Efficacy of Treatment for Metastatic Hormone-Sensitive Prostate Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
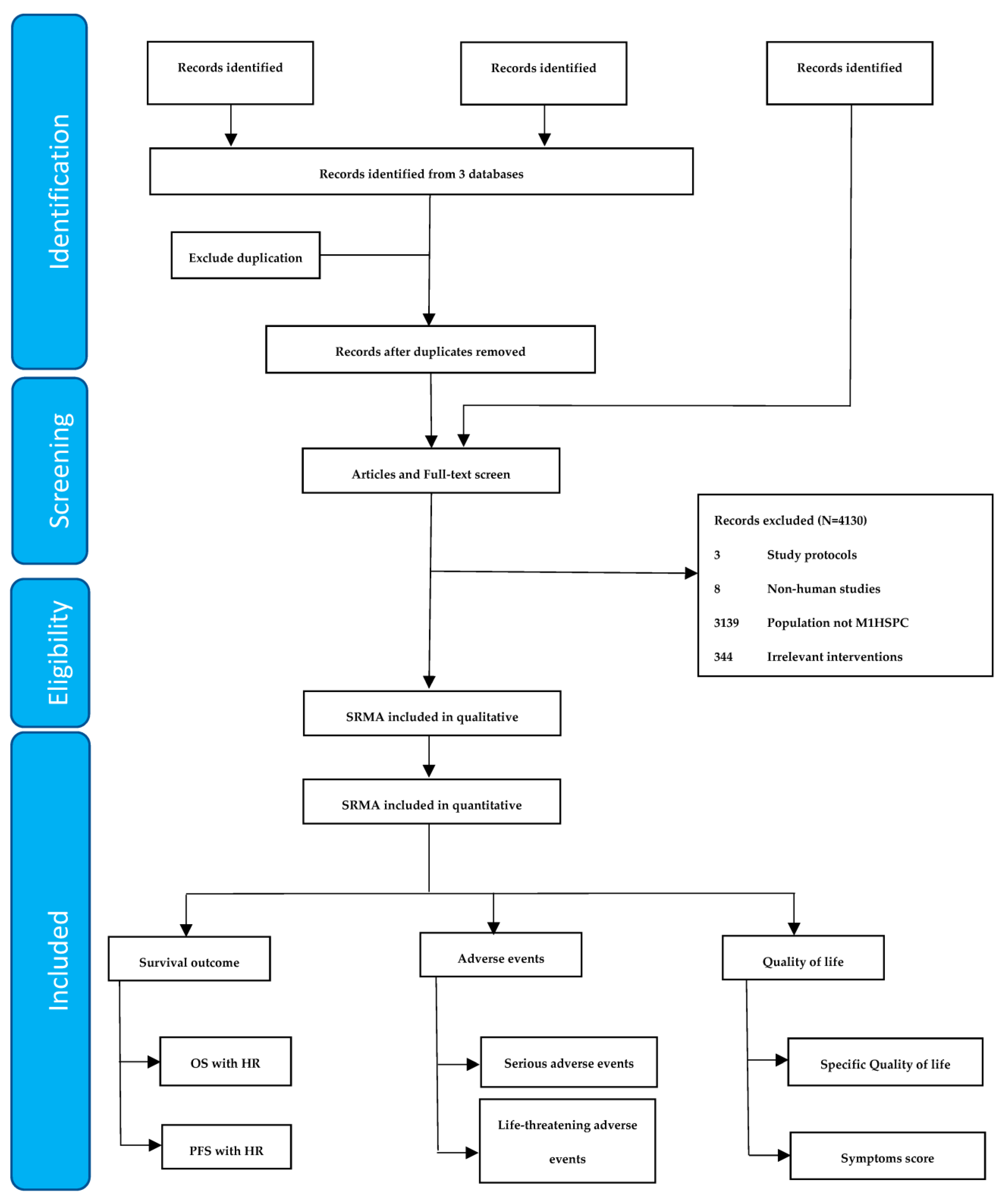
2.1. Search Strategy and Criteria for Study Inclusion
2.2. Interventions and Outcomes
2.3. Data Extraction and Study Quality Assessment
2.4. Data Synthesis and Statistical Analyses in the Umbrella Review
3. Results
3.1. Mortality Rate
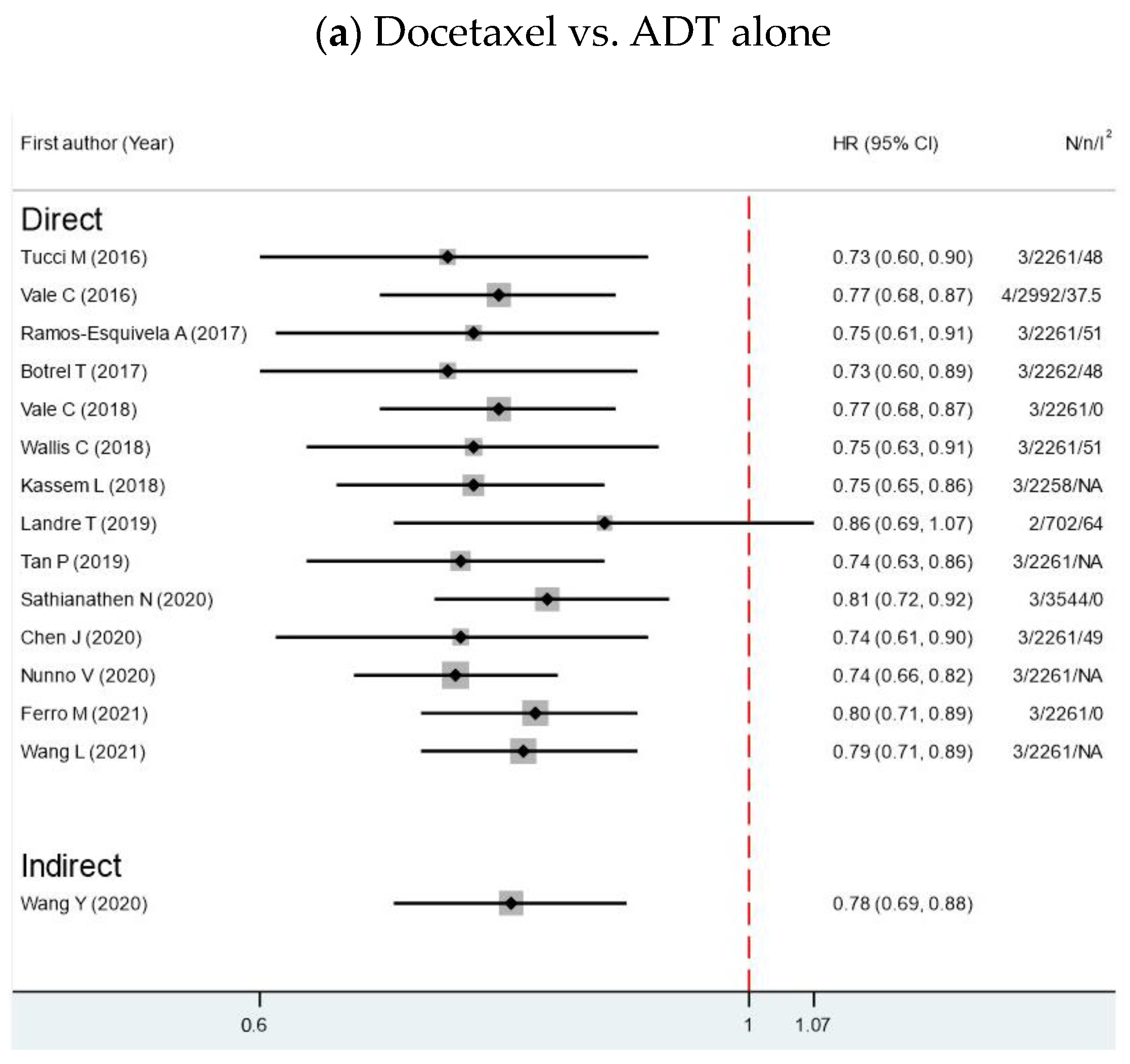
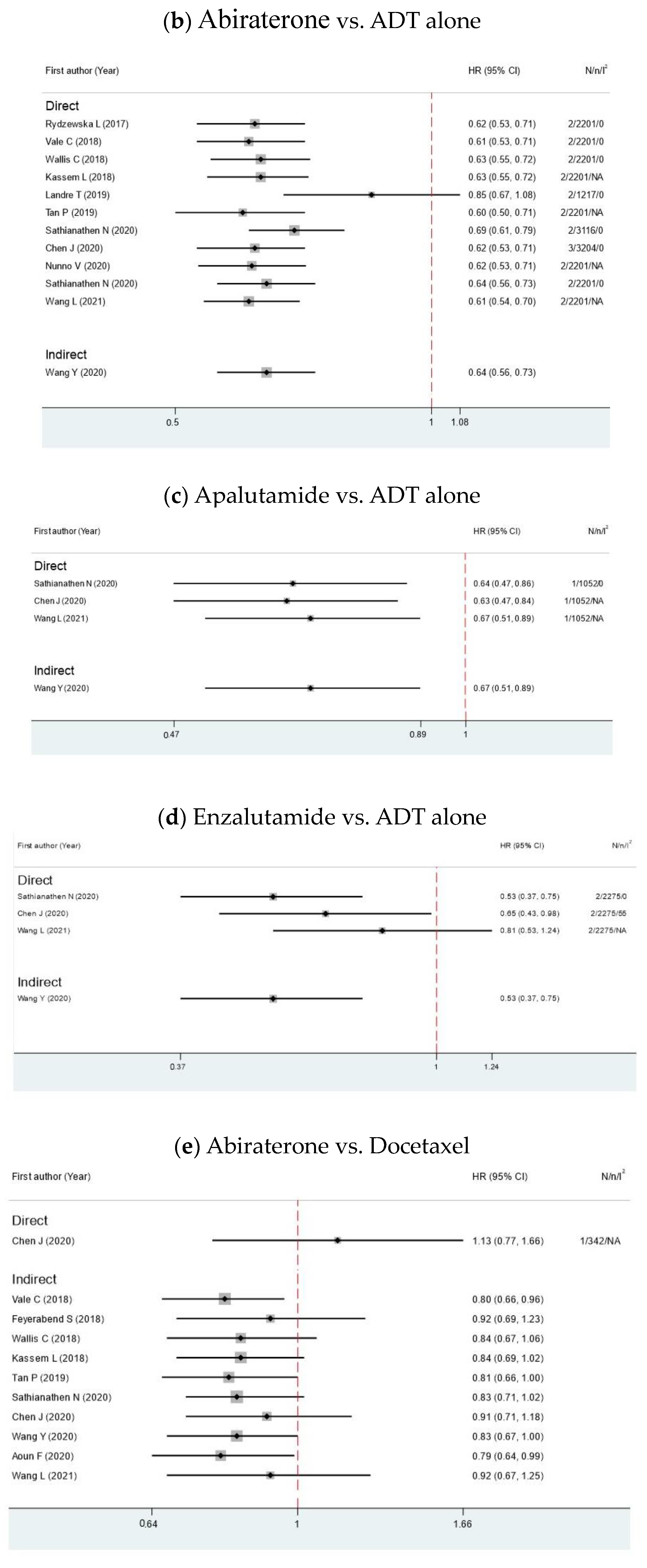
3.2. Overall Survival
3.3. Progression-Free Survival
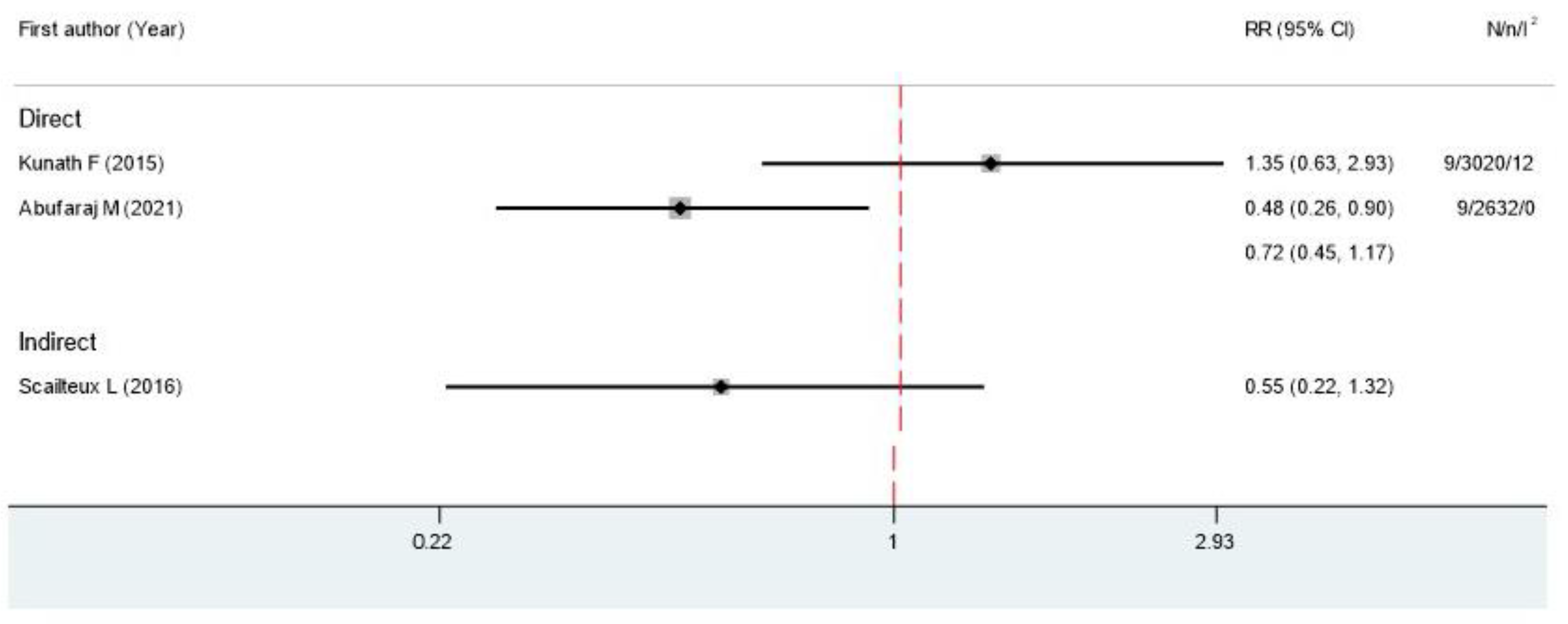
3.4. Adverse Events
3.5. Excess Significance Test
3.6. Degree of Overlap
3.7. Risk of Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wong, M.C.; Goggins, W.B.; Wang, H.H.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Center, M.M.; Jemal, A.; Lortet-Tieulent, J.; Ward, E.; Ferlay, J.; Brawley, O.; Bray, F. International variation in prostate cancer incidence and mortality rates. Eur. Urol. 2012, 61, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; Biganzoli, D.; Rossi, R.S.; Palmisano, F.; Bussetti, M.; Verzotti, E.; Gregori, A.; Bianchi, F.; Maggioni, M.; Ceriotti, F.; et al. Individual risk prediction of high grade prostate cancer based on the combination between total prostate-specific antigen (PSA) and free to total PSA ratio. Clin. Chem. Lab. Med. 2023, 61, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Seidenfeld, J.; Samson, D.J.; Hasselblad, V.; Aronson, N.; Albertsen, P.C.; Bennett, C.L.; Wilt, T.J. Single-therapy androgen suppression in men with advanced prostate cancer: A systematic review and meta-analysis. Ann. Intern. Med. 2000, 132, 566–577. [Google Scholar] [CrossRef]
