Dosimetric Comparison of Conventional Radiotherapy, Volumetric Modulated Arc Therapy, and Proton Beam Therapy for Palliation of Thoracic Spine Metastases Secondary to Breast or Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient and Treatment Characteristics
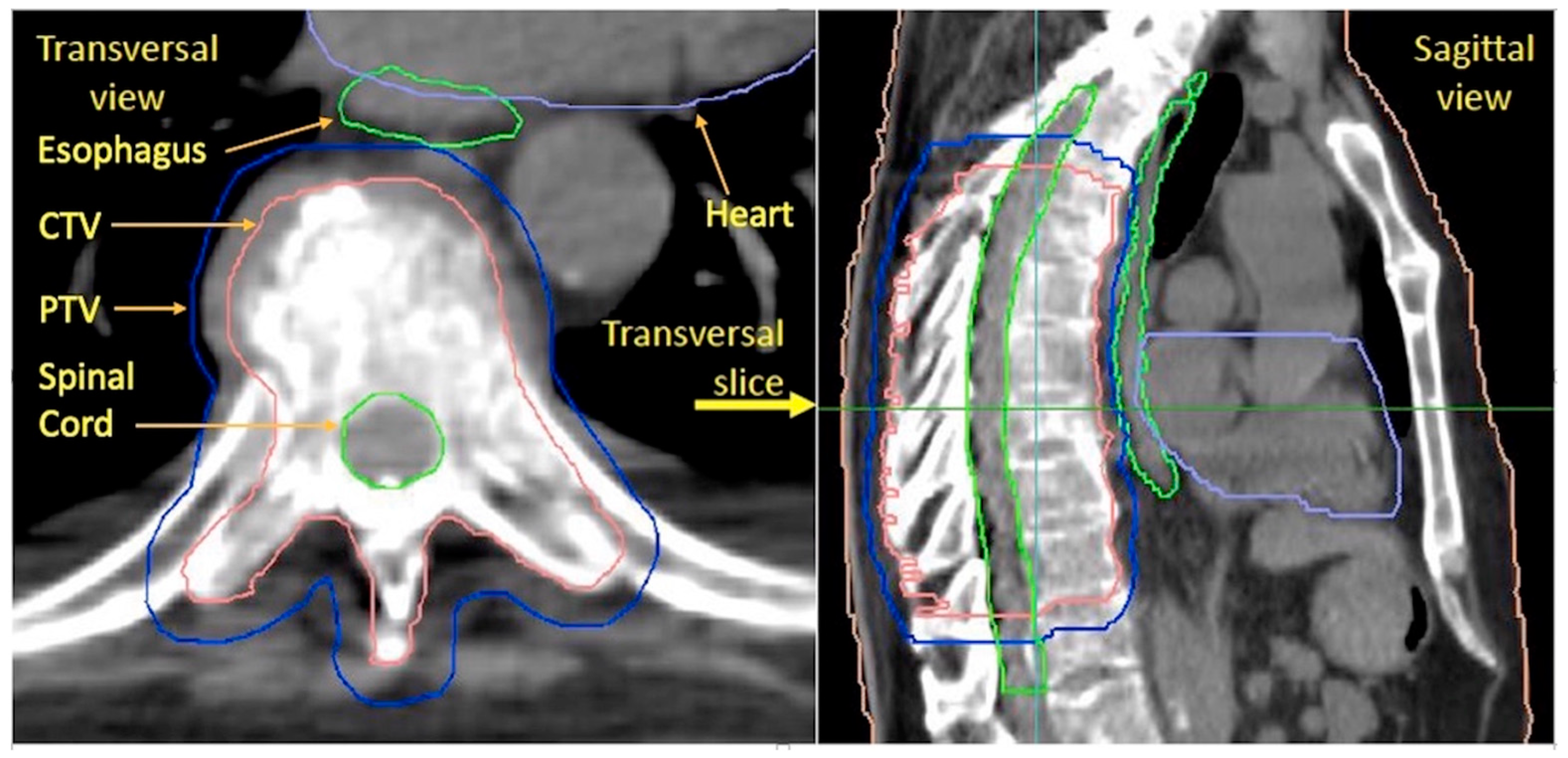
2.2. Treatment, Setup, and Structure Delineation
2.3. Treatment Planning
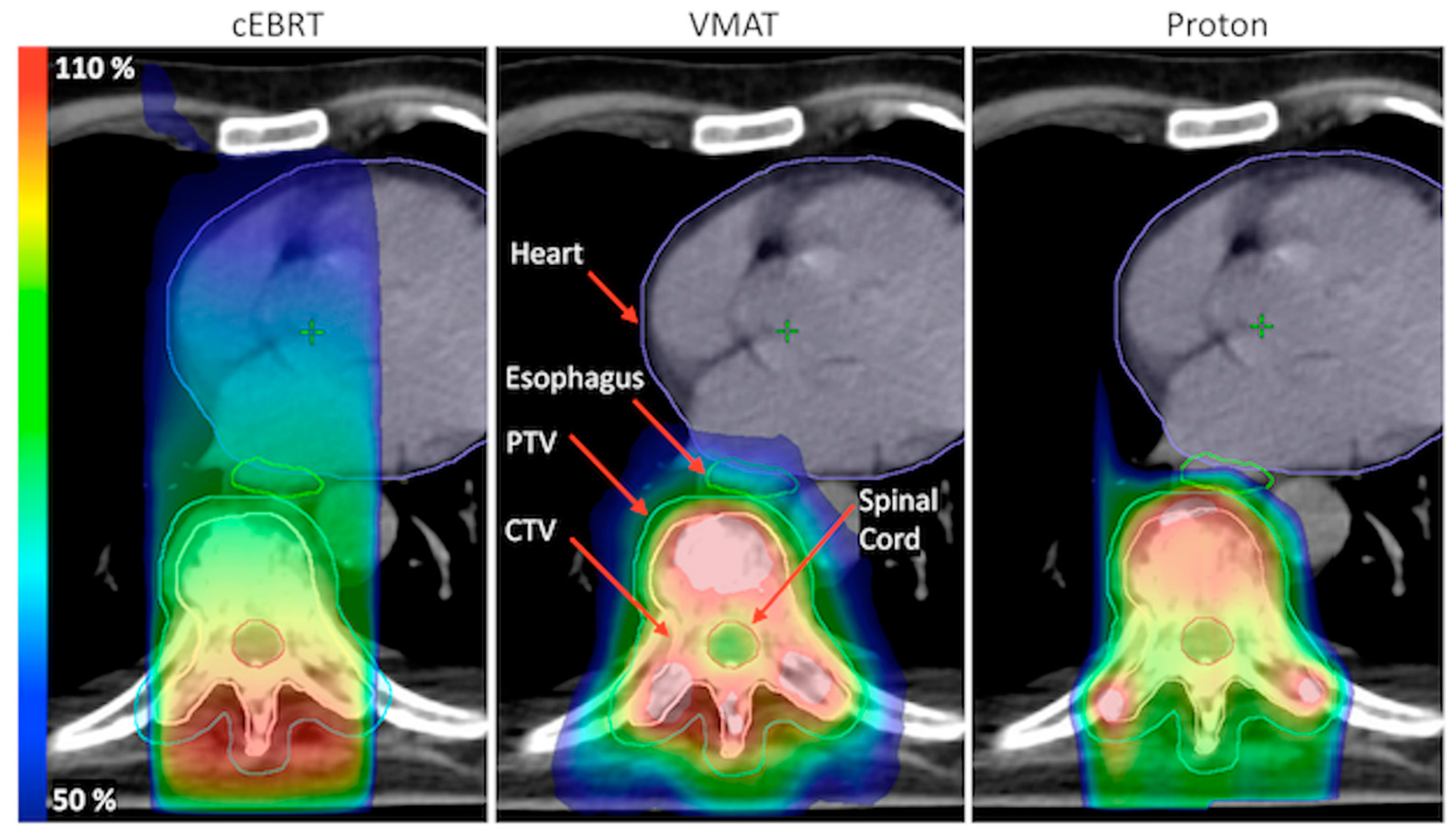
2.4. Plan Evaluation
2.5. Statistics
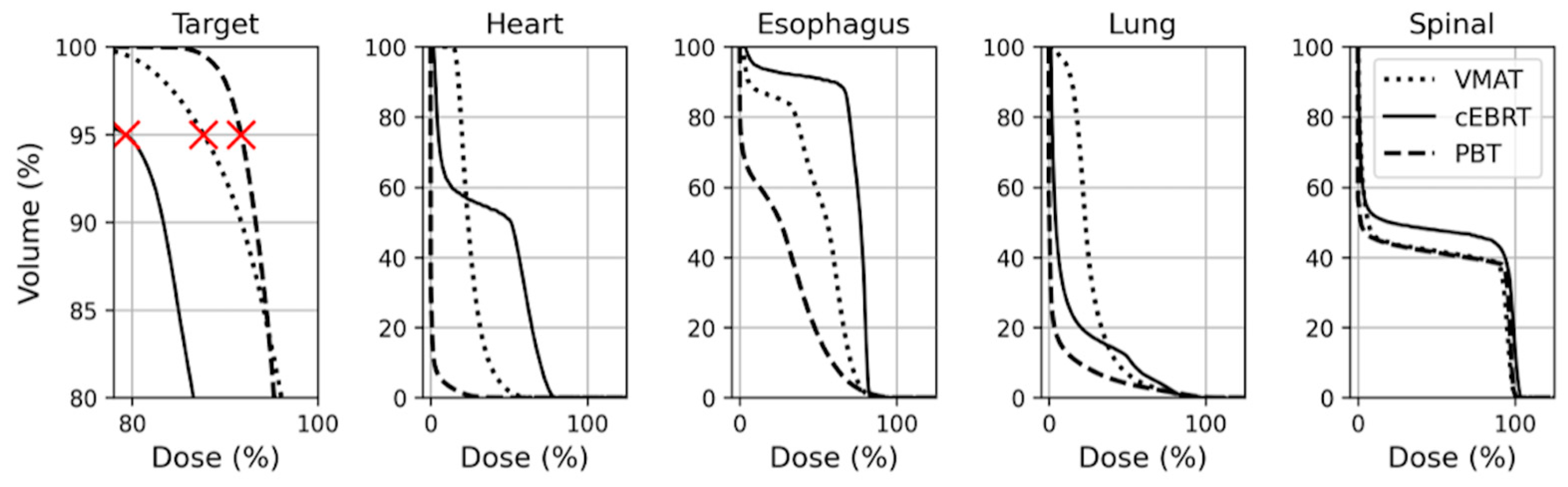
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.F.; Shen, J.; Li, X.; Rengan, R.; Silvestris, N.; Wang, M.; Derosa, L.; Zheng, X.; Belli, A.; Zhang, X.L.; et al. Incidence of patients with bone metastases at diagnosis of solid tumors in adults: A large population-based study. Ann. Transl. Med. 2020, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Norgaard, M.; Jensen, A.O.; Jacobsen, J.B.; Cetin, K.; Fryzek, J.P.; Sorensen, H.T. Skeletal related events, bone metastasis and survival of prostate cancer: A population based cohort study in Denmark (1999 to 2007). J. Urol. 2010, 184, 162–167. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, W.; Biskup, E.; Yang, W.; Yang, Z.; Wang, H.; Qiu, X.; Zhang, C.; Hu, G.; Hu, G. Incidence, risk factors and prognostic characteristics of bone metastases and skeletal-related events (SREs) in breast cancer patients: A systematic review of the real world data. J. Bone Oncol. 2018, 11, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Steinauer, K.; Gross, M.W.; Huang, D.J.; Eppenberger-Castori, S.; Guth, U. Radiotherapy in patients with distant metastatic breast cancer. Radiat. Oncol. 2014, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic Body Radiation Therapy for Spinal Metastases: Benefits and Limitations. Semin. Radiat. Oncol. 