Charlson–Deyo Comorbidity Index as a Novel Predictor for Recurrence in Non-Muscle-Invasive Bladder Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Variables of Interest and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
3.2. Overall Recurrence of the Study Population
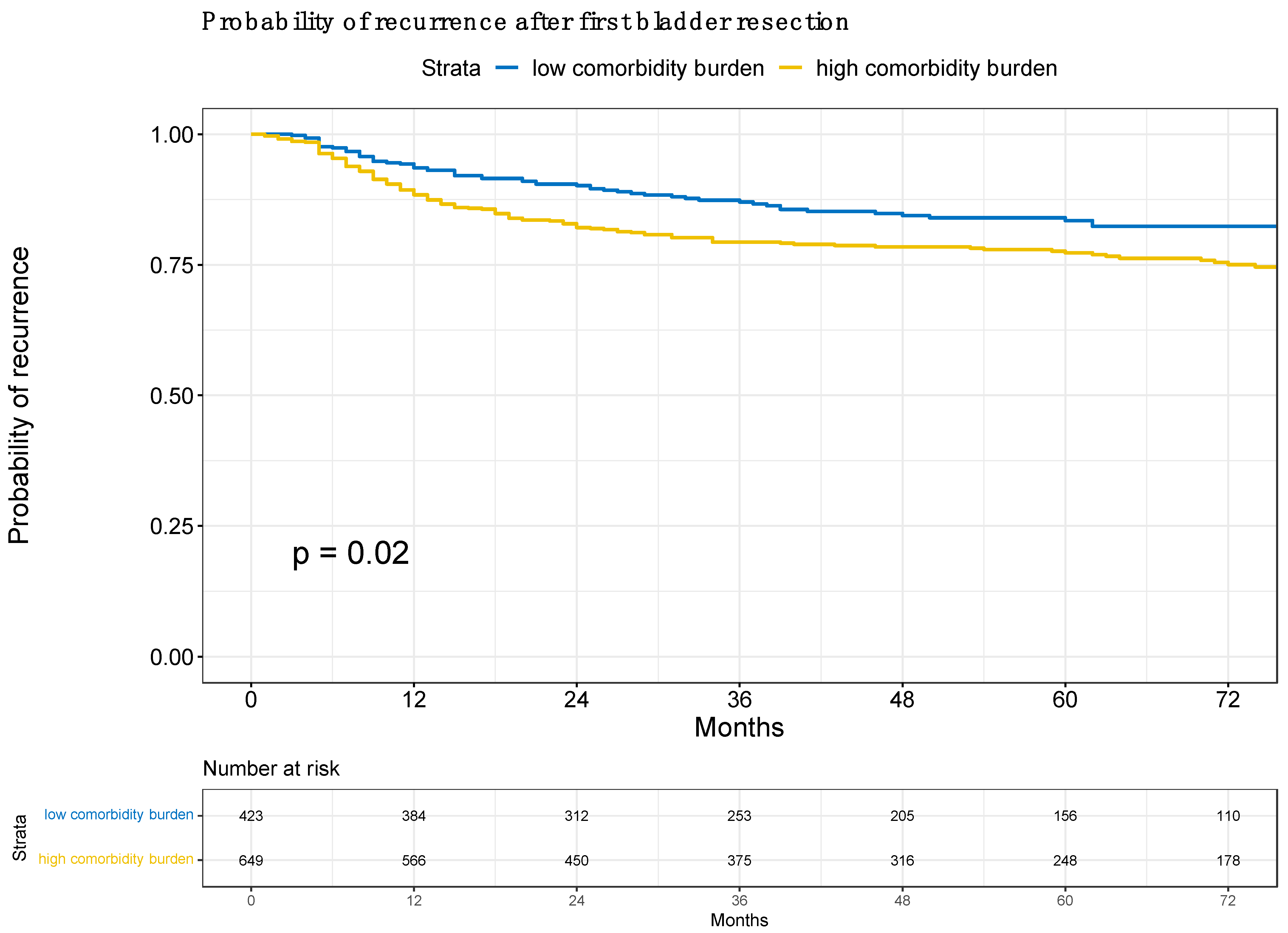
3.3. Risk of Recurrence According to Low vs. High Comorbidity Burden
3.4. Risk of Recurrence According to Charlson–Deyo Comorbidity Index as a Continuous Scale
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and progression of disease in non-muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Stenzl, A.; Hennenlotter, J.; Schilling, D. Can we still afford bladder cancer? Curr. Opin. Urol. 2008, 18, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Sievert, K.D.; Amend, B.; Nagele, U.; Schilling, D.; Bedke, J.; Horstmann, M.; Hennenlotter, J.; Kruck, S.; Stenzl, A. Economic aspects of bladder cancer: What are the benefits and costs? World J. Urol. 2009, 27, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Cambier, S.; Sylvester, R.J.; Collette, L.; Gontero, P.; Brausi, M.A.; van Andel, G.; Kirkels, W.J.; Da Silva, F.C.; Oosterlinck, W.; Prescott, S.; et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1-3 Years of Maintenance Bacillus Calmette-Guérin. Eur. Urol. 2016, 69, 60–69. [Google Scholar] [CrossRef]
- Ferro, M.; Tătaru, O.S.; Musi, G.; Lucarelli, G.; Abu Farhan, A.R.; Cantiello, F.; Damiano, R.; Hurle, R.; Contieri, R.; Busetto, G.M.; et al. Modified Glasgow Prognostic Score as a Predictor of Recurrence in Patients with High Grade Non-Muscle Invasive Bladder Cancer Undergoing Intravesical Bacillus Calmette–Guerin Immunotherapy. Diagnostics 2022, 12, 586. [Google Scholar] [CrossRef]
- Panigrahi, G.; Ambs, S. How comorbidities shape cancer biology and survival. Trends Cancer 2021, 7, 488–495. [Google Scholar] [CrossRef]
- Megwalu, I.I.; Vlahiotis, A.; Radwan, M.; Piccirillo, J.F.; Kibel, A.S. Prognostic Impact of Comorbidity in Patients with Bladder Cancer. Eur. Urol. 2008, 53, 581–589. [Google Scholar] [CrossRef]
