Self-Reported Baseline Quality of Life Mirrors Treatment-Specific Characteristics of Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Patients and Primary Objective
2.2. Ethical Approval and Consent to Participate
2.3. Data Collection
2.4. Sample Size Determination
2.5. Statistical Analyses
3. Results
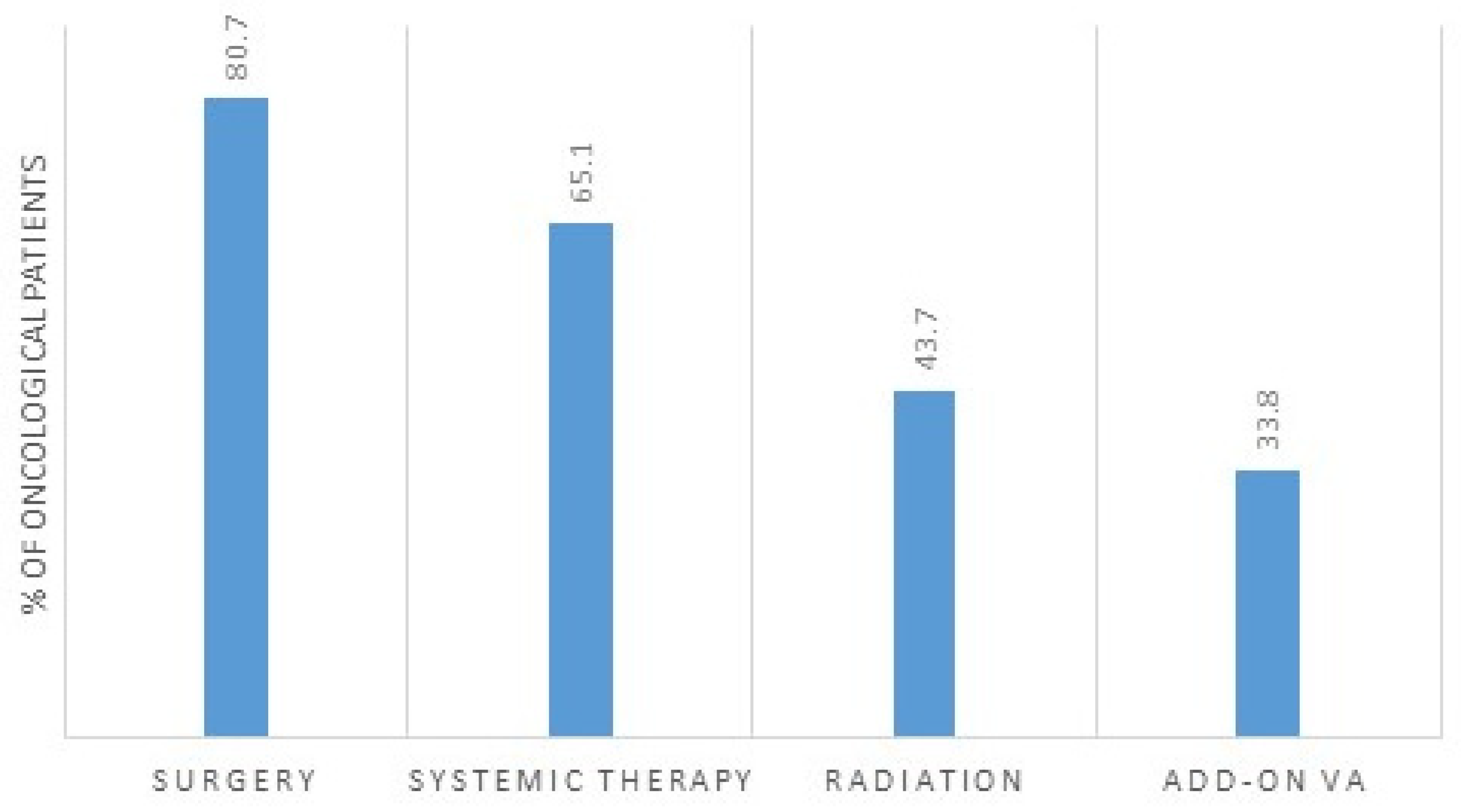