- Weckermann, D.; Harzmann, R. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. Eur. Urol. 2004, 46, 279–283; discussion 83–84. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Rydzewska, L.H.M.; Burdett, S.; Vale, C.L.; Clarke, N.W.; Fizazi, K.; Kheoh, T.; Mason, M.D.; Miladinovic, B.; James, N.D.; Parmar, M.K.B.; et al. Adding abiraterone to androgen deprivation therapy in men with metastatic hormone-sensitive prostate cancer: A systematic review and meta-analysis. Eur. J. Cancer 2017, 84, 88–101. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef]
- Hennessy, E.A.; Johnson, B.T. Examining overlap of included studies in meta-reviews: Guidance for using the corrected covered area index. Res. Synth. Methods 2020, 11, 134–145. [Google Scholar] [CrossRef]
- Ioannidis, J.P.; Trikalinos, T.A. An exploratory test for an excess of significant findings. Clin. Trials 2007, 4, 245–253. [Google Scholar] [CrossRef]
- Scailteux, L.M.; Naudet, F.; Alimic, Q.; Vincendeau, S.; Oger, E. Mortality, cardiovascular risk, and androgen deprivation therapy for prostate cancer: A systematic review with direct and network metaanalyses of randomized controlled trials and observational studies. Medicine 2016, 95, e3873. [Google Scholar] [CrossRef] [PubMed]