2023, 33, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.K.; Jiang, J.; Shih, Y.T.; Lei, X.; Xu, Y.; Hoffman, K.E.; Giordano, S.H.; Smith, B.D. Trends in Radiation for Bone Metastasis During a Period of Multiple National Quality Improvement Initiatives. J. Oncol. Pract. 2019, 15, e356–e368. [Google Scholar] [CrossRef] [PubMed]
- Howell, D.D.; James, J.L.; Hartsell, W.F.; Suntharalingam, M.; Machtay, M.; Suh, J.H.; Demas, W.F.; Sandler, H.M.; Kachnic, L.A.; Berk, L.B. Single-fraction radiotherapy versus multifraction radiotherapy for palliation of painful vertebral bone metastases-equivalent efficacy, less toxicity, more convenient: A subset analysis of Radiation Therapy Oncology Group trial 97-14. Cancer 2013, 119, 888–896. [Google Scholar] [CrossRef]
- Rades, D.; Stalpers, L.J.; Veninga, T.; Schulte, R.; Hoskin, P.J.; Obralic, N.; Bajrovic, A.; Rudat, V.; Schwarz, R.; Hulshof, M.C.; et al. Evaluation of five radiation schedules and prognostic factors for metastatic spinal cord compression. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 3366–3375. [Google Scholar] [CrossRef]
- Rich, S.E.; Chow, R.; Raman, S.; Liang Zeng, K.; Lutz, S.; Lam, H.; Silva, M.F.; Chow, E. Update of the systematic review of palliative radiation therapy fractionation for bone metastases. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 547–557. [Google Scholar] [CrossRef]
- van der Velden, J.; Willmann, J.; Spalek, M.; Oldenburger, E.; Brown, S.; Kazmierska, J.; Andratschke, N.; Menten, J.; van der Linden, Y.; Hoskin, P. ESTRO ACROP guidelines for external beam radiotherapy of patients with uncomplicated bone metastases. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 173, 197–206. [Google Scholar] [CrossRef]