3.1. Oncological Treatment
3.2. Association between bQL and Standard-Oncological Treatment
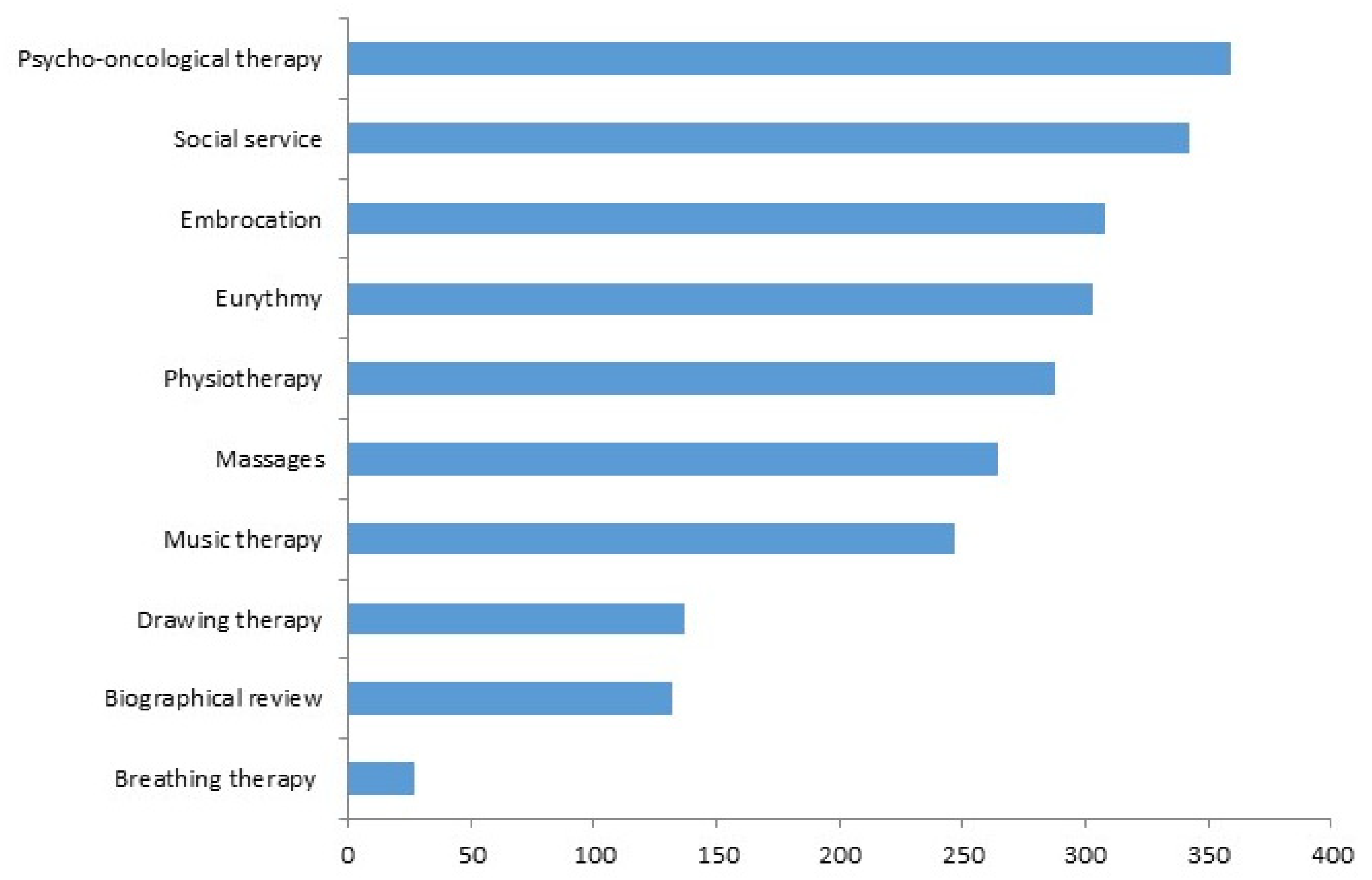
3.3. Association between bQL and Add-On Complementary Therapies
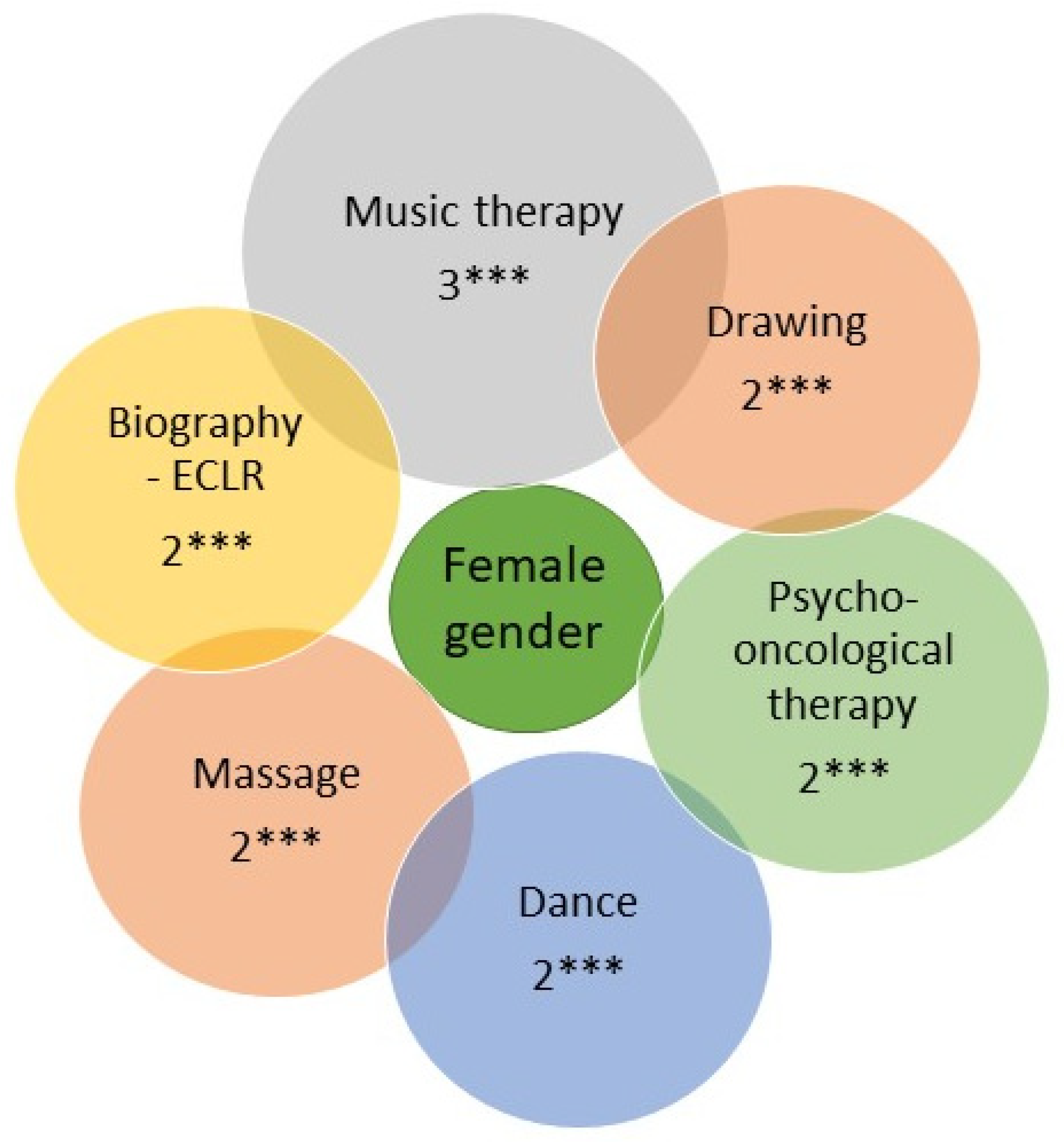
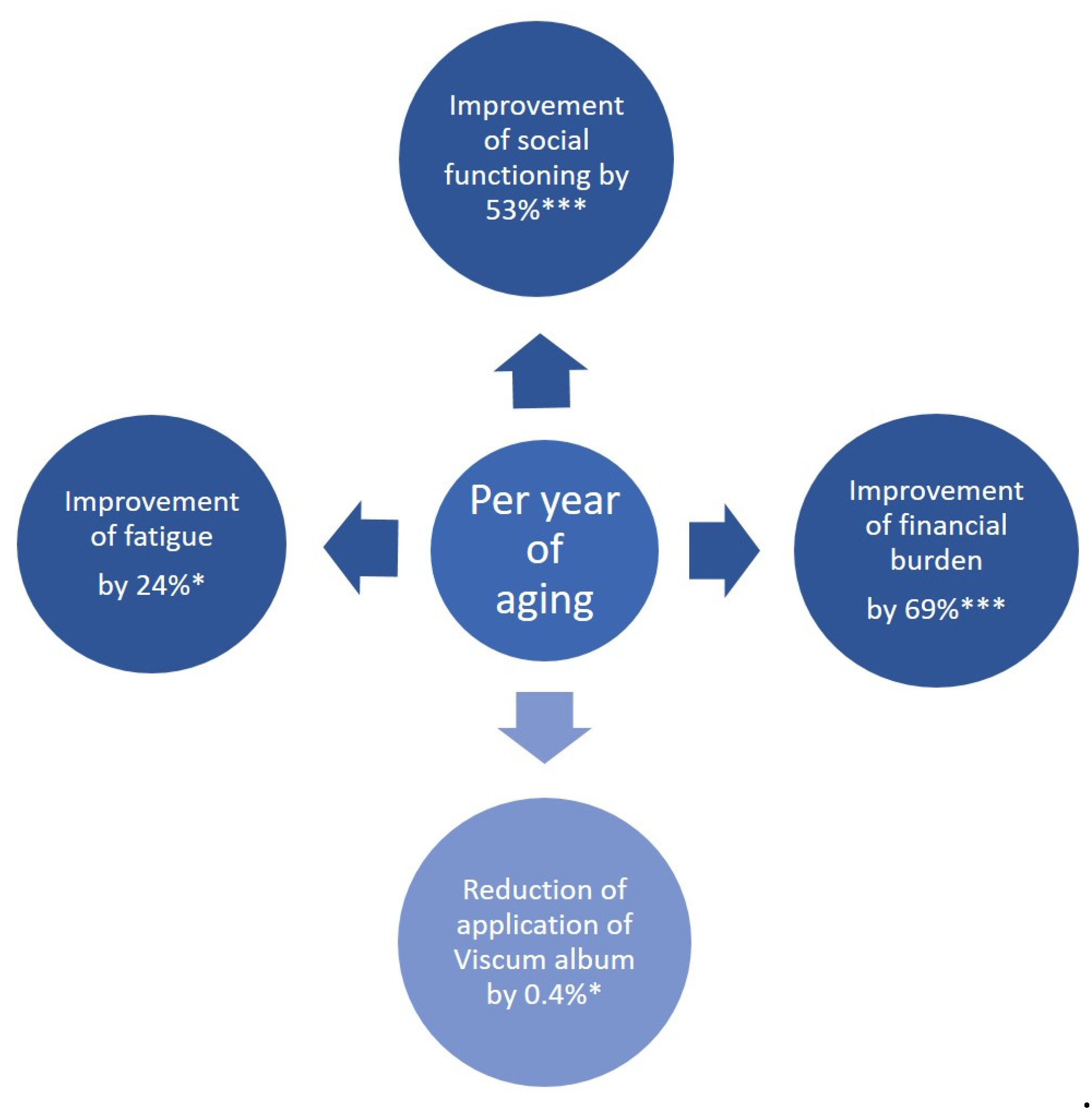
3.4. Gender- and Age-Mediated Association Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DKG, Deutsche Krebshilfe, AWMF. Erweiterte S3-Leitlinie Palliativmedizin für Patienten mit einer nicht-heilbaren Krebserkrankung Langversion 2.2—September 2020 AWMF-Registernummer: 128/001OL. Available online: https://register.awmf.org/de/leitlinien/detail/128-001OL (accessed on 7 December 2023).
- DKG, Deutsche Krebshilfe, AWMF. S3-Leitlinie Supportive Therapie bei Onkologischen Patient Innen Langversion 1.3—Februar 2020 AWMF-Registernummer: 032/054OL. Available online: https://register.awmf.org/assets/guidelines/032-054OLl_S3_Supportiv_2020-07-abgelaufen.pdf (accessed on 7 December 2023).
