A Comparison of 3D Conformal and Deep Inspiratory Breath Holding vs. 4D-CT Intensity-Modulated Radiation Therapy for Patients with Left Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting Study Design
2.2. Subjects
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Primary Outcomes
2.6. Data Collection
2.7. Statistical Analysis of the Data
3. Results
3.1. Baseline Characteristics
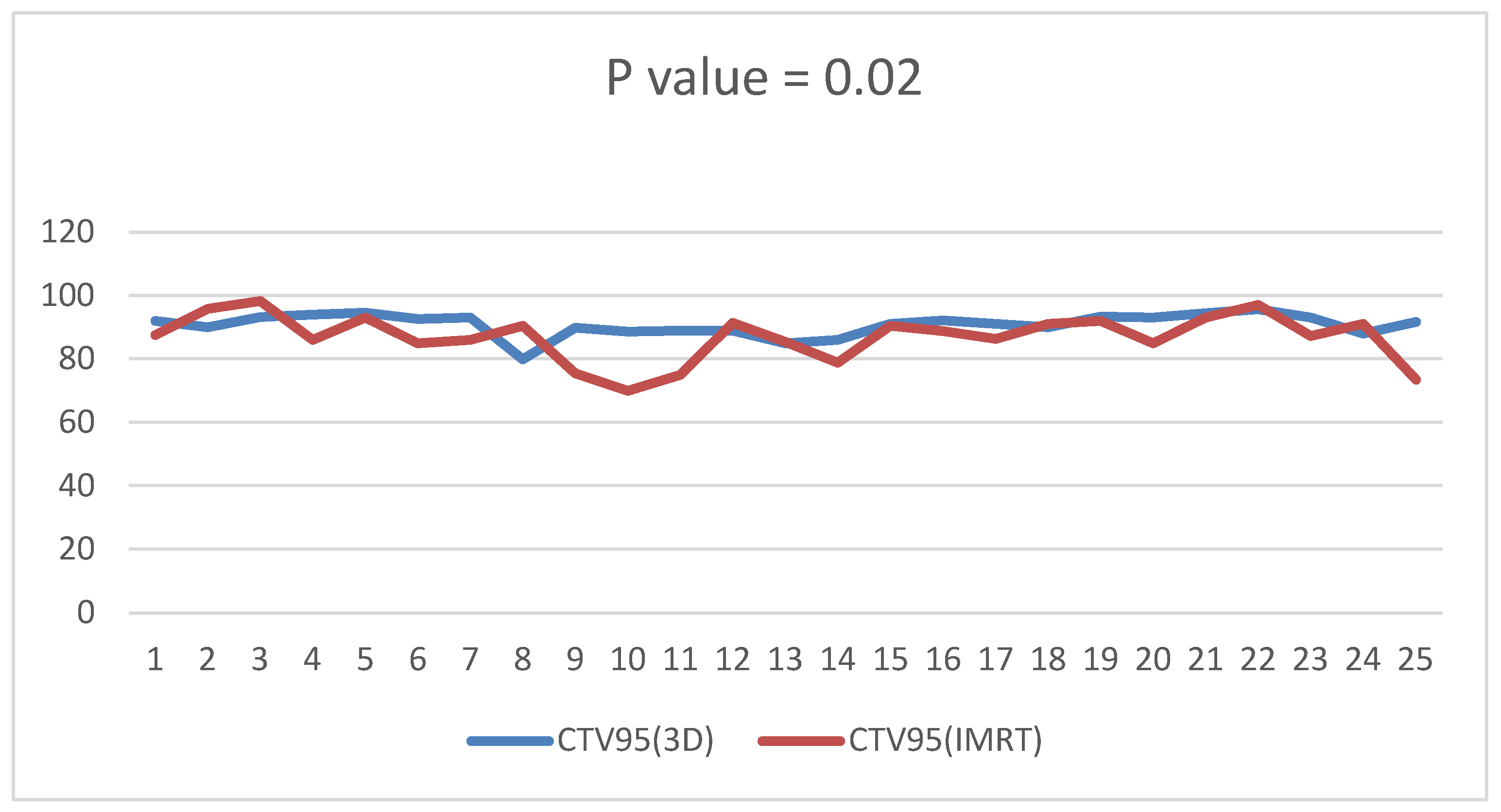
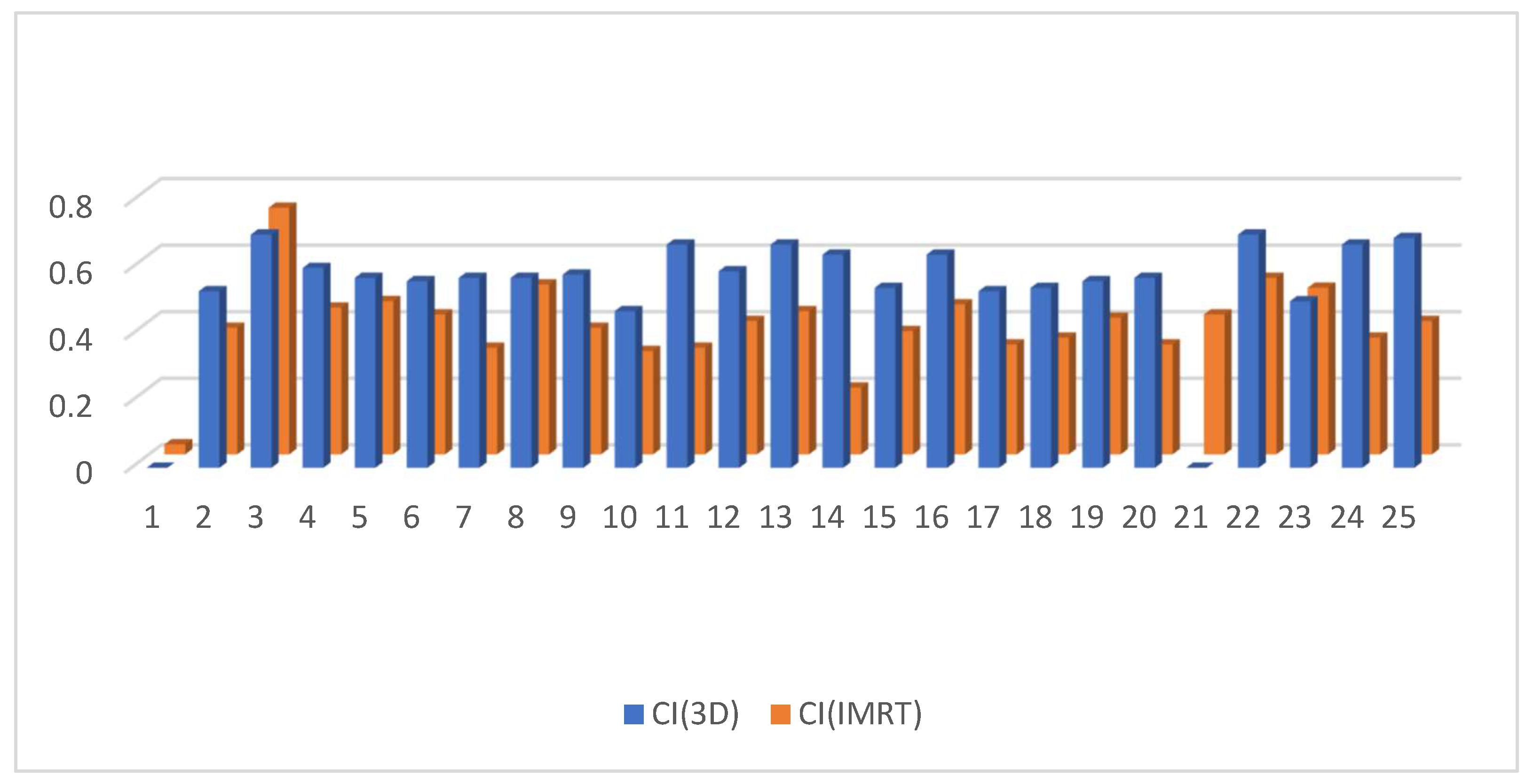
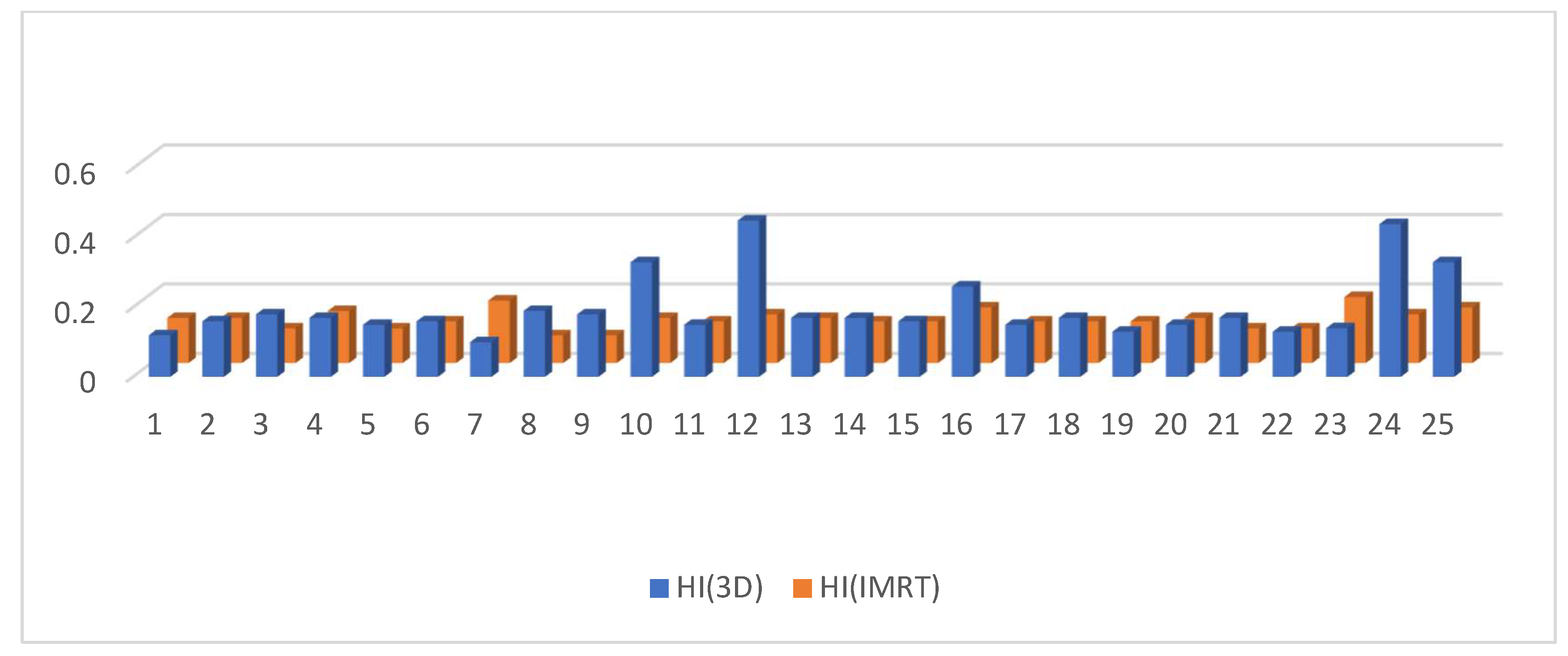
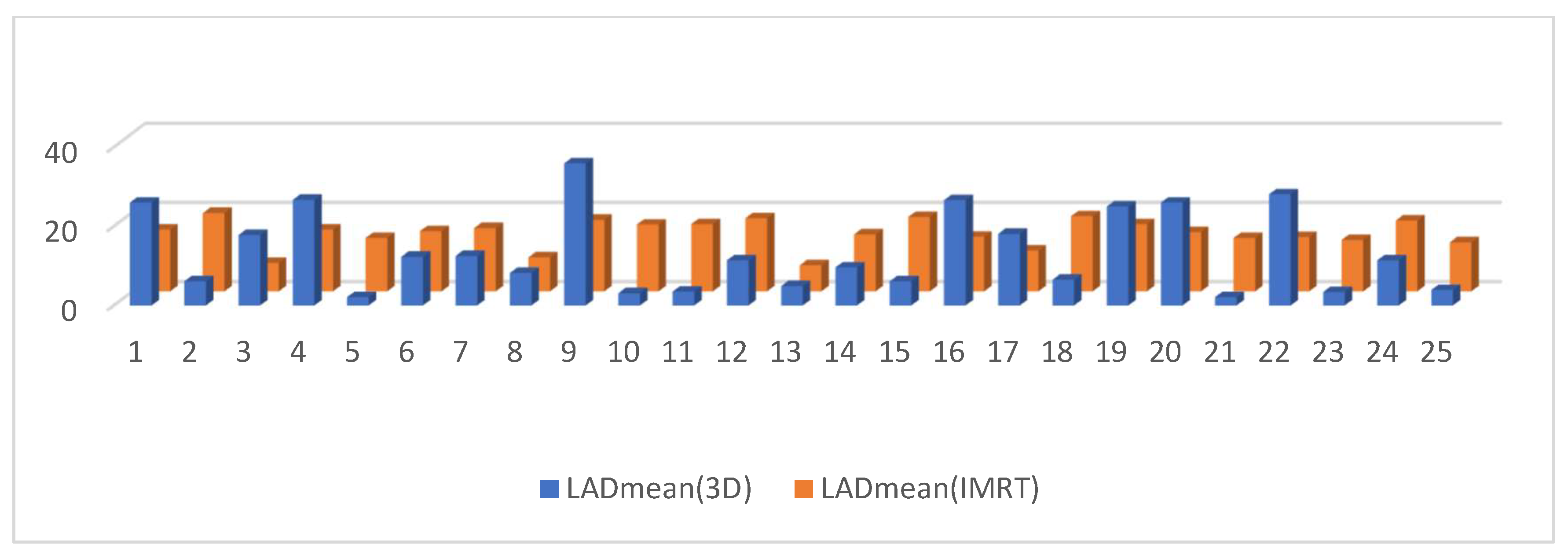
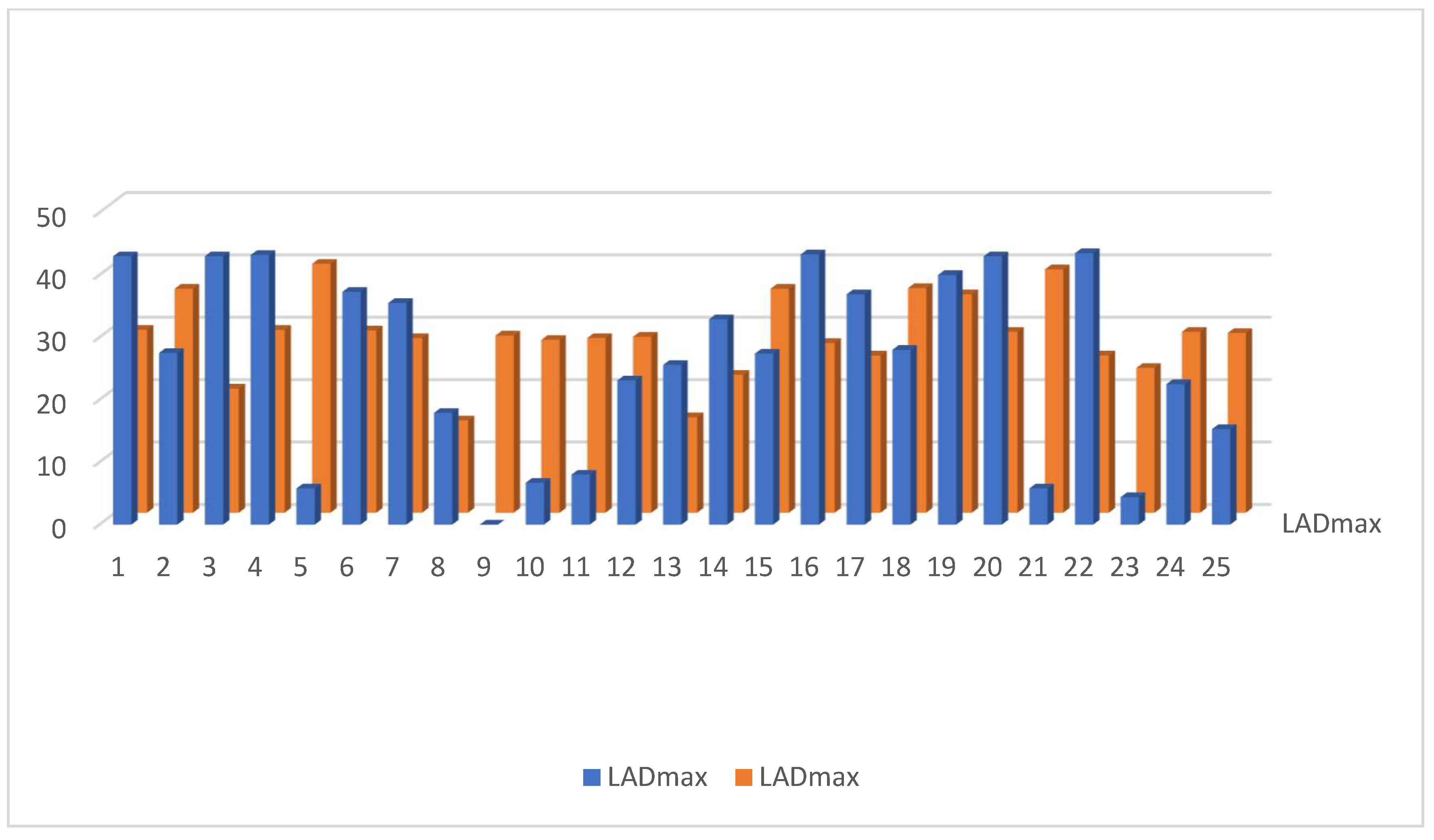
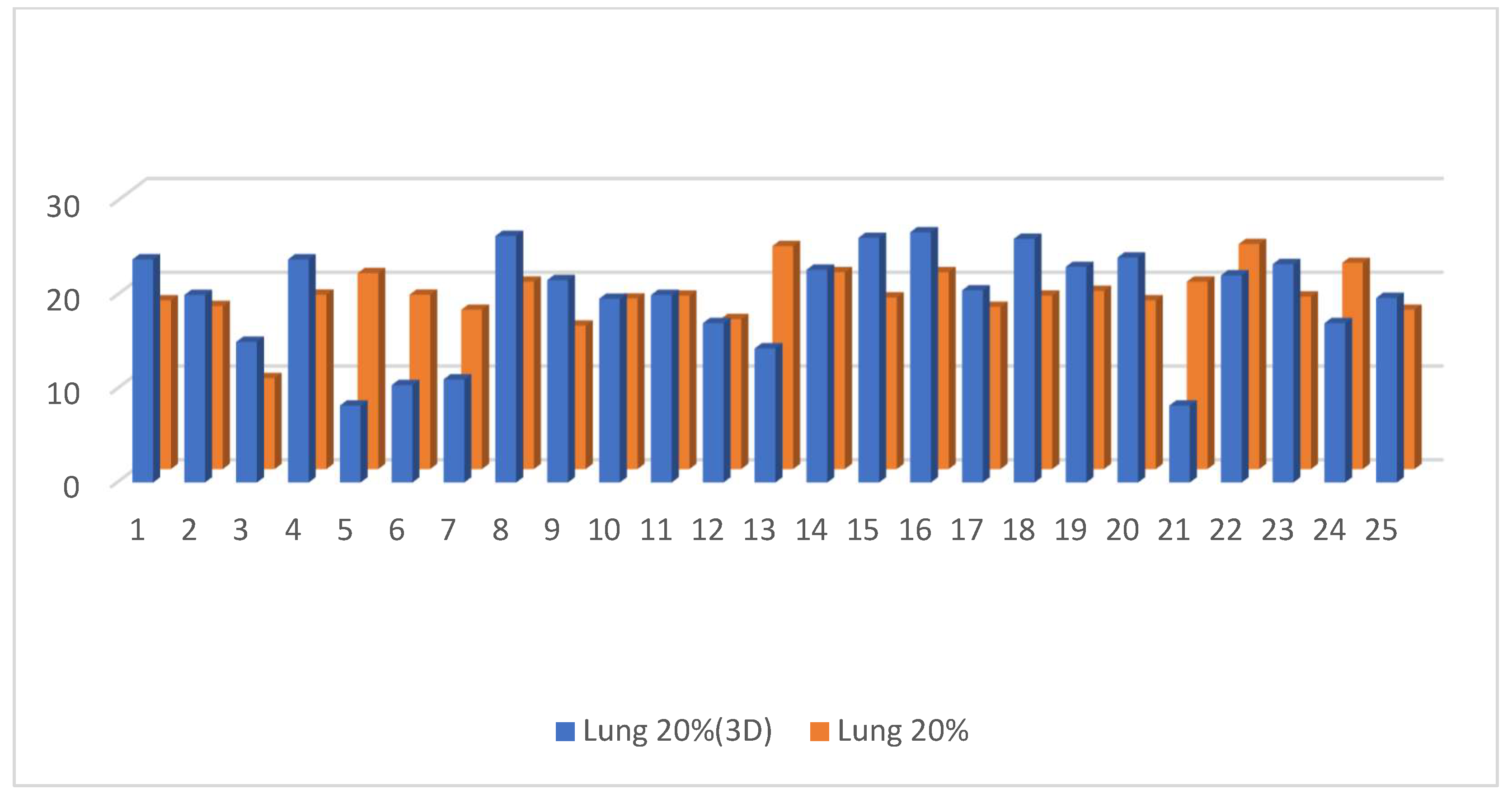
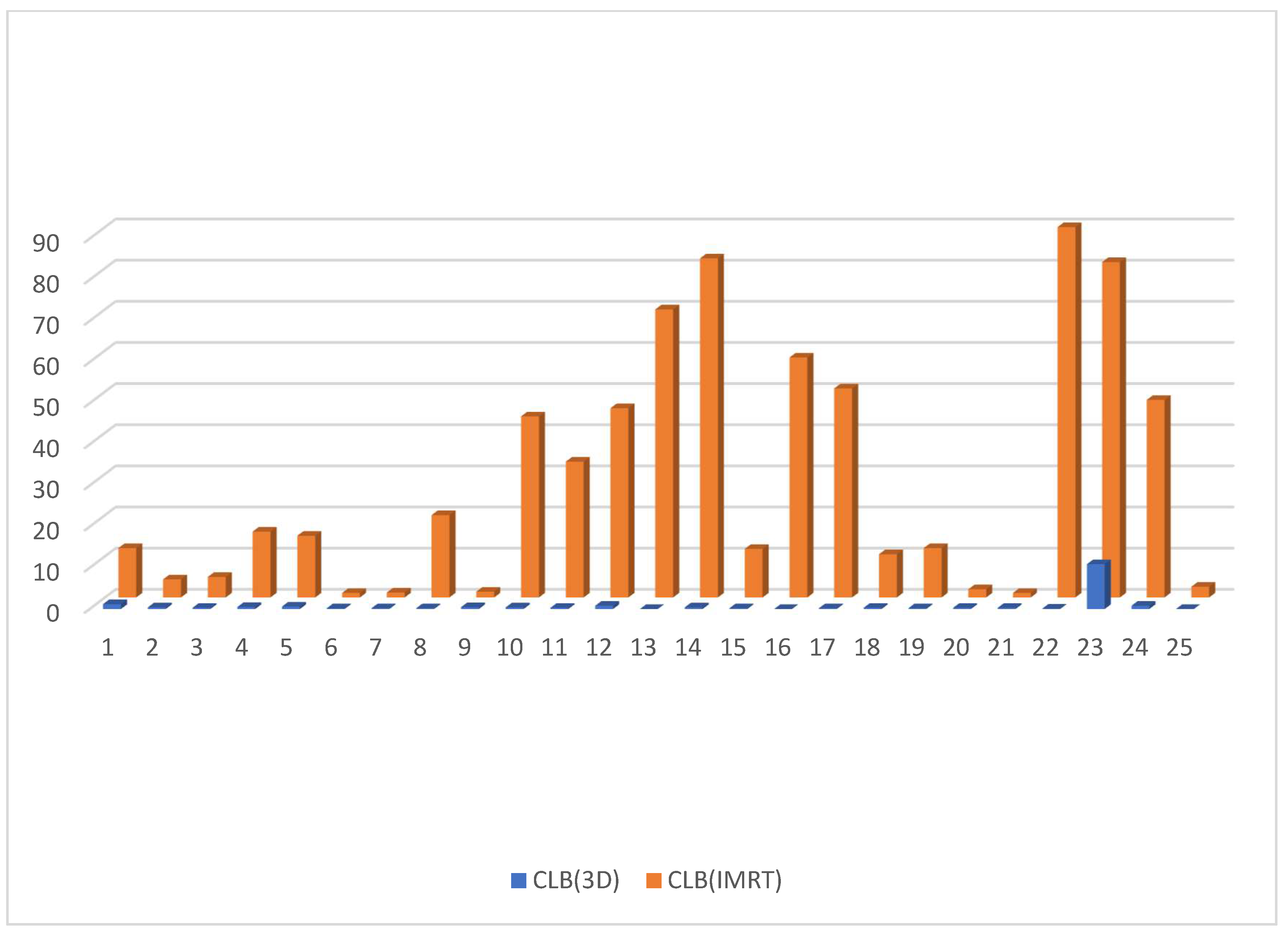
3.2. Dosimetric Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GLOBOCAN 2020: New Global Cancer Data. 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 1 September 2023).
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A.J. Cancer statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Kohler, B.A.; Sherman, R.L.; Howlader, N.; Jemal, A.; Ryerson, A.B.; Henry, K.A.; Boscoe, F.P.; Cronin, K.A.; Lake, A.; Noone, A.-M.; et al. Annual report to the nation on the status of cancer, 1975–2011, featuring incidence of breast cancer subtypes by race/ethnicity, poverty, and state. J. Natl. Cancer Inst. 2015, 107, djv048. [Google Scholar] [CrossRef] [PubMed]