- Zhang, H.R.; Li, J.K.; Yang, X.G.; Qiao, R.Q.; Hu, Y.C. Conventional Radiotherapy and Stereotactic Radiosurgery in the Management of Metastatic Spine Disease. Technol. Cancer Res. Treat. 2020, 19, 1533033820945798. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Yorke, E.D.; Jackson, A.; Ten Haken, R.K.; Constine, L.S.; Eisbruch, A.; Bentzen, S.M.; Nam, J.; Deasy, J.O. Use of normal tissue complication probability models in the clinic. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S10–S19. [Google Scholar] [CrossRef] [PubMed]
- Gottumukkala, S.; Srivastava, U.; Brocklehurst, S.; Mendel, J.T.; Kumar, K.; Yu, F.F.; Agarwal, A.; Shah, B.R.; Vira, S.; Raj, K.M. Fundamentals of Radiation Oncology for Treatment of Vertebral Metastases. Radiographics 2021, 41, 2136–2156. [Google Scholar] [CrossRef]
- Hunte, S.O.; Clark, C.H.; Zyuzikov, N.; Nisbet, A. Volumetric modulated arc therapy (VMAT): A review of clinical outcomes-what is the clinical evidence for the most effective implementation? Br. J. Radiol. 2022, 95, 20201289. [Google Scholar] [CrossRef] [PubMed]
- Patyal, B. Dosimetry aspects of proton therapy. Technol. Cancer Res. Treat. 2007, 6, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Brandal, P.; Bergfeldt, K.; Aggerholm-Pedersen, N.; Backstrom, G.; Kerna, I.; Gubanski, M.; Bjornlinger, K.; Evensen, M.E.; Kuddu, M.; Pettersson, E.; et al. A Nordic-Baltic perspective on indications for proton therapy with strategies for identification of proper patients. Acta Oncol. 2020, 59, 1157–1163. [Google Scholar] [CrossRef]
- Korevaar, E.W.; Habraken, S.J.M.; Scandurra, D.; Kierkels, R.G.J.; Unipan, M.; Eenink, M.G.C.; Steenbakkers, R.; Peeters, S.G.; Zindler, J.D.; Hoogeman, M.; et al. Practical robustness evaluation in radiotherapy—>A photon and proton-proof alternative to PTV-based plan evaluation. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2019, 141, 267–274. [Google Scholar] [CrossRef]