- Williams, S.B.; Kamat, A.M.; Chamie, K.; Froehner, M.; Wirth, M.P.; Wiklund, P.N.; Black, P.C.; Steinberg, G.D.; Boorjian, S.A.; Daneshmand, S.; et al. Systematic Review of Comorbidity and Competing-risks Assessments for Bladder Cancer Patients. Eur. Urol. Oncol. 2018, 1, 91–100. [Google Scholar] [CrossRef]
- Barone, B.; Finati, M.; Cinelli, F.; Fanelli, A.; Del Giudice, F.; De Berardinis, E.; Sciarra, A.; Russo, G.; Mancini, V.; D’altilia, N.; et al. Bladder Cancer and Risk Factors: Data from a Multi-Institutional Long-Term Analysis on Cardiovascular Disease and Cancer Incidence. J. Pers. Med. 2023, 13, 512. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Newman, A.A.C.; Afonso, M.S.; van Solingen, C.; Corr, E.M.; Brown, E.J.; Albers, K.B.; Yamaguchi, N.; Narke, D.; Schlegel, M.; et al. Myocardial infarction accelerates breast cancer via innate immune reprogramming. Nat. Med. 2020, 26, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Anwar, S.L.; Cahyono, R.; Prabowo, D.; Avanti, W.S.; Choridah, L.; Dwianingsih, E.K.; Harahap, W.A.; Aryandono, T. Metabolic comorbidities and the association with risks of recurrent metastatic disease in breast cancer survivors. BMC Cancer 2021, 21, 590. [Google Scholar] [CrossRef] [PubMed]
- Charlson Comorbidity Index (CCI)—Strokengine. Available online: https://strokengine.ca/en/assessments/charlson-comorbidity-index-cci/ (accessed on 7 September 2023).
- Deyo, R.A.; Cherkin, D.C.; Ciol, M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 1992, 45, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Gontero, P.; Compérat, E.; Dominguez, J.L.; Liedberg, P.; Mariappan, A.; Masson-Lecomte, A.H.; Mostafid, B.W.G.; van Rhijn, M.; Roupret, T.; Seisen, S.F.; et al. Non-Muscle-invasive Bladder Cancer (TaT1 and CIS). EAU Guidelines. Available online: https://uroweb.org/guidelines/non-muscle-invasive-bladder-cancer (accessed on 1 October 2023).
- Concept: Charlson Comorbidity Index. Available online: http://mchp-appserv.cpe.umanitoba.ca/viewConcept.php?printer=Y&conceptID=1098 (accessed on 9 October 2023).