- Abufaraj, M.; Iwata, T.; Kimura, S.; Haddad, A.; Al-Ani, H.; Abusubaih, L.; Moschini, M.; Briganti, A.; Karakiewicz, P.I.; Shariat, S.F. Differential Impact of Gonadotropin-releasing Hormone Antagonist Versus Agonist on Clinical Safety and Oncologic Outcomes on Patients with Metastatic Prostate Cancer: A Meta-analysis of Randomized Controlled Trials. Eur. Urol. 2021, 79, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Rajabi, F.; Sari, A.A.; Ayati, M.; Heidari, S.; Ghamary, F. Degarelix for the treatment of advanced prostate cancer compared with GnRh-Agonists: A systematic review and meta-analysis. Med. J. Islam. Repub. Iran 2016, 30, 317. [Google Scholar] [PubMed]
- Sciarra, A.; Fasulo, A.; Ciardi, A.; Petrangeli, E.; Gentilucci, A.; Maggi, M.; Innocenzi, M.; Pierella, F.; Gentile, V.; Salciccia, S.; et al. A meta-analysis and systematic review of randomized controlled trials with degarelix versus gonadotropin-releasing hormone agonists for advanced prostate cancer. Medicine 2016, 95, e3845. [Google Scholar] [CrossRef] [PubMed]
- Kunath, F.; Borgmann, H.; Blümle, A.; Keck, B.; Wullich, B.; Schmucker, C.; Sikic, D.; Roelle, C.; Schmidt, S.; Wahba, A.; et al. Gonadotropin-releasing hormone antagonists versus standard androgen suppression therapy for advanced prostate cancer A systematic review with meta-analysis. BMJ Open 2015, 5, e008217. [Google Scholar] [CrossRef]