- de Gelder, R.; Heijnsdijk, E.A.; Fracheboud, J.; Draisma, G.; de Koning, H.J. The effects of population-based mammography screening starting between age 40 and 50 in the presence of adjuvant systemic therapy. Int. J. Cancer 2014, 137, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Munoz, D.; Near, A.M.; van Ravesteyn, N.T.; Lee, S.J.; Schechter, C.B.; Alagoz, O.; Berry, D.A.; Burnside, E.S.; Chang, Y.; Chisholm, G.; et al. Effects of screening and systemic adjuvant therapy on ER-specific us breast cancer mortality. JNCI J. Natl. Cancer Inst. 2014, 106, dju289. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.; Hunter, M.; McKinnon, K.; Casey, T.; Turner, M.; Taylor, S.; Paterson, C. A systematic review of multimodal prehabilitation in breast cancer. Breast Cancer Res. Treat. 2023, 197, 1–37. [Google Scholar] [CrossRef]
- Halyard, M.Y.; Brown, L.C.; Mutter, R.W. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int. J. Women’s Health 2015, 7, 449–458. [Google Scholar] [CrossRef]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Darby, S.C.; Ewertz, M.; McGale, P.; Bennet, A.M.; Blom-Goldman, U.; Brønnum, D.; Correa, C.; Cutter, D.; Gagliardi, G.; Gigante, B.; et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N. Engl. J. Med. 2013, 368, 987–998. [Google Scholar] [CrossRef]