- van Zutphen, M.; Beeren, I.; Aben, K.K.H.; van der Heijden, A.G.; Witjes, J.A.; Kiemeney, L.A.L.M.; Vrieling, A. Body mass index and waist circumference in relation to risk of recurrence and progression after non-muscle invasive bladder cancer. Cancer Med. 2023, 12, 20459–20469. [Google Scholar] [CrossRef]
- Kwan, M.L.; Haque, R.; Young-Wolff, K.C.; Lee, V.S.; Roh, J.M.; Ergas, I.J.; Wang, Z.; Cannavale, K.L.; Ambrosone, C.B.; Loo, R.K.; et al. Smoking Behaviors and Prognosis in Patients with Non-Muscle-Invasive Bladder Cancer in the Be-Well Study. JAMA Netw. Open 2022, 5, E2244430. [Google Scholar] [CrossRef] [PubMed]
- Bilski, K.; Kozikowski, M.; Skrzypczyk, M.A.; Dobruch, A.; Hendricksen, K.; D’andrea, D.; Czech, A.K.; Dobruch, J. Sex Remains Negative Prognostic Factor in Contemporary Cohort of High-Risk Non-Muscle-Invasive Bladder Cancer. Cancers 2022, 14, 6110. [Google Scholar] [CrossRef]
- Contieri, R.; Grajales, V.; Tan, W.S.; Martini, A.; Sood, A.; Hensley, P.; Bree, K.; Lobo, N.; Nogueras-Gonzalez, G.M.; Guo, C.C.; et al. Impact of age >70 years on oncological outcomes in patients with non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guérin. BJU Int. 2023. [Google Scholar] [CrossRef]
- Ferro, M.; Chiujdea, S.; Musi, G.; Lucarelli, G.; Del Giudice, F.; Hurle, R.; Damiano, R.; Cantiello, F.; Mari, A.; Minervini, A.; et al. Impact of Age on Outcomes of Patients with Pure Carcinoma In Situ of the Bladder: Multi-Institutional Cohort Analysis. Clin. Genitourin. Cancer 2022, 20, e166–e172. [Google Scholar] [CrossRef]
- Kishore, J.; Goel, M.; Khanna, P. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Methodol. 1992, 34, 527–541. [Google Scholar] [CrossRef]
- Vickers, A.J.; Assel, M.J.; Sjoberg, D.D.; Qin, R.; Zhao, Z.; Koyama, T.; Botchway, A.; Wang, X.; Huo, D.; Kattan, M.; et al. Guidelines for Reporting of Figures and Tables for Clinical Research in Urology. BJU Int. 2020, 126, 14–25. [Google Scholar] [CrossRef]
- Assel, M.; Sjoberg, D.; Elders, A.; Wang, X.; Huo, D.; Botchway, A.; Delfino, K.; Fan, Y.; Zhao, Z.; Koyama, T.; et al. Outcomes/Epidemiology/Socioeconomics Guidelines for Reporting of Statistics for Clinical Research in Urology. J. Urol. 2019, 201, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Association of Smoking Status and Recurrence of Non-Muscle Invasive Bladder Cancer among Patients Managed with Blue Light Cystoscopy—ClinicalKey. Available online: https://www.clinicalkey.com/#!/content/playContent/1-s2.0-S107814392100185X?returnurl=https:%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS107814392100185X%3Fshowall%3Dtrue&referrer= (accessed on 12 September 2023).
- Miyake, M.; Nishinihon Uro-oncology Extensive Collaboration group; Matsuyama, H.; Teramukai, S.; Kinoshita, F.; Yokota, I.; Matsumoto, H.; Shimada, K.; Kinjyo, M.; Shimokama, T.; et al. A new risk stratification model for intravesical recurrence, disease progression, and cancer-specific death in patients with non-muscle invasive bladder cancer: The J-NICE risk tables. Int. J. Clin. Oncol. 2020, 25, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Teoh, J.Y.-C.; Kamat, A.M.; Black, P.C.; Grivas, P.; Shariat, S.F.; Babjuk, M. Recurrence mechanisms of non-muscle-invasive bladder cancer—A clinical perspective. Nat. Rev. Urol. 2022, 19, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Kikuchi, E.; Horiguchi, Y.; Tanaka, N.; Miyajima, A.; Nakagawa, K.; Nakashima, J.; Oya, M. Late recurrence and progression in non-muscle-invasive bladder cancers after 5-year tumor-free periods. Urology 2010, 75, 1385–1390. [Google Scholar] [CrossRef]