- Chen, M.N.; Ho, K.Y.; Hung, Y.N.; Su, C.C.; Kuan, C.H.; Tai, H.C.; Cheng, N.C.; Lin, C.C. Pre-treatment quality of life as a predictor of distant metastasis-free survival and overall survival in patients with head and neck cancer who underwent free flap reconstruction. Eur. J. Oncol. Nurs. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- van Nieuwenhuizen, A.J.; Buffart, L.M.; Brug, J.; Leemans, C.R.; Verdonck-de Leeuw, I.M. The association between health related quality of life and survival in patients with head and neck cancer: A systematic review. Oral Oncol. 2015, 51, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, A. Quality of life data as prognostic indicators of survival in cancer patients: An overview of the literature from 1982 to 2008. Health Qual. Life Outcomes 2009, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mo, F.K.; Chan, S.L.; Hui, E.P.; Tang, N.S.; Koh, J.; Leung, L.K.; Poon, A.N.; Hui, J.; Chu, C.M.; et al. Prognostic values of EORTC QLQ-C30 and QLQ-HCC18 index-scores in patients with hepatocellular carcinoma—Clinical application of health-related quality-of-life data. BMC Cancer 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Husson, O.; de Rooij, B.H.; Kieffer, J.; Oerlemans, S.; Mols, F.; Aaronson, N.K.; van der Graaf, W.T.A.; van de Poll-Franse, L.V. The EORTC QLQ-C30 Summary Score as Prognostic Factor for Survival of Patients with Cancer in the “Real-World”: Results from the Population-Based PROFILES Registry. Oncologist 2020, 25, e722–e732. [Google Scholar] [CrossRef]
- Quinten, C.; Coens, C.; Mauer, M.; Comte, S.; Sprangers, M.A.; Cleeland, C.; Osoba, D.; Bjordal, K.; Bottomley, A.; EORTC Clinical Groups. Baseline quality of life as a prognostic indicator of survival: A meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009, 10, 865–871. [Google Scholar] [CrossRef]
- Saxton, A.; Velanovich, V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann. Surg. 2011, 253, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Mierzynska, M.; Piccinin, C.; Pe, M.; Martinelli, F.; Gotay, C.; Coens, C.; Mauer, M.; Eggermont, A.; Groenvold, M.; Bjordal, K.; et al. Prognostic value of patient-reported outcomes from international randomised clinical trials on cancer: A systematic review. Lancet Oncol. 2019, 20, e683–e696. [Google Scholar] [CrossRef]
- Asplund, D.; Bisgaard, T.; Bock, D.; Burcharth, J.; González, E.; Haglind, E.; Kolev, Y.; Matthiessen, P.; Rosander, C.; Rosenberg, J.; et al. Pretreatment quality of life in patients with rectal cancer is associated with intrusive thoughts and sense of coherence. Int. J. Color. Dis. 2017, 32, 1639–1647. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Nat. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Schoenfeld, D.A. Sample-size formula for the proportional-hazards regression model. Biometrics 1983, 39, 499–503. [Google Scholar] [CrossRef]
- David, E.A.; Andersen, S.W.; Beckett, L.A.; Melnikow, J.; Clark, J.M.; Brown, L.M.; Cooke, D.T.; Kelly, K.; Canter, R.J. Survival benefits associated with surgery for advanced non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 157, 1620–1628. [Google Scholar] [CrossRef]
- Hu, H.M.; Tsai, H.J.; Ku, H.Y.; Lo, S.S.; Shan, Y.S.; Chang, H.C.; Chao, Y.; Chen, J.S.; Chen, S.C.; Chiang, C.J.; et al. Survival outcomes of management in metastatic gastric adenocarcinoma patients. Sci. Rep. 2021, 11, 23142. [Google Scholar] [CrossRef] [PubMed]
- S3-Leitlinie. S3-Leitlinie Prävention, Diagnostik, Therapie und Nachsorge des Lungenkarzinoms. Leitlinienprogramm Onkologie. p. 658. 2022. (Version 2.1—Dezember 2022). Available online: https://register.awmf.org/de/leitlinien/detail/020-007OL (accessed on 7 December 2023).
