Local Recurrence and Development of Spinal Cord Syndrome during Follow-Up after Surgical Treatment of Metastatic Spine Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Demographics
3.2. Spinal Scores
3.3. Additional Treatment
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ortiz Gómez, J.A. The Incidence of Vertebral Body Metastases. Int. Orthop. 1995, 19, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.G.; Gaisendrees, C.; Kernich, N.; Weber, M.; Scheyerer, M.J.; Eysel, P.; Siewe, J.; Zarghooni, K. Epidemiology of Surgically Treated Spinal Tumors: A Multicenter Surveillance Study of 9686 Patients from the German Spine Registry (DWG Register). Z. Orthop. Unfall. 2023. ahead of print. [Google Scholar] [CrossRef]
- Van den Brande, R.; MJ Cornips, E.; Peeters, M.; Ost, P.; Billiet, C.; Van de Kelft, E. Epidemiology of Spinal Metastases, Metastatic Epidural Spinal Cord Compression and Pathologic Vertebral Compression Fractures in Patients with Solid Tumors: A Systematic Review. J. Bone Oncol. 2022, 35, 100446. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, K.; Kanda, Y.; Yurube, T.; Takeoka, Y.; Miyazaki, K.; Ohnishi, H.; Matsuo, T.; Ryu, M.; Kuroshima, K.; Kumagai, N.; et al. The Identification of Risk Factors for Symptomatic Spinal Metastasis Onset: A Prospective Cohort Study of 128 Asymptomatic Spinal Metastasis Patients. Cancers 2023, 15, 1251. [Google Scholar] [CrossRef] [PubMed]
- Shiber, M.; Kimchi, G.; Knoller, N.; Harel, R. The Evolution of Minimally Invasive Spine Tumor Resection and Stabilization: From K-Wires to Navigated One-Step Screws. J. Clin. Med. 2023, 12, 536. [Google Scholar] [CrossRef]
- Barzilai, O.; Bilsky, M.H.; Laufer, I. The Role of Minimal Access Surgery in the Treatment of Spinal Metastatic Tumors. Glob. Spine J. 2020, 10, 79S. [Google Scholar] [CrossRef]
- Bludau, F.; Winter, L.; Welzel, G.; Obertacke, U.; Schneider, F.; Wenz, F.; Ruder, A.M.; Giordano, F.A. Long-Term Outcome after Combined Kyphoplasty and Intraoperative Radiotherapy (Kypho-IORT) for Vertebral Tumors. Radiat. Oncol. 2020, 15, 263. [Google Scholar] [CrossRef]
- Sayed, D.; Jacobs, D.; Sowder, T.; Haines, D.; Orr, W. Prospective Evaluation Spinal Radiofrequency Ablation Combined with Cement Augmentation for Painful Spinal Vertebral Metastasis: A Single-Center Prospective Study. Pain Physician 2019, 22, E441–E449. [Google Scholar] [CrossRef]