- Bryan, R.T.; Collins, S.I.; Daykin, M.C.; Zeegers, M.P.; Cheng, K.; Wallace, D.M.A.; Sole, G.M. Mechanisms of recurrence of Ta/T1 bladder cancer. Ind. Mark. Manag. 2010, 92, 519–524. [Google Scholar] [CrossRef]
| Characteristic | N | CCI Low, N = 423 (39%) 1 | CCI High, N = 649 (61%) 1 | p-Value 2 |
|---|---|---|---|---|
| Age | 1072 | 62 (54, 68) | 77 (71, 83) | <0.001 |
| CCI | 1072 | 2.00 (1.00, 3.00) | 6.00 (4.00, 7.00) | <0.001 |
| Female sex | 1072 | 108 (26%) | 148 (23%) | 0.3 |
| BMI | 1022 | 27.1 (24.5, 29.6) | 26.7 (24.2, 29.3) | 0.2 |
| Smoking status | 1072 | <0.001 | ||
| Yes | 166 (39%) | 173 (27%) | ||
| No | 52 (12%) | 70 (11%) | ||
| Unknown | 205 (48%) | 406 (63%) | ||
| Stage | 1072 | <0.001 | ||
| pTa low | 263 (62%) | 303 (47%) | ||
| pTa high | 35 (8.3%) | 59 (9.1%) | ||
| pT1 | 89 (21%) | 206 (32%) | ||
| pTis | 12 (2.8%) | 17 (2.6%) | ||
| Unknown | 24 (5.7%) | 64 (9.9%) | ||
| EAU risk classification | 1072 | <0.001 | ||
| low | 38 (9.0%) | 25 (3.9%) | ||
| intermediate | 212 (50%) | 259 (40%) | ||
| high | 153 (36%) | 331 (51%) | ||
| very high | 18 (4.3%) | 30 (4.6%) | ||
| Unknown | 2 (0.5%) | 4 (0.6%) | ||
| Concomitant CIS | 1072 | 0.8 | ||
| yes | 28 (6.6%) | 45 (6.9%) | ||
| no | 387 (91%) | 595 (92%) | ||
| Unknown | 8 (1.9%) | 9 (1.4%) | ||
| Detrusor muscle in histology | 1072 | 0.9 | ||
| yes | 200 (47%) | 307 (47%) | ||
| no | 144 (34%) | 213 (33%) | ||
| Unknown | 79 (19%) | 129 (20%) | ||
| Postoperative chemotherapy | 1072 | <0.001 | ||
| yes | 246 (58%) | 308 (47%) | ||
| No | 159 (38%) | 320 (49%) | ||
| Unknown | 18 (4.3%) | 21 (3.2%) | ||
| Adjuvant BCG | 1072 | 81 (19%) | 125 (19%) | >0.9 |
| Univariable | Multivariable 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR 1 | 95% CI 1 | p-Value | OR 1 | 95% CI 1 | p-Value |
| Comorbidity burden | ||||||
| low | — | — | — | — | ||
| high | 1.40 | 1.05, 1.87 | 0.02 | 1.42 | 1.06, 1.92 | 0.018 |
| Univariable | Multivariable 2 | |||||
|---|---|---|---|---|---|---|
| Characteristic | OR 1 | 95% CI 1 | p-Value | OR 1 | 95% CI 1 | p-Value |
| CCI | 1.05 | 1.00, 1.10 | 0.03 | 1.05 | 1.00, 1.10 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheipner, L.; Zurl, H.; Altziebler, J.V.; Pichler, G.P.; Schöpfer-Schwab, S.; Jasarevic, S.; Gaisl, M.; Pohl, K.C.; Pemberger, K.; Andlar, S.; et al. Charlson–Deyo Comorbidity Index as a Novel Predictor for Recurrence in Non-Muscle-Invasive Bladder Cancer. Cancers 2023, 15, 5770. https://doi.org/10.3390/cancers15245770
Scheipner L, Zurl H, Altziebler JV, Pichler GP, Schöpfer-Schwab S, Jasarevic S, Gaisl M, Pohl KC, Pemberger K, Andlar S, et al. Charlson–Deyo Comorbidity Index as a Novel Predictor for Recurrence in Non-Muscle-Invasive Bladder Cancer. Cancers. 2023; 15(24):5770. https://doi.org/10.3390/cancers15245770
Chicago/Turabian StyleScheipner, Lukas, Hanna Zurl, Julia V. Altziebler, Georg P. Pichler, Stephanie Schöpfer-Schwab, Samra Jasarevic, Michael Gaisl, Klara C. Pohl, Karl Pemberger, Stefan Andlar, and et al. 2023. "Charlson–Deyo Comorbidity Index as a Novel Predictor for Recurrence in Non-Muscle-Invasive Bladder Cancer" Cancers 15, no. 24: 5770. https://doi.org/10.3390/cancers15245770
APA StyleScheipner, L., Zurl, H., Altziebler, J. V., Pichler, G. P., Schöpfer-Schwab, S., Jasarevic, S., Gaisl, M., Pohl, K. C., Pemberger, K., Andlar, S., Hutterer, G. C., Bele, U., Leitsmann, C., Leitsmann, M., Augustin, H., Zigeuner, R., Ahyai, S., & Mischinger, J. (2023). Charlson–Deyo Comorbidity Index as a Novel Predictor for Recurrence in Non-Muscle-Invasive Bladder Cancer. Cancers, 15(24), 5770. https://doi.org/10.3390/cancers15245770