- Tucci, M.; Bertaglia, V.; Vignani, F.; Buttigliero, C.; Fiori, C.; Porpiglia, F.; Scagliotti, G.V.; Di Maio, M. Addition of docetaxel to androgen deprivation therapy for patients with hormone-sensitive metastatic prostate cancer: A systematic review and meta-analysis. Eur. Urol. 2016, 69, 563–573. [Google Scholar] [CrossRef]
- Vale, C.L.; Burdett, S.; Rydzewska, L.H.M.; Albiges, L.; Clarke, N.W.; Fisher, D.; Fizazi, K.; Gravis, G.; James, N.D.; Mason, M.D.; et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016, 17, 243–256. [Google Scholar] [CrossRef]
- Ramos-Esquivel, A.; Fernández, C.; Zeledón, Z. Androgen-deprivation therapy plus chemotherapy in metastatic hormone-sensitive prostate cancer. A systematic review and meta-analysis of randomized clinical trials. Urol. Oncol. Semin. Orig. Investig. 2016, 34, 335.e9–335.e19. [Google Scholar] [CrossRef]
- Botrel, T.E.A.; Clark, O.; Pompeo, A.C.L.; Bretas, F.F.H.; Sadi, M.V.; Ferreira, U.; Reis, R.B.D. Efficacy and safety of combined androgen deprivation therapy (ADT) and docetaxel compared with ADT alone for metastatic hormone-naive prostate cancer: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0157660. [Google Scholar] [CrossRef]
- Vale, C.L.; Fisher, D.J.; White, I.R.; Carpenter, J.R.; Burdett, S.; Clarke, N.W.; Fizazi, K.; Gravis, G.; James, N.D.; Mason, M.D.; et al. What is the optimal systemic treatment of men with metastatic, hormone-naive prostate cancer? A STOPCAP systematic review and network meta-analysis. Ann. Oncol. 2018, 29, 1249–1257. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Klaassen, Z.; Bhindi, B.; Goldberg, H.; Chandrasekar, T.; Farrell, A.M.; Boorjian, S.A.; Kulkarni, G.S.; Karnes, R.J.; Satkunasivam, R. Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naïve Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2018, 73, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Kassem, L.; Shohdy, K.S.; Abdel-Rahman, O. Abiraterone acetate/androgen deprivation therapy combination versus docetaxel/androgen deprivation therapy combination in advanced hormone-sensitive prostate cancer: A network meta-analysis on safety and efficacy. Curr. Med. Res. Opin. 2018, 34, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.S.; Aguiar, P.; Haaland, B.; Lopes, G. Addition of abiraterone, docetaxel, bisphosphonate, celecoxib or combinations to androgen-deprivation therapy (ADT) for metastatic hormone-sensitive prostate cancer (mHSPC): A network meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Sathianathen, N.J.; Koschel, S.; Thangasamy, I.A.; Teh, J.; Alghazo, O.; Butcher, G.; Howard, H.; Kapoor, J.; Lawrentschuk, N.; Siva, S.; et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur. Urol. 2020, 77, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ni, Y.; Sun, G.; Liao, B.; Zhang, X.; Zhao, J.; Zhu, S.; Wang, Z.; Shen, P.; Zeng, H. Comparison of Current Systemic Combination Therapies for Metastatic Hormone-Sensitive Prostate Cancer and Selection of Candidates for Optimal Treatment: A Systematic Review and Bayesian Network Meta-Analysis. Front. Oncol. 2020, 10, 519388. [Google Scholar] [CrossRef]