- Halperin, E.C.; Brady, L.W.; Perez, C.A.; Wazer, D.E. Perez & Brady’s Principles and Practice of Radiation Oncology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Conway, J.L.; Conroy, L.; Harper, L.; Scheifele, M.; Li, H.; Smith, W.L.; Graham, T.; Phan, T.; Olivotto, I.A. Deep inspiration breath-hold produces a clinically meaningful reduction in ipsilateral lung dose during locoregional radiation therapy for some women with right-sided breast cancer. Pract. Radiat. Oncol. 2017, 7, 147–153. [Google Scholar] [CrossRef]
- Arabaci, A.; Solak, N. Investigation of Thermal Decomposition Behavior of Cerium (III) Acetate Hydrate. Gazi Univ. J. Sci. 2012, 25, 777–782. [Google Scholar]
- Eber, J.; Blondet, C.; Schmitt, M.; Cox, D.G.; Vit, C.; Le Fèvre, C.; Antoni, D.; Hubele, F.; Noel, G. Efficacity of Deep Inspiration Breath Hold and Intensity-Modulated Radiotherapy in Preventing Perfusion Defect for Left Sided Breast Cancer (EDIPE): A Prospective Cohort Study Protocol. Cancers 2023, 15, 2467. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.H.; Berg, M.; Pedersen, A.N.; Andersen, K.; Glavicic, V.; Jakobsen, E.H.; Jensen, I.; Josipovic, M.; Lorenzen, E.L.; Nielsen, H.M.; et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: National guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013, 52, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, M.; Thor, M.; Thornqvist, S.; Sondergaard, J.; Lassen-Ramshad, Y.; Muren, L.P. Advances in radiotherapy: From 2D to 4D. Cancer Imaging 2011, 11, S145–S152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Comsa, D.; Barnett, E.; Le, K.; Mohamoud, G.; Zaremski, D.; Fenkell, L.; Kassam, Z. Introduction of moderate deep inspiration breath hold for radiation therapy of left breast: Initial experience of a regional cancer center. Pract. Radiat. Oncol. 2014, 4, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Cante, D.; Franco, P.; Sciacero, P.; Girelli, G.; Pasquino, M.; Borca, V.C.; Tofani, S.; La Porta, M.R.; Ricardi, U. Hypofractionated whole-breast radiotherapy and concomitant boost after breast conservation in elderly patients. Tumori J. 2016, 102, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Hodapp, N. The ICRU Report 83: Prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). Strahlenther. Onkol. 2012, 188, 97–99. [Google Scholar] [CrossRef]
- Roach, M., III; Nam, J.; Gagliardi, G.; El Naqa, I.; Deasy, J.O.; Marks, L.B. radiation dose–volume effects and the penile bulb. Int. J. Radiat. Oncol. 2010, 76, S130–S134. [Google Scholar] [CrossRef]
- Emami, B. Tolerance of normal tissue to therapeutic radiation. Rep. Radiother. Oncol. 2013, 1, 123–127. [Google Scholar]