- Makhlouf, S.M.; Pini, S.; Ahmed, S.; Bennett, M.I. Managing Pain in People with Cancer-a Systematic Review of the Attitudes and Knowledge of Professionals, Patients, Caregivers and Public. J. Cancer Educ. 2020, 35, 214–240. [Google Scholar] [CrossRef] [PubMed]
- Verhaar, S.; Vissers, P.A.; Maas, H.; van de Poll-Franse, L.V.; van Erning, F.N.; Mols, F. Treatment-related differences in health related quality of life and disease specific symptoms among colon cancer survivors: Results from the population-based PROFILES registry. Eur. J. Cancer 2015, 51, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Van Lancker, A.; Velghe, A.; Van Hecke, A.; Verbrugghe, M.; Van Den Noortgate, N.; Grypdonck, M.; Verhaeghe, S.; Bekkering, G.; Beeckman, D. Prevalence of symptoms in older cancer patients receiving palliative care: A systematic review and meta-analysis. J. Pain Symptom. Manag. 2014, 47, 90–104. [Google Scholar] [CrossRef]
- Bossi, P.; Antonuzzo, A.; Cherny, N.I.; Rosengarten, O.; Pernot, S.; Trippa, F.; Schuler, U.; Snegovoy, A.; Jordan, K.; Ripamonti, C.I.; et al. Diarrhoea in adult cancer patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv126–iv142. [Google Scholar] [CrossRef]
- Smith, G.L.; Lopez-Olivo, M.A.; Advani, P.G.; Ning, M.S.; Geng, Y.; Giordano, S.H.; Volk, R.J. Financial Burdens of Cancer Treatment: A Systematic Review of Risk Factors and Outcomes. J. Nat. Comp. Can. Net. 2019, 17, 1184–1192. [Google Scholar] [CrossRef]
- Dodson, R.M.; McQuellon, R.P.; Mogal, H.D.; Duckworth, K.E.; Russell, G.B.; Votanopoulos, K.I.; Shen, P.; Levine, E.A. Quality-of-life evaluation after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Ann. Surg. Oncol. 2016, 23, 772–783. [Google Scholar] [CrossRef]
- Thronicke, A.; von Trott, P.; Kröz, M.; Grah, C.; Matthes, B.; Schad, F. Health-Related Quality of Life in Patients with Lung Cancer Applying Integrative Oncology Concepts in a Certified Cancer Centre. Evid. Based Complement. Altern. Med. 2020, 2020, 5917382. [Google Scholar] [CrossRef]
- Dilworth, S.; Higgins, I.; Parker, V.; Kelly, B.; Turner, J. Patient and health professional’s perceived barriers to the delivery of psychosocial care to adults with cancer: A systematic review. Psychooncology 2014, 23, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Ball, H.; Moore, S.; Leary, A. A systematic literature review comparing the psychological care needs of patients with mesothelioma and advanced lung cancer. Eur. J. Oncol. Nurs. 2016, 25, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Braamse, A.; Schuurhuizen, C.; Beekman, A.T.; van Linde, M.; Sprangers, M.A.; Verheul, H.M. Distress in patients with cancer—On the need to distinguish between adaptive and maladaptive emotional responses. Acta Oncol. 2017, 56, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Zabora, J.; Brintzenhofeszoc, K.; Curbow, B.; Hooker, C.; Piantadosi, S. The prevalence of psychological distress by cancer site. Psycho. Oncol. 2001, 10, 19–28. [Google Scholar] [CrossRef]