- Di Nunno, V.; Santoni, M.; Mollica, V.; Conti, A.; Montironi, R.; Battelli, N.; Ardizzoni, A.; Massari, F. Systemic Treatment for Metastatic Hormone Sensitive Prostate Cancer: A Comprehensive Meta-Analysis Evaluating Efficacy and Safety in Specific Sub-Groups of Patients. Clin. Drug Investig. 2020, 40, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.; Lucarelli, G.; Crocetto, F.; Dolce, P.; Verde, A.; La Civita, E.; Zappavigna, S.; de Cobelli, O.; Di Lorenzo, G.; Facchini, B.A.; et al. First-line systemic therapy for metastatic castration-sensitive prostate cancer: An updated systematic review with novel findings. Crit. Rev. Oncol./Hematol. 2021, 157, 103198. [Google Scholar] [CrossRef]
- Wang, L.; Paller, C.J.; Hong, H.; De Felice, A.; Alexander, G.C.; Brawley, O. Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2021, 7, 412–420. [Google Scholar] [CrossRef]
- Wang, Y.; Gui, H.; Wang, J.; Tian, J.; Wang, H.; Liang, C.; Hao, Z.; Rodriguez, R.; Wang, Z. Comparative Efficacy of Combined Radiotherapy, Systemic Therapy, and Androgen Deprivation Therapy for Metastatic Hormone-Sensitive Prostate Cancer: A Network Meta-Analysis and Systematic Review. Front. Oncol. 2020, 10, 567616. [Google Scholar] [CrossRef]