- Guo, M.; Kolberg, K.L.; Smith, E.C.; Smith, B.W.; Yousif, J.E.; Kessler, J.L.; Linzey, J.R.; Calinescu, A.A.; Clines, G.A.; Spratt, D.E.; et al. Predominance of Spinal Metastases Involving the Posterior Vertebral Body. World Neurosurg. 2018, 119, e991–e996. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef]
- Fizazi, K.; Gillessen, S. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann. Oncol. 2023, 34, 557–563. [Google Scholar] [CrossRef]
- Olson, R.; Schlijper, R.; Chng, N.; Matthews, Q.; Arimare, M.; Mathews, L.; Hsu, F.; Berrang, T.; Louie, A.; Mou, B.; et al. SUPR-3D: A randomized phase iii trial comparing simple unplanned palliative radiotherapy versus 3d conformal radiotherapy for patients with bone metastases: Study protocol. BMC Cancer 2019, 19, 1011. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, W.V.O. Comparison between 3D-CRT and modulated techniques for head-and-neck and breast. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2018. [Google Scholar]
- Rozanec, N.; Allibhai, Z.; Bhatti, M.; Chan, E.; McIntosh, M.; Moseley, D.; Taremi, M.; Abbas, A. Palliation of Vertebral Metastases with Radiotherapy: Exploration of Volumetric-Modulated Arc Therapy from Development to Implementation in Routine Clinical Practice. J. Med. Imaging Radiat. Sci. 2019, 50, 68–73. [Google Scholar] [CrossRef]
- Thomas, E.M.; Popple, R.A.; Prendergast, B.M.; Clark, G.M.; Dobelbower, M.C.; Fiveash, J.B. Effects of flattening filter-free and volumetric-modulated arc therapy delivery on treatment efficiency. J. Appl. Clin. Med. Phys. 2013, 14, 4328. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, P.A.; Kerstiens, J.; Richard, H. Proton facility economics: The importance of “simple” treatments. J. Am. Coll. Radiol. 2012, 9, 560–563. [Google Scholar] [CrossRef]