- Pembroke, C.; Hudson, E.; Hanna, L. Management of cancer of the body of. Pract. Clin. Oncol. 2015, 360. [Google Scholar]
- Hayden, A.J.; Rains, M.; Tiver, K. Deep inspiration breath hold technique reduces heart dose from radiotherapy for left-sided breast cancer. J. Med. Imaging Radiat. Oncol. 2012, 56, 464–472. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Kanaan, M.H.G.; Abdollah, S.S.; Vakil, M.K.; Marzi, M.; Mazarzaei, A.; Ghasemian, A. Metallic Nanoparticles: Their Potential Role in Breast Cancer Immunotherapy via Trained Immunity Provocation. Biomedicines 2023, 11, 1245. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yang, D.; Zhang, X.; Teng, Y.; Yuan, W.; Zhang, Y.; He, R.; Tang, F.; Pang, J.; Han, B.; et al. Comparison of deep inspiration breath hold versus free breathing in radiotherapy for left sided breast cancer. Front. Oncol. 2022, 12, 845037. [Google Scholar] [CrossRef]
- Beaton, L.; Bergman, A.; Nichol, A.; Aparicio, M.; Wong, G.; Gondara, L.; Speers, C.; Weir, L.; Davis, M.; Tyldesley, S. Cardiac death after breast radiotherapy and the QUANTEC cardiac guidelines. Clin. Transl. Radiat. Oncol. 2019, 19, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lu, Z.; Liu, Z.; Luo, W.; Shao, S.; Tan, L.; Ma, X.; Liu, J.; Drokow, E.K.; Ren, J. Dosimetric comparison between three- and four-dimensional computerised tomography radiotherapy for breast cancer. Oncol. Lett. 2019, 18, 1800–1814. [Google Scholar] [CrossRef] [PubMed]