- Landre, T.; Guetz, G.D.; Chouahnia, K.; Fossey-Diaz, V.; Taleb, C.; Culine, S. Is There a Benefit of Addition Docetaxel, Abiraterone, Celecoxib, or Zoledronic Acid in Initial Treatments for Patients Older than 70 Years with Hormone-sensitive Advanced Prostate Cancer? A Meta-analysis. Clin. Genitourin. Cancer 2019, 17, e806–e813. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Oestreich, M.C.; Brown, S.J.; Gupta, S.; Konety, B.R.; Dahm, P.; Kunath, F. Abiraterone acetate in combination with androgen deprivation therapy compared to androgen deprivation therapy only for metastatic hormone-sensitive prostate cancer. Cochrane Database Syst. Rev. 2020, 12, CD013245. [Google Scholar] [CrossRef] [PubMed]
- Aoun, F.; Rassy, E.E.; Sleilaty, G.; Assi, T.; Bakouny, Z.; Kattan, J. The optimal treatment of metastatic hormone-naive prostate cancer: Abirateron acetate or docetaxel? Future Oncol. 2017, 13, 2785–2790. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, S.; Saad, F.; Li, T.; Ito, T.; Diels, J.; Van Sanden, S.; De Porre, P.; Roiz, J.; Abogunrin, S.; Koufopoulou, M.; et al. Survival benefit, disease progression and quality-of-life outcomes of abiraterone acetate plus prednisone versus docetaxel in metastatic hormone-sensitive prostate cancer: A network meta-analysis. Eur. J. Cancer 2018, 103, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Sciarra, A.; Busetto, G.M.; Salciccia, S.; Del Giudice, F.; Maggi, M.; Crocetto, F.; Ferro, M.; De Berardinis, E.; Scarpa, R.M.; Porpiglia, F.; et al. Does Exist a Differential Impact of Degarelix Versus LHRH Agonists on Cardiovascular Safety? Evidences From Randomized and Real-World Studies. Front. Endocrinol. 2021, 12, 695170. [Google Scholar] [CrossRef]