- Dea, N.; Versteeg, A.L.; Sahgal, A.; Verlaan, J.J.; Charest-Morin, R.; Rhines, L.D.; Sciubba, D.M.; Schuster, J.M.; Weber, M.H.; Lazary, A.; et al. Metastatic Spine Disease: Should Patients with Short Life Expectancy Be Denied Surgical Care? An International Retrospective Cohort Study. Neurosurgery 2020, 87, 303. [Google Scholar] [CrossRef]
- Wai, E.K.; Finkelstein, J.A.; Tangente, R.P.; Holden, L.; Chow, E.; Ford, M.; Yee, A. Quality of Life in Surgical Treatment of Metastatic Spine Disease. Spine 2003, 28, 508–512. [Google Scholar] [CrossRef]
- Quan, G.M.Y.; Vital, J.M.; Aurouer, N.; Obeid, I.; Palussière, J.; Diallo, A.; Pointillart, V. Surgery Improves Pain, Function and Quality of Life in Patients with Spinal Metastases: A Prospective Study on 118 Patients. Eur. Spine J. 2011, 20, 1970. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Küchler, J.; Graumüller, L.; Abusamha, A.; Schild, S.E.; Gliemroth, J. Radiotherapy with or without Decompressive Surgery for Metastatic Spinal Cord Compression: A Retrospective Matched-Pair Study Including Data from Prospectively Evaluated Patients. Cancers 2022, 14, 1260. [Google Scholar] [CrossRef] [PubMed]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS Framework: Approach to the Treatment of Spinal Metastatic Tumors. Oncologist 2013, 18, 744. [Google Scholar] [CrossRef]
- Fisher, C.G.; Dipaola, C.P.; Ryken, T.C.; Bilsky, M.H.; Shaffrey, C.I.; Berven, S.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; Chou, D.; et al. A Novel Classification System for Spinal Instability in Neoplastic Disease: An Evidence-Based Approach and Expert Consensus from the Spine Oncology Study Group. Spine 2010, 35, E1221–E1229. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.L.; Myrehaug, S.; Soliman, H.; Husain, Z.A.; Tseng, C.L.; Detsky, J.; Ruschin, M.; Atenafu, E.G.; Witiw, C.D.; Larouche, J.; et al. Mature Local Control and Reirradiation Rates Comparing Spine Stereotactic Body Radiation Therapy with Conventional Palliative External Beam Radiation Therapy. Int. J. Radiat. Oncol. 2022, 114, 293–300. [Google Scholar] [CrossRef]
- Frankel, H.L.; Hancock, D.O.; Hyslop, G.; Melzak, J.; Michaelis, L.S.; Ungar, G.H.; Vernon, J.D.S.; Walsh, J.J. The Value of Postural Reduction in the Initial Management of Closed Injuries of the Spine with Paraplegia and Tetraplegia. Spinal Cord 1969, 7, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bilsky, M.H.; Laufer, I.; Fourney, D.R.; Groff, M.; Schmidt, M.H.; Varga, P.P.; Vrionis, F.D.; Yamada, Y.; Gerszten, P.C.; Kuklo, T.R. Reliability Analysis of the Epidural Spinal Cord Compression Scale. J. Neurosurg. Spine 2010, 13, 324–328. [Google Scholar] [CrossRef]
