, 14, e310–e320. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Shin, J.H.; Yamada, Y.J.; Mesfin, A.; Fehlings, M.G.; Rhines, L.D.; Sahgal, A. Stereotactic Body Radiotherapy for Spinal Metastases: What are the Risks and How Do We Minimize Them? Spine 2017, 41, 1–15. [Google Scholar] [CrossRef]
- Fourney, D.; DiPaola, C.; Fisher, C. P153. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence Based Approach and Expert Consensus from the Spine Oncology Study Group. Spine J. 2009, 9, 193S. [Google Scholar] [CrossRef]
- Mantel, F.; Sweeney, R.A.; Klement, R.J.; Hawkins, M.A.; Belderbos, J.; Ahmed, M.; Toussaint, A.; Polat, B.; Flentje, M.; Guckenberger, M. Risk Factors for Vertebral Compression Fracture after Spine Stereotactic Body Radiation Therapy: Long-Term Results of a Prospective Phase 2 Study. Radiother. Oncol. 2019, 141, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Adzersen, K.H. Allgemeine Terminologie und Merkmale Unerwünschter Ereignisse Common Terminology Criteria for Adverse Events (CTCAE); Deutsche Krebsforschungszentrum: Heidelberg, Germany, 2016; pp. 1–174. [Google Scholar]
- Cellini, F.; Manfrida, S.; Deodato, F.; Cilla, S.; Maranzano, E.; Pergolizzi, S.; Arcidiacono, F.; Di Franco, R.; Pastore, F.; Muto, M.; et al. Pain Reduction with Bone Metastases Stereotactic Radiotherapy (PREST): A Phase III Randomized Multicentric Trial. Trials 2019, 20, 609. [Google Scholar] [CrossRef]
- Sprave, T.; Welte, S.E.; Bruckner, T.; Förster, R.; Bostel, T.; Schlampp, I.; Nicolay, N.H.; Debus, J.; Rief, H. Intensity-Modulated Radiotherapy with Integrated-Boost in Patients with Bone Metastasis of the Spine: Study Protocol for a Randomized Controlled Trial. Trials 2018, 19, 59. [Google Scholar] [CrossRef]
- Potkrajcic, V.; Mueller, A.C.; Frey, B.; Gani, C.; Zips, D.; Hoffmann, R.; Frantz, S.; Warm, V.; Paulsen, F.; Eckert, F. Dose-Escalated Radiotherapy with Simultaneous Integrated Boost for Bone Metastases in Selected Patients with Assumed Favourable Prognosis. Radiol. Oncol. 2022, 56, 515–524. [Google Scholar] [CrossRef]
- Rogowski, P.; Trapp, C.; von Bestenbostel, R.; Schmidt-Hegemann, N.S.; Shi, R.; Ilhan, H.; Kretschmer, A.; Stief, C.; Ganswindt, U.; Belka, C.; et al. Outcomes of Metastasis-Directed Therapy of Bone Oligometastatic Prostate Cancer. Radiat. Oncol. 2021, 16, 125. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gui, C.; Grimm, J.; Huang, E.; Kleinberg, L.; Lo, L.; Sciubba, D.; Khan, M.; Redmond, K.J. Normal Tissue Complication Probability of Vertebral Compression Fracture after Stereotactic Body Radiotherapy for de Novo Spine Metastasis. Radiother. Oncol. 2020, 150, 142–149. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | n | % |
|---|---|---|
| Gender + | ||
| male | 43 | 69.35 |
| female | 19 | 30.65 |
| Age at primary diagnosis + | ||
| 20–40 | 7 | 11.29 |
| 41–60 | 24 | 38.71 |
| 61–80 | 29 | 46.77 |
| >80 | 2 | 3.23 |
| median 60.5 (R 20–91) | ||
| Age at SIB–SBRT + | ||
| 30–40 | 2 | 3.23 |
| 41–60 | 22 | 35.48 |
| 61–80 | 33 | 53.23 |
| >80 | 5 | 8.06 |
| median 66.5 (R 30–91) | ||
| Years from initial diagnosis to development of metastases | ||
| ≤1 | 31 | 50 |
| >1 | 31 | 50 |
| median 0.96 (R 0–27) | ||
| Primary tumor + | ||
| prostate | 31 | 50.00 |
| breast | 9 | 14.52 |
| lung | 7 | 11.29 |
| head and neck cancer | 4 | 6.45 |
| melanoma | 3 | 4.84 |
| other | 8 | 12.90 |
| Karnofsky Performance Score + | ||
| 90–100 | 48 | 77.42 |
| 70–80 | 8 | 12.90 |
| 50–60 | 5 | 8.06 |
| NA | 1 | 1.61 |
| median 100 (60–100) | ||
| Postmenopausal status + | ||
| male gender | 43 | 69.35 |
| premenopausal | 1 | 1.61 |
| postmenopausal | 8 | 12.90 |
| NA | 10 | 16.13 |
| Diagnosed osteoporosis + | ||
| yes | 1 | 1.61 |
| no | 61 | 98.39 |
| Oligometastatic subgroup + | ||
| primary oligometastasis | 10 | 16.13 |
| secondary oligometastasis | 31 | 50.00 |
| oligoprogression | 19 | 30.65 |
| oligopersistence | 2 | 3.23 |
| Number of active metastases present at SIB–SBRT + | ||
| 1 | 39 | 62.90 |
| 2 | 15 | 24.19 |
| 3 | 8 | 12.90 |
| Location of spinal metastasis * | ||
| cervical spine | 4 | 5.63 |
| thoracic spine | 34 | 47.89 |
| lumbar spine | 30 | 42.25 |
| sacrum | 3 | 4.23 |
| Type of metastasis * | ||
| osteolytic | 17 | 23.94 |
| osteoblastic | 45 | 63.38 |
| mixed | 9 | 12.68 |
| Baseline pain (VAS) * | ||
| 0 | 62 | 87.32 |
| 2 | 4 | 5.63 |
| 4–6 | 3 | 4.23 |
| 8–10 | 2 | 2.82 |
| Spinal Instability Neoplastic Score (SINS) * | ||
| 1–6 (stable) | 67 | 94.37 |
| 7–9 (potentially unstable) | 4 | 5.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waltenberger, M.; Strick, C.; Vogel, M.M.E.; Diehl, C.; Combs, S.E. SBRT of Spinal Metastases Using a Simultaneous Integrated Boost Concept in Oligometastatic Cancer Patients Is Safe and Effective. Cancers 2023, 15, 5813. https://doi.org/10.3390/cancers15245813
Waltenberger M, Strick C, Vogel MME, Diehl C, Combs SE. SBRT of Spinal Metastases Using a Simultaneous Integrated Boost Concept in Oligometastatic Cancer Patients Is Safe and Effective. Cancers. 2023; 15(24):5813. https://doi.org/10.3390/cancers15245813
Chicago/Turabian StyleWaltenberger, Maria, Christian Strick, Marco M. E. Vogel, Christian Diehl, and Stephanie E. Combs. 2023. "SBRT of Spinal Metastases Using a Simultaneous Integrated Boost Concept in Oligometastatic Cancer Patients Is Safe and Effective" Cancers 15, no. 24: 5813. https://doi.org/10.3390/cancers15245813
APA StyleWaltenberger, M., Strick, C., Vogel, M. M. E., Diehl, C., & Combs, S. E. (2023). SBRT of Spinal Metastases Using a Simultaneous Integrated Boost Concept in Oligometastatic Cancer Patients Is Safe and Effective. Cancers, 15(24), 5813. https://doi.org/10.3390/cancers15245813