- Freedman, G.M.; Anderson, P.R.; Goldstein, L.J.; Ma, C.-M.; Li, J.; Swaby, R.F.; Litwin, S.; Watkins-Bruner, D.; Sigurdson, E.R.; Morrow, M. Four-week course of radiation for breast cancer using hypofractionated intensity modulated radiation therapy with an incorporated boost. Int. J. Radiat. Oncol. 2007, 68, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Reardon, K.A.; Read, P.W.; Morris, M.M.; Reardon, M.A.; Geesey, C.; Wijesooriya, K. A comparative analysis of 3D conformal deep inspiratory–breath hold and free-breathing intensity-modulated radiation therapy for left-sided breast cancer. Med. Dosim. 2013, 38, 190–195. [Google Scholar] [CrossRef]
- Petit, C.; Escande, A.; Sarrade, T.; Vaugier, L.; Kirova, Y.; Tallet, A. Radiation therapy in the thoracic region: Radio-induced cardiovascular disease, cardiac delineation and sparing, cardiac dose constraints, and cardiac implantable electronic devices. Cancer/Radiothérapie 2023, 27, 588–598. [Google Scholar] [CrossRef]
- Das Majumdar, S.K.; Amritt, A.; Dhar, S.S.; Barik, S.; Beura, S.S.; Mishra, T.; Muduly, D.K.; Dash, A.; Parida, D.K. A Dosimetric study comparing 3D-CRT vs. IMRT vs. VMAT in left-sided breast cancer patients after mastectomy at a tertiary care centre in eastern india. Cureus 2022, 14, e23568. [Google Scholar] [CrossRef]