- McNamara, A.L.; Willers, H.; Paganetti, H. Modelling variable proton relative biological effectiveness for treatment planning. Br. J. Radiol. 2020, 93, 20190334. [Google Scholar] [CrossRef]
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99-117. [Google Scholar] [CrossRef]
- Traneus, E.; Oden, J. Introducing Proton Track-End Objectives in Intensity Modulated Proton Therapy Optimization to Reduce Linear Energy Transfer and Relative Biological Effectiveness in Critical Structures. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 747–757. [Google Scholar] [CrossRef]
- Rief, H.; Chaudhri, N.; Tonndorf-Martini, E.; Bruckner, T.; Rieken, S.; Bostel, T.; Forster, R.; Schlampp, I.; Debus, J.; Sterzing, F. Intensity-modulated radiotherapy versus proton radiotherapy versus carbon ion radiotherapy for spinal bone metastases: A treatment planning study. J. Appl. Clin. Med. Phys. 2015, 16, 186–194. [Google Scholar] [CrossRef]
- Gram, V.R.; Gram, D.; Persson, G.F.; Suppli, M.H.; Barrett, S. Reduction of oesophageal toxicity with VMAT dose-sparing radiotherapy in thoracic metastatic spinal cord compression: A feasibility study. Tech. Innov. Patient Support. Radiat. Oncol. 2022, 23, 8–14. [Google Scholar] [CrossRef]
- Rades, D.; Hansen, O.; Jensen, L.H.; Dziggel, L.; Staackmann, C.; Doemer, C.; Cacicedo, J.; Conde-Moreno, A.J.; Segedin, B.; Ciervide-Jurio, R.; et al. Radiotherapy for metastatic spinal cord compression with increased radiation doses (RAMSES-01): A prospective multicenter study. BMC Cancer 2019, 19, 1163. [Google Scholar] [CrossRef]