- Paulino Pereira, N.R.; Groot, O.Q.; Verlaan, J.J.; Bongers, M.E.R.; Twining, P.K.; Kapoor, N.D.; Van Dijk, C.N.; Schwab, J.H.; Bramer, J.A.M. Quality of Life Changes After Surgery for Metastatic Spinal Disease: A Systematic Review and Meta-Analysis. Clin. Spine Surg. 2022, 35, 38–48. [Google Scholar] [CrossRef]
- Luksanapruksa, P.; Buchowski, J.M.; Zebala, L.P.; Kepler, C.K.; Singhatanadgige, W.; Bumpass, D.B. Perioperative Complications of Spinal Metastases Surgery. Clin. Spine Surg. 2017, 30, 4–13. [Google Scholar] [CrossRef]
- Paulino Pereira, N.R.; Ogink, P.T.; Groot, O.Q.; Ferrone, M.L.; Hornicek, F.J.; van Dijk, C.N.; Bramer, J.A.M.; Schwab, J.H. Complications and Reoperations after Surgery for 647 Patients with Spine Metastatic Disease. Spine J. 2019, 19, 144–156. [Google Scholar] [CrossRef]
- Sundaresan, N.; Rothman, A.; Manhart, K.; Kelliher, K. Surgery for Solitary Metastases of the Spine: Rationale and Results of Treatment. Spine 2002, 27, 1802–1806. [Google Scholar] [CrossRef] [PubMed]
- Igoumenou, V.G.; Mavrogenis, A.F.; Angelini, A.; Baracco, R.; Benzakour, A.; Benzakour, T.; Bork, M.; Vazifehdan, F.; Nena, U.; Ruggieri, P. Complications of Spine Surgery for Metastasis. Eur. J. Orthop. Surg. Traumatol. 2019, 30, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Walter, S.G.; Lenz, M.; Gaisendrees, C.; Schlachtenberger, G.; Sircar, K.; Knöll, P.; Siewe, J.; Brenke, C.; Rommelspacher, Y.; Shiban, E.; et al. Complications Associated to Wound Drainages in Tumor Spine Surgery: A Multicenter Surveillance Study from the German Spine Registry (DWG-Register). Sci. Rep. 2022, 12, 19983. [Google Scholar] [CrossRef] [PubMed]
- Curtin, M.; Piggott, R.P.; Murphy, E.P.; Munigangaiah, S.; Baker, J.F.; McCabe, J.P.; Devitt, A. Spinal Metastatic Disease: A Review of the Role of the Multidisciplinary Team. Orthop. Surg. 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Dea, N.; Detsky, J.S.; Sahgal, A. Management of Recurrent or Progressive Spinal Metastases: Reirradiation Techniques and Surgical Principles. Neuro-Oncol. Pract. 2020, 7, i45. [Google Scholar] [CrossRef]
- Wise, J.J.; Fischgrund, J.S.; Herkowitz, H.N.; Montgomery, D.; Kurz, L.T. Complication, Survival Rates, and Risk Factors of Surgery for Metastatic Disease of the Spine. Spine 1999, 24, 1943–1951. [Google Scholar] [CrossRef]