- Figlia, V.; Simonetto, C.; Eidemüller, M.; Naccarato, S.; Sicignano, G.; De Simone, A.; Ruggieri, R.; Mazzola, R.; Matuschek, C.; Bölke, E.; et al. Mammary Chain Irradiation in Left-Sided Breast Cancer: Can We Reduce the Risk of Secondary Cancer and Ischaemic Heart Disease with Modern Intensity-Modulated Radiotherapy Techniques? Breast Care 2021, 16, 358–367. [Google Scholar] [CrossRef]





| Parameter | Total, n = 25 (%) |
|---|---|
| Median age (range) | 45 (24–68) |
| Mean weight | 79.6 kg |
| Mean BMI | 31.4 |
| Premenopausal | 10 |
| Postmenopausal | 15 |
| Education level | |
| Illiterate or low literacy | 13 |
| High literacy | 12 |
| T.N.M. Stage | |
| T1 | 8 (32) |
| T2 | 12 (48) |
| T3 | 3 (12) |
| T4 | 2 (8) |
| N0 N1 | 9 (36.7) |
| N2–3 | 8 (26.7) |
| Chemotherapy | |
| Adjuvant | 20 (80) |
| Neoadjuvant | 5 (20) |
| Trastuzumab | 6 (24) |
| Surgery | |
| Breast conservative | 10 (40) |
| Modified radical mastectomy | 15 (60) |
| Radiotherapy | |
| Breast and boost | 10 (40) |
| Breast and chest wall | 3 (12) |
| Postmastectomy chest wall | 15 (60) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldaly, M.; Hussien, A.; El-nadi, I.M.; Laz, N.I.; Said, A.S.A.; Al-Ahmad, M.M.; Hussein, R.R.S.; Rabie, A.S.I.; Shaaban, A.H. A