- Greenlee, H.; Shi, Z. Implementing Integrative Oncology: Hopes and Challenges. J. Oncol. Pract. 2019, 15, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Lyman, G.H.; Greenlee, H.; Bohlke, K.; Bao, T.; DeMichele, A.M.; Deng, G.E.; Fouladbakhsh, J.M.; Gil, B.; Hershman, D.L.; Mansfield, S.; et al. Integrative Therapies during and after Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 2647–2655. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.M.; Balneaves, L.G.; Cardoso, M.J.; Cohen, L.; Greenlee, H.; Johnstone, P.; Kücük, Ö.; Mailman, J.; Mao, J.J. A Comprehensive Definition for Integrative Oncology. J. Nat. Cancer Inst. Monogr. 2017, 2017, lgx012. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.J.; Hunter, J.; Seely, D.; Balneaves, L.G.; Rossi, E.; Bao, T. Integrative Oncology: International Perspectives. Integr Cancer Ther. 2019, 18, 1534735418823266. [Google Scholar] [CrossRef]
- Stussman, B.J.; Nahin, R.R.; Barnes, P.M.; Ward, B.W. U.S. Physician Recommendations to Their Patients About the Use of Complementary Health Approaches. J. Altern. Complement. Med. 2019, 26, 25–33. [Google Scholar] [CrossRef]
- Warren, Y.; Hecksher, A.; Schepel, C.; Trufan, S.; Greiner, R.; Yaguda, S.; Bailey-Dorton, C.; Fisher, J.; White, R.; Hadzikadic-Gusic, L. Integrative Oncology in Young Women with Breast Cancer. Oncology 2022, 36, 658–663. [Google Scholar] [CrossRef]
| All Patients (n = 538) | |
|---|---|
| Age in years, median (IQR) | 65 (54.0−72.0) |
| Gender, female, n (%) | 382 (71.0) |
| Gender, male, n (%) | 156 (29.0) |
| UICC 0, n (%) | 14 (2.6) |
| UICC I, n (%) | 128 (23.8) |
| UICC II, n (%) | 148 (27.5) |
| UICC III, n (%) | 88 (16.4) |
| UICC IV, n (%) | 70 (13.0) |
| Breast cancer | 233(43.3) |
| Lung cancer | 92 (17.1) |
| Colon cancer | 82 (15.2) |
| Rectum cancer | 49 (9.1) |
| Other cancer | 82 (15.2) |
| SURG | RAD | SYS | VA | NPI | PHY | DAN | EMB | DRA | MAS | ECLR | PSY | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Global health | -- | -- | -- | -- | 1.03 * | -- | -- | -- | -- | -- | -- | -- |
| Appetite loss | -- | -- | -- | -- | 1.01 * | -- | -- | -- | -- | -- | -- | -- |
| Insomnia | -- | -- | -- | -- | 0.99 * | -- | -- | -- | -- | -- | -- | -- |
| Fatigue | -- | -- | -- | -- | -- | -- | 1.01 * | -- | -- | 1.02 * | 1.03 *** | 1.01 * |
| Social functioning | -- | -- | -- | 1.24 * | 1.35 * | 1.4 *** | 1.3 * | 1.39 ** | 1.4 ** | 1.24 * | 1.46 *** | -- |
| Phys. functioning | 1.03 ** | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Pain | -- | 1.01 * | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Financial burden | 0.99 * | 0.99 * | -- | -- | -- | -- | -- | 1.01 * | -- | -- | -- | |
| Diarrhea | 0.99 * | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | |
| Dyspnea | -- | 1.01 * | -- | -- | -- | -- | -- | -- | 0.99 * | -- | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thronicke, A.; Oei, S.L.; Grieb, G.; Grabowski, P.; Roos, J.; Schad, F. Self-Reported Baseline Quality of Life Mirrors Treatment-Specific Characteristics of Cancer Patients. Cancers 2023, 15, 5763. https://doi.org/10.3390/cancers15245763
Thronicke A, Oei SL, Grieb G, Grabowski P, Roos J, Schad F. Self-Reported Baseline Quality of Life Mirrors Treatment-Specific Characteristics of Cancer Patients. Cancers. 2023; 15(24):5763. https://doi.org/10.3390/cancers15245763
Chicago/Turabian StyleThronicke, Anja, Shiao Li Oei, Gerrit Grieb, Patricia Grabowski, Juliane Roos, and Friedemann Schad. 2023. "Self-Reported Baseline Quality of Life Mirrors Treatment-Specific Characteristics of Cancer Patients" Cancers 15, no. 24: 5763. https://doi.org/10.3390/cancers15245763
APA StyleThronicke, A., Oei, S. L., Grieb, G., Grabowski, P., Roos, J., & Schad, F. (2023). Self-Reported Baseline Quality of Life Mirrors Treatment-Specific Characteristics of Cancer Patients. Cancers, 15(24), 5763. https://doi.org/10.3390/cancers15245763