- Bishop, A.J.; Tao, R.; Rebueno, N.C.; Christensen, E.N.; Allen, P.K.; Wang, X.A.; Amini, B.; Tannir, N.M.; Tatsui, C.E.; Rhines, L.D.; et al. Outcomes for Spine Stereotactic Body Radiation Therapy and an Analysis of Predictors of Local Recurrence. Int. J. Radiat. Oncol. 2015, 92, 1016–1026. [Google Scholar] [CrossRef]
- Laufer, I.; Iorgulescu, J.B.; Chapman, T.; Lis, E.; Shi, W.; Zhang, Z.; Cox, B.W.; Yamada, Y.; Bilsky, M.H. Local Disease Control for Spinal Metastases Following “Separation Surgery” and Adjuvant Hypofractionated or High-Dose Single-Fraction Stereotactic Radiosurgery: Outcome Analysis in 186 Patients. J. Neurosurg. Spine 2013, 18, 207–214. [Google Scholar] [CrossRef]
- Li, R.F.; Qiao, R.Q.; Xu, M.Y.; Ma, R.X.; Hu, Y.C. Separation Surgery in the Treatment of Spinal Metastasis. Technol. Cancer Res. Treat. 2022, 21, 15330338221107208. [Google Scholar] [CrossRef]
- Lenschow, M.; Lenz, M.; von Spreckelsen, N.; Ossmann, J.; Meyer, J.; Keßling, J.; Nadjiri, L.; Telentschak, S.; Zarghooni, K.; Knöll, P.; et al. Impact of Spinal Instrumentation on Neurological Outcome in Patients with Intermediate Spinal Instability Neoplastic Score (SINS). Cancers 2022, 14, 2193. [Google Scholar] [CrossRef]
- Hsu, F.-M.; Xiao, F.; Lin, P.-C.; Chen, Y.-H. Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases Medical Images View Project Neurocognitive Outcome of Conformal WBRT w/Wo Hippocampal Avoidance for Brain Metastases View Project Neoadjuvant Stereotactic Body Radiation Therapy for Spine Metastases. Artic. J. Spine Neurosurg. 2018, 7. [Google Scholar] [CrossRef]
| Total | No Spinal Cord Syndrome during Follow-Up | Spinal Cord Syndrome during Follow-Up | p-Value | |
|---|---|---|---|---|
| Number of cases n (%) | 746 | 690 (92.5%) | 56 (7.5%) | |
| Tumor entity n (%) 1 = Renal Ca 2 = Mamma Ca 3 = Lymphoma 4 = SCLC 5 = NSCLC 6 = Thyroid Ca 7 = Multiple Myeloma 8 = Prostate Ca 9 = Sarcoma 10 = CUP 11 = GI 12 = Urothel 13 = Malign. Melanoma 14 = other | 45 (6.0%) 116 (15.5%) 35 (4.7%) 17 (2.3%) 115 (15.4%) 17 (2.3%) 71 (9.5%) 117 (15.7%) 22 (2.9%) 35 (4.7%) 73 (9.8%) 21 (2.8%) 17 (2.3%) 45 (6.0%) | 41 (5.9%) 111 (16.1%) 33 (4.8%) 15 (2.2%) 106 (15.3%) 15 (2.2%) 63 (9.1%) 106 (15.3%) 21 (3.0%) 33 (4.8%) 70 (10.1%) 19 (2.7%) 15 (2.2%) 41 (5.9%) | 4 (7.1%) 5 (8.9%) 2 (3.6%) 2 (3.6%) 9 (10.1%) 2 (3.6%) 8 (14.2%) 11 (19.6%) 1 (1.8%) 2 (3.6%) 3 (5.4%) 1 (1.8%) 2 (3.6%) 4 (7.1%) | 0.56 |
| Gender (M/F) | 463/283 | 423/267 | 39/17 | 0.31 |
| Age (in yrs.) | 63.7 (50.5, 77.0) | 64.0 (51.3, 76.8) | 60.6 (51.8, 74.2) | 0.54 |
| t to radiation (d; 95%CI) | 31.8 (12.3, 51.3) | 22.6 (10.6, 34.7) | 106.2 (44.9, 167.1) | * < 0.001 |