- Ryu, S.; Deshmukh, S.; Timmerman, R.D.; Movsas, B.; Gerszten, P.; Yin, F.F.; Dicker, A.; Abraham, C.D.; Zhong, J.; Shiao, S.L.; et al. Stereotactic Radiosurgery vs Conventional Radiotherapy for Localized Vertebral Metastases of the Spine: Phase 3 Results of NRG Oncology/RTOG 0631 Randomized Clinical Trial. JAMA Oncol. 2023, 9, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, A.; Myrehaug, S.D.; Siva, S.; Masucci, G.L.; Maralani, P.J.; Brundage, M.; Butler, J.; Chow, E.; Fehlings, M.G.; Foote, M.; et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: An open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021, 22, 1023–1033. [Google Scholar] [CrossRef] [PubMed]

| Patient | Target | No. of Vertebrae within Target | RT-Regimen |
|---|---|---|---|
| 1 | T4-T8 | 5 | 8 Gy × 1 |
| 2 | T3-T12 | 10 | 8 Gy × 2 |
| 3 | T5-T7 | 3 | 8 Gy × 2 |
| 4 | T2-T5 | 4 | 7 Gy × 1 |
| 5 | T2-T11 | 10 | 4 Gy × 5 |
| 6 | T3-T8 | 6 | 8 Gy × 2 |
| 7 | T5-T9 | 5 | 8 Gy × 2 |
| 8 | T1-T5 | 5 | 8 Gy × 1 |
| 9 | T5-T9 | 5 | 4 Gy × 5 |
| 10 | T7-T9 | 3 | 8 Gy × 2 |
| 11 | T5-T8 | 4 | 4 Gy × 5 |
| 12 | T4-T7 | 4 | 8 Gy × 2 |
| 13 | T5-T8 | 4 | 4 Gy × 5 |
| 14 | T5-T10 | 6 | 4 Gy × 5 |
| 15 | T2-T8 | 7 | 5 Gy × 5 |
| 16 | T4-T7 | 4 | 8 Gy × 2 |
| 17 | T3-T8 | 6 | 4 Gy × 5 |
| 18 | T7-T10 | 4 | 8 Gy × 2 |
| 19 | T5-T8 | 4 | 8 Gy × 2 |
| 20 | T2-T4/ T8-10 | 6 | 4 Gy × 5 |
| 21 | T2-T4 | 3 | 8 Gy × 2 |
| 22 | T7-T9 | 3 | 8 Gy × 1 |
| 23 | T2-T6 | 5 | 4 Gy × 5 |
| 24 | T2-T6 | 5 | 8 Gy × 2 |
| 25 | T6-T9 | 4 | 8 Gy × 2 |
| 26 | T1-T6 | 6 | 4 Gy × 5 |
| 27 | T5-T7 | 3 | 8 Gy × 1 |
| 28 | T7-T11 | 5 | 8 Gy × 2 |
| 29 | C6-T11 | 13 | 8 Gy × 1 |
| Target/OAR | Variable (%) | VMAT | cEBRT | PBT 1 | p-Value | ||
|---|---|---|---|---|---|---|---|
| VMAT vs. cEBRT | VMAT vs. PBT | cEBRT vs. PBT | |||||
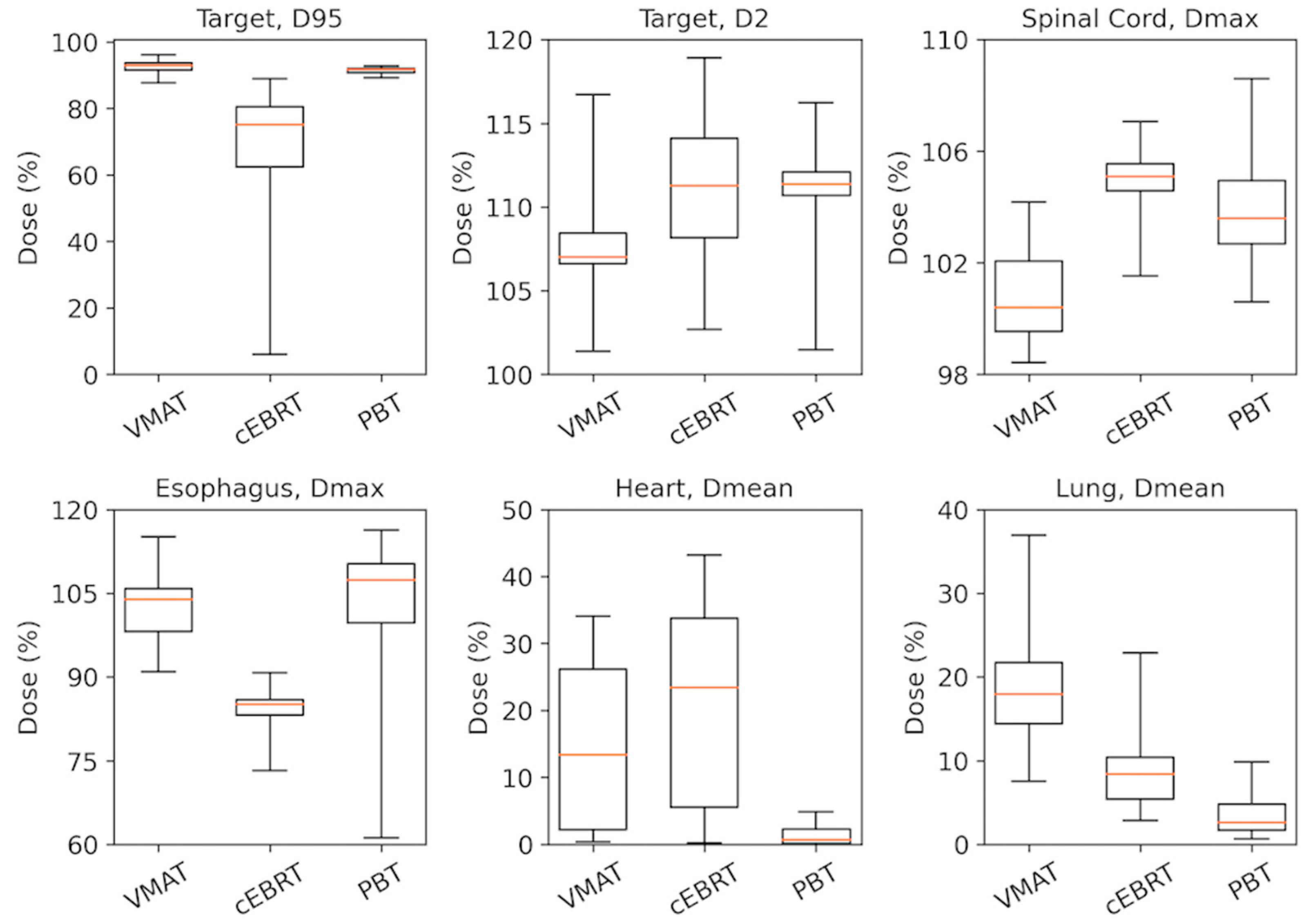
| CTV | D98 | 93.4 (92.7–94.1) | 82.0 (79.9–84.0) | 92.3 (91.7–93.0) | <0.001 | <0.001 | <0.001 |