Comparison of 3D Conformal and Deep Inspiratory Breath Holding vs. 4D-CT Intensity-Modulated Radiation Therapy for Patients with Left Breast Cancer. Cancers 2023, 15, 5799. https://doi.org/10.3390/cancers15245799
Aldaly M, Hussien A, El-nadi IM, Laz NI, Said ASA, Al-Ahmad MM, Hussein RRS, Rabie ASI, Shaaban AH. A Comparison of 3D Conformal and Deep Inspiratory Breath Holding vs. 4D-CT Intensity-Modulated Radiation Therapy for Patients with Left Breast Cancer. Cancers. 2023; 15(24):5799. https://doi.org/10.3390/cancers15245799
Chicago/Turabian StyleAldaly, Moustafa, Azza Hussien, Inas Mohsen El-nadi, Nabila Ibrahim Laz, Amira S. A. Said, Mohammad M. Al-Ahmad, Raghda R. S. Hussein, Al Shaimaa Ibrahim Rabie, and Ahmed Hassan Shaaban. 2023. "A Comparison of 3D Conformal and Deep Inspiratory Breath Holding vs. 4D-CT Intensity-Modulated Radiation Therapy for Patients with Left Breast Cancer" Cancers 15, no. 24: 5799. https://doi.org/10.3390/cancers15245799
APA StyleAldaly, M., Hussien, A., El-nadi, I. M., Laz, N. I., Said, A. S. A., Al-Ahmad, M. M., Hussein, R. R. S., Rabie, A. S. I., & Shaaban, A. H. (2023). A Comparison of 3D Conformal and Deep Inspiratory Breath Holding vs. 4D-CT Intensity-Modulated Radiation Therapy for Patients with Left Breast Cancer. Cancers, 15(24), 5799. https://doi.org/10.3390/cancers15245799