| KPS | 61.0 ± 14.4 | 61.0 ± 14.4 | 62.5 ± 13.4 | 0.44 |
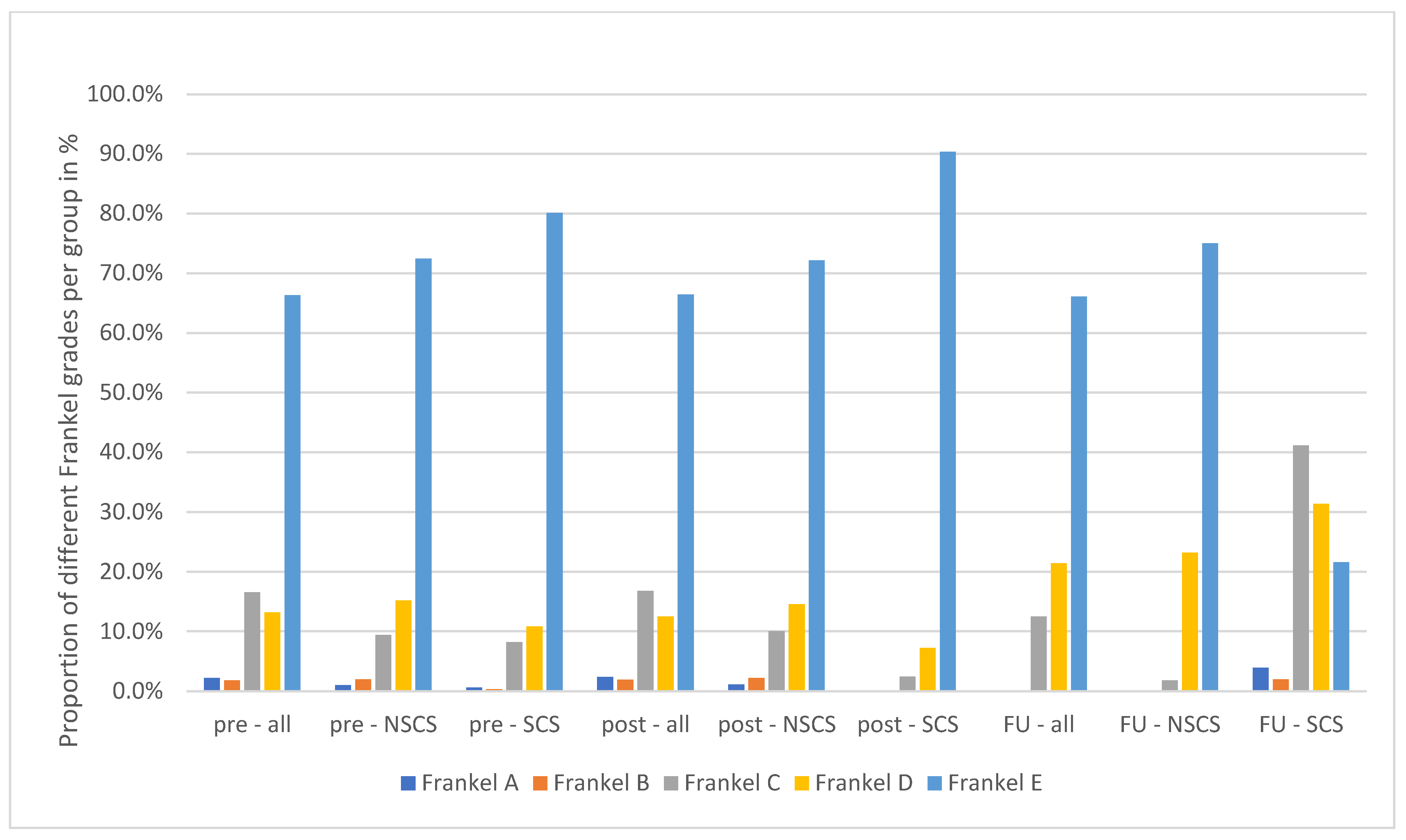
| Frankel grade presurgery (in %) A/B/C/D/E | 2/2/17/13/66 | 2/2/17/13/66 | 0/0/13/21/66 | 0.47 |
| Frankel grade post-surgery (in %) A/B/C/D/E | 1/2/9/15/72 | 1/2/10/15/72 | 0/0/2/23/75 | 0.16 |
| Frankel grade follow-up (in %) A/B/C/D/E | 1/1/8/11/79 | 0/0/2/7/90 | 4/2/41/31/22 | * 0.02 |
| Tumor Main Location Cervical Thoracic Lumbar Sacral | 11.8% 53.9% 32.6% 1.9% | 12.8% 56.1% 29.3% 1.8% | 16.1% 53.6% 25.0% 5.4% | 0.76 |
| Type of surgery Decompression surgery (DS) Hemilaminectomy Laminectomy No decompressive surgery Vertebrectomy Debulking surgery | 3.4% 7.2% 68.7% 10.3% 9.4% 1.1% | 3.4% 7.3% 68.7% 10.2% 9.4% 1.0% | 3.6% 5.4% 67.9% 10.7% 10.7% 1.8% | 0.82 |
| Spinal Cord Syndrome during Follow-Up | |
|---|---|
| Spinal cord syndrome without prior SCS | 40 (76.9%) |
| Spinal cord syndrome with prior history of SCS | 7 (13.5%) |
| Aggravated SCS | 9 (17.3%) |
| ESCC score (I/II/III in %) | 25.2/39.1/35.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knöll, P.; Lenschow, M.; Lenz, M.; Neuschmelting, V.; von Spreckelsen, N.; Telentschak, S.; Olbrück, S.; Weber, M.; Rosenbrock, J.; Eysel, P.; et al. Local Recurrence and Development of Spinal Cord Syndrome during Follow-Up after Surgical Treatment of Metastatic Spine Disease. Cancers 2023, 15, 4749. https://doi.org/10.3390/cancers15194749
Knöll P, Lenschow M, Lenz M, Neuschmelting V, von Spreckelsen N, Telentschak S, Olbrück S, Weber M, Rosenbrock J, Eysel P, et al. Local Recurrence and Development of Spinal Cord Syndrome during Follow-Up after Surgical Treatment of Metastatic Spine Disease. Cancers. 2023; 15(19):4749. https://doi.org/10.3390/cancers15194749
Chicago/Turabian StyleKnöll, Peter, Moritz Lenschow, Maximilian Lenz, Volker Neuschmelting, Niklas von Spreckelsen, Sergej Telentschak, Sebastian Olbrück, Maximilian Weber, Johannes Rosenbrock, Peer Eysel, and et al. 2023. "Local Recurrence and Development of Spinal Cord Syndrome during Follow-Up after Surgical Treatment of Metastatic Spine Disease" Cancers 15, no. 19: 4749. https://doi.org/10.3390/cancers15194749
APA StyleKnöll, P., Lenschow, M., Lenz, M., Neuschmelting, V., von Spreckelsen, N., Telentschak, S., Olbrück, S., Weber, M., Rosenbrock, J., Eysel, P., & Walter, S. G. (2023). Local Recurrence and Development of Spinal Cord Syndrome during Follow-Up after Surgical Treatment of Metastatic Spine Disease. Cancers, 15(19), 4749. https://doi.org/10.3390/cancers15194749