| D2 | 107.6 (107.0–109.0) | 109.0 (106.8–111.5) | 110.3 (109.5–111.0) | 0.254 | 0.006 | 0.116 | |
| Dmean | 101.8 (101.1–103.2) | 95.4 (94.2–96.5) | 100.9 (100.5–101.2) | <0.001 | 0.006 | <0.001 | |
| PTV 2 | D95 | 92.9 (91.6–93.7) | 75.2 (60.6–81.0) | 91.7 2 (90.8–92.0) | <0.001 | 0.001 | <0.001 |
| D2 | 107.0 (106.6–108.6) | 111.3 (108.0–114.4) | 111.4 2 (110.7–112.1) | 0.001 | <0.001 | 0.829 | |
| Dmean | 100.5 (99.5–101.3) | 93.2 (90.8–95.2) | 99.4 2 (98.6–99.6) | <0.001 | 0.001 | <0.001 | |
| Spinal Cord | Dmax | 100.4 (99.5–102.2) | 105.1 (104.6–105.6) | 103.6 (102.6–105.0) | <0.001 | <0.001 | 0.002 |
| Dmean | 78.5 (74.4–82.6) | 85.3 (79.3–90.6) | 75.2 (68.7–78.7) | 0.001 | <0.001 | <0.001 | |
| Esophagus | Dmax | 103.9 (97.7–106.1) | 85.1 (83.2–86.0) | 107.4 (99.4–110.7) | <0.001 | 0.043 | <0.001 |
| Dmean | 31.2 (23.9–41.4) | 33.7 (28.5–41.4) | 19.7 (12.4–29.1) | 0.001 | <0.001 | <0.001 | |
| V20 | 42.5 (30.5–55.1) | 45.8 (37.6–56.8) | 32.1 (24.5–47.1) | <0.001 | <0.001 | <0.001 | |
| V30 | 40.7 (30.5–53.6) | 44.4 (36.0–55.5) | 29.1 (19.9–44.7) | <0.001 | <0.001 | <0.001 | |
| V40 | 36.9 (29.0–52.0) | 43.9 (34.4–54.4) | 25.5 (14.5–38.4) | <0.001 | <0.001 | <0.001 | |
| V50 | 32.4 (26.4–44.7) | 42.6 (33.2–53.1) | 21.4 (10.2–32.3) | <0.001 | <0.001 | <0.001 | |
| Heart | Dmean | 13.4 (2.0–26.6) | 23.4 (4.2–34.6) | 0.68 (0.10–2.4) | <0.001 | <0.001 | <0.001 |
| V20 | 31.5 (0.23–66.7) | 39.5 (5.3–58.3) | 0.97 (0.00–4.3) | 0.831 | <0.001 | <0.001 | |
| V30 | 14.1 (0.00–38.1) | 36.5 (4.5–56.0) | 0.28 (0.00–2.5) | <0.001 | <0.001 | <0.001 | |
| V40 | 6.0 (0.00–14.7) | 32.7 (3.8–54.1) | 0.09 (0.00–1.5) | <0.001 | <0.001 | <0.001 | |
| V50 | 2.0 (0.00–6.6) | 24.7 (2.5–44.6) | 0.02 (0.00–0.83) | <0.001 | <0.001 | <0.001 | |
| Lung Total | Dmean | 17.9 (14.0–22.3) | 8.4 (5.3–10.7) | 2.6 (1.7–4.9) | <0.001 | <0.001 | <0.001 |
| V20 | 41.0 (31.4–55.1) | 10.3 (6.8–14.1) | 4.1 (2.5–7.4) | <0.001 | <0.001 | <0.001 | |
| V30 | 20.6 (15.1–26.5) | 8.4 (5.3–12.0) | 3.3 (1.8–6.0) | <0.001 | <0.001 | <0.001 | |
| V40 | 11.0 (7.9–14.0) | 7.1 (4.3–9.8) | 2.6 (1.4–4.8) | <0.001 | <0.001 | <0.001 | |
| V50 | 6.8 (4.6–8.8) | 4.8 (2.8–7.8) | 2.1 (1.0–4.0) | 0.039 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lideståhl, A.; Fredén, E.; Siegbahn, A.; Johansson, G.; Lind, P.A. Dosimetric Comparison of Conventional Radiotherapy, Volumetric Modulated Arc Therapy, and Proton Beam Therapy for Palliation of Thoracic Spine Metastases Secondary to Breast or Prostate Cancer. Cancers 2023, 15, 5736. https://doi.org/10.3390/cancers15245736
Lideståhl A, Fredén E, Siegbahn A, Johansson G, Lind PA. Dosimetric Comparison of Conventional Radiotherapy, Volumetric Modulated Arc Therapy, and Proton Beam Therapy for Palliation of Thoracic Spine Metastases Secondary to Breast or Prostate Cancer. Cancers. 2023; 15(24):5736. https://doi.org/10.3390/cancers15245736
Chicago/Turabian StyleLideståhl, Anders, Emil Fredén, Albert Siegbahn, Gracinda Johansson, and Pehr A. Lind. 2023. "Dosimetric Comparison of Conventional Radiotherapy, Volumetric Modulated Arc Therapy, and Proton Beam Therapy for Palliation of Thoracic Spine Metastases Secondary to Breast or Prostate Cancer" Cancers 15, no. 24: 5736. https://doi.org/10.3390/cancers15245736
APA StyleLideståhl, A., Fredén, E., Siegbahn, A., Johansson, G., & Lind, P. A. (2023). Dosimetric Comparison of Conventional Radiotherapy, Volumetric Modulated Arc Therapy, and Proton Beam Therapy for Palliation of Thoracic Spine Metastases Secondary to Breast or Prostate Cancer. Cancers, 15(24), 5736. https://doi.org/10.3390/cancers15245736