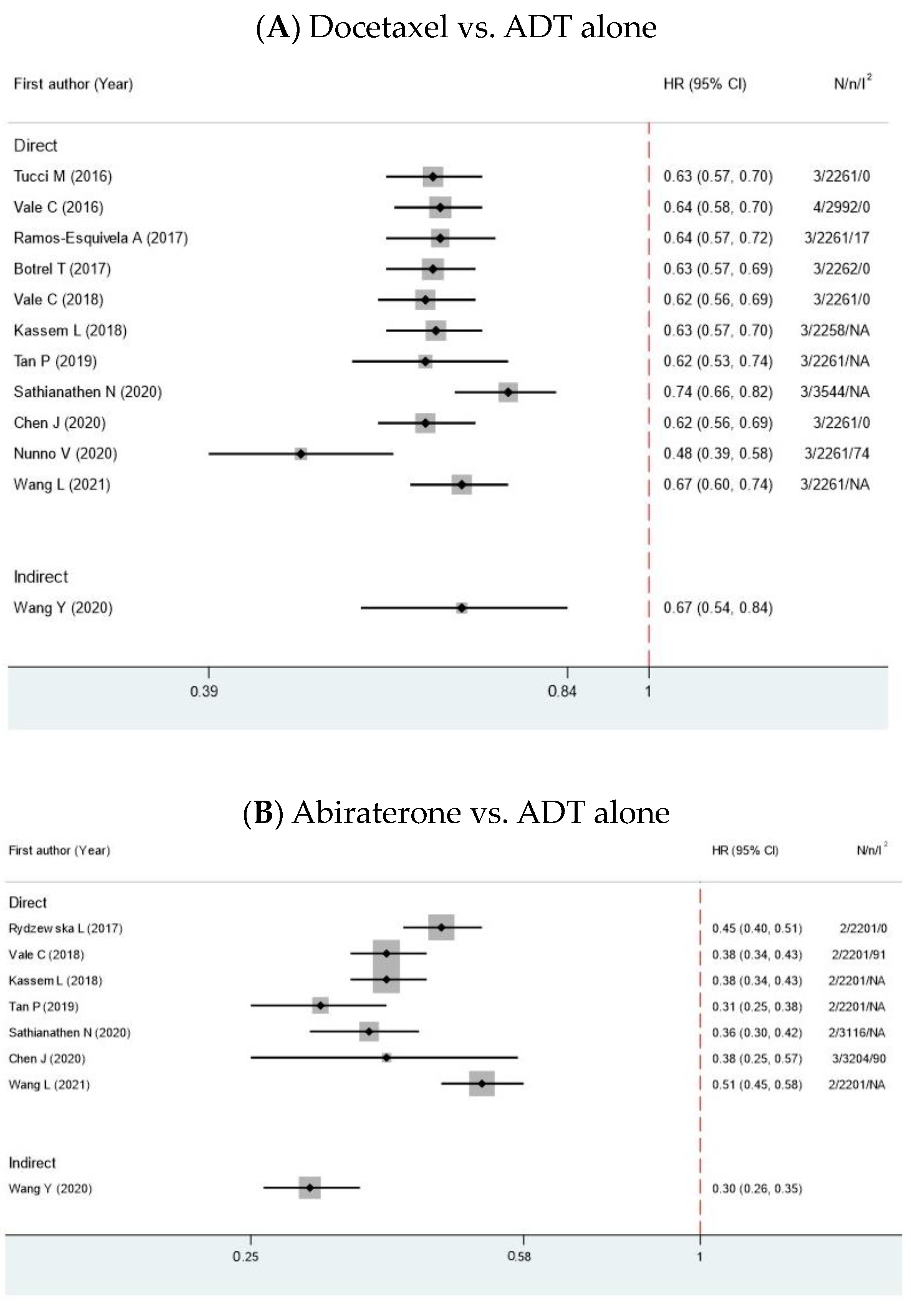
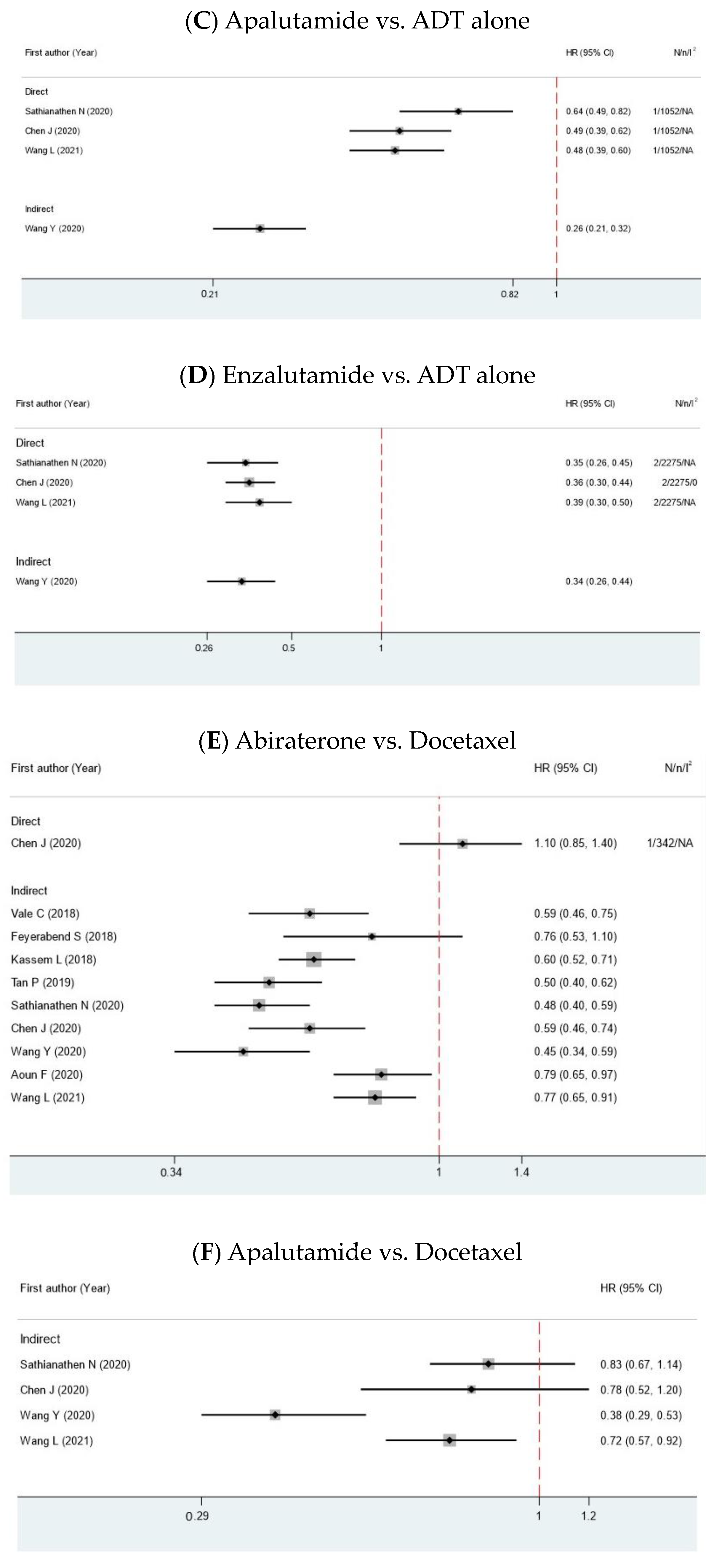

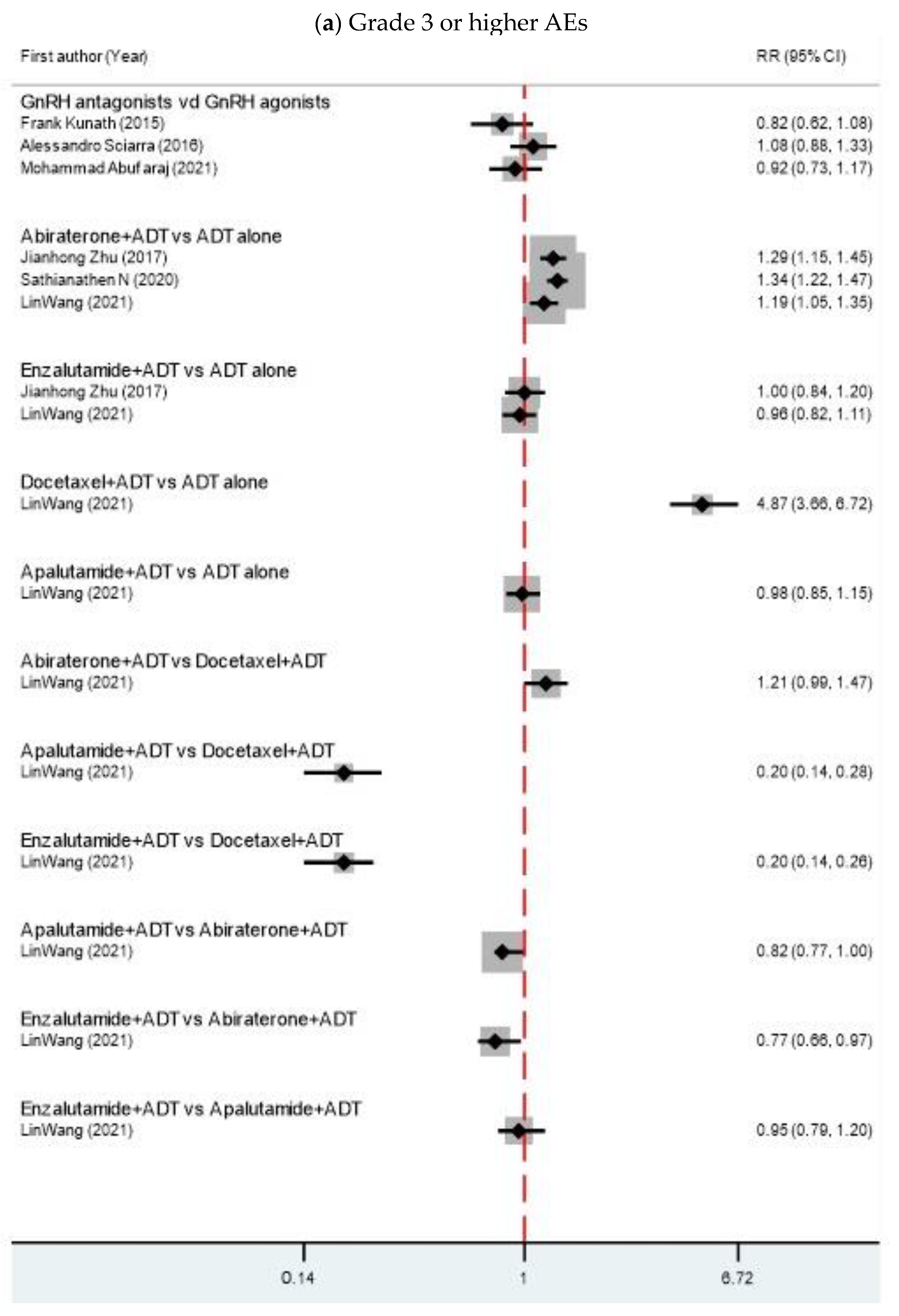
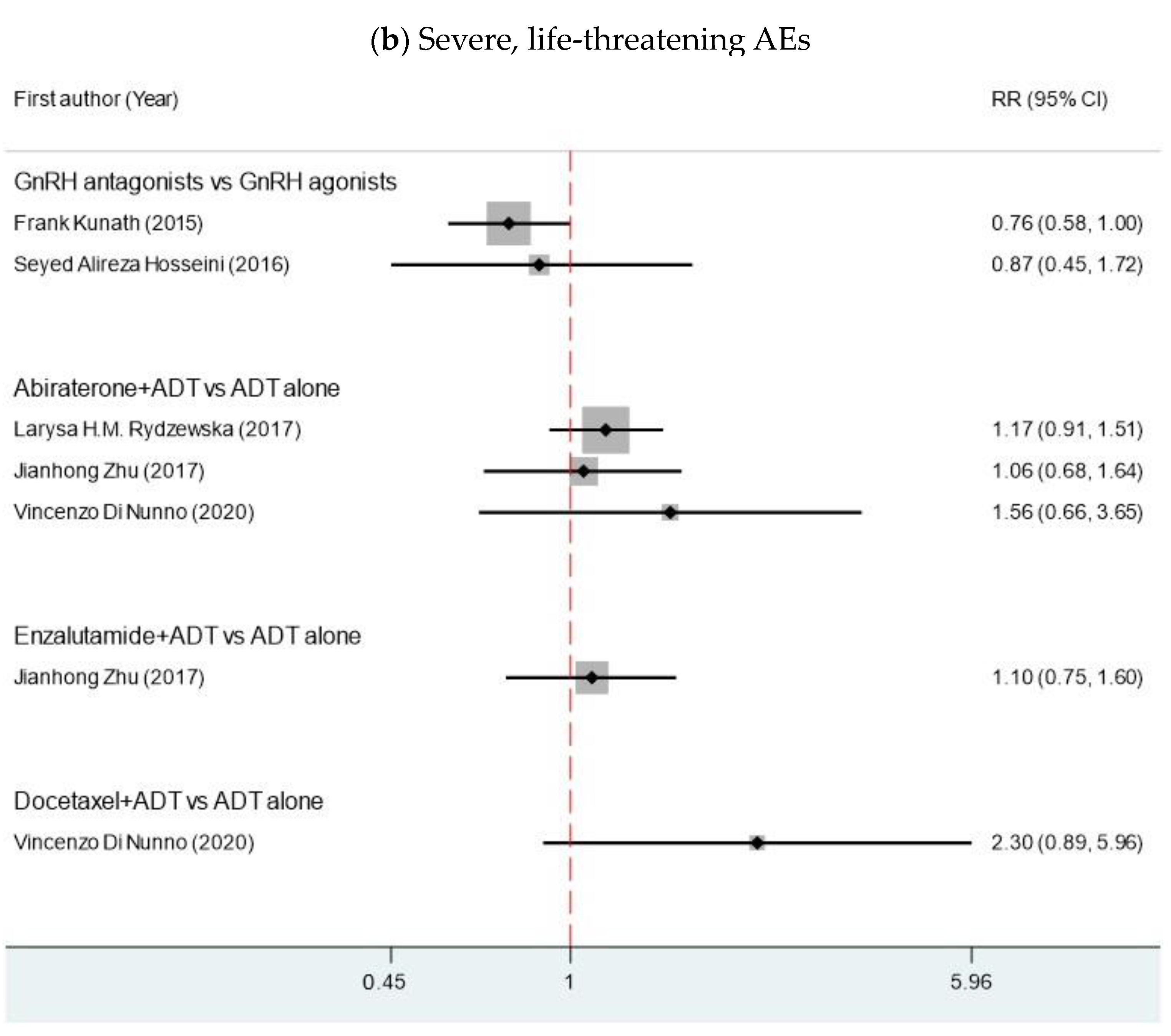
| Author | Year | MA Type | Comparison | Search Time | N | n | Years RCT Published | Interested Outcome | Median Age (IQR) | Median PSA (IQR) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ferro M | 2021 | NMA | ADT vs. Docetaxel | 30 October 2020 | 7 | 2261 | 2016–2020 | OS | 66.9 (36.0–88.0) | 50.9 (0.2–8540.1) |
| Wang L | 2021 | NMA | ADT vs. Docetaxel vs. Abiraterone vs. Apalutamide vs. Enzalutamide | 5 November 2019 | 19 | 7287 | 2013–2019 | OS, PFS, AE | N/A | N/A |
| Abufaruj M | 2021 | DMA | GnRH agonists vs. GnRH antagonists | April 2020 | 8 | 2632 | 2008–2019 | AE, PSA-progression, overall mortality | N/A | N/A |
| Sathianathen N | 2020 | NMA | ADT vs. Docetaxel vs. Abiraterone vs. Apalutamide vs. Enzalutamide | 5 November 2019 | 10 | 7287 | 2013–2019 | OS, PFS, AE | 67.0 (33.0–92.0) | 51.0 (Range 0.2–8450.1) |
| Chen J | 2020 | NMA | ADT vs. Docetaxel vs. Abiraterone vs. Apalutamide vs. Enzalutamide | 25 September 2019 | 11 | 11,174 | 2013–2019 | OS, PFS | 67.0 | 51.0 |
| Wang Y | 2020 | NMA | ADT vs. Docetaxel vs. Abiraterone vs. Apalutamide vs. Enzalutamide * | 7 May 2020 | 8 | 8701 | 2015–2019 | OS, PFS | N/A | N/A |
| Nunno V | 2020 | DMA | ADT vs. Docetaxel vs. Abiraterone * | 15 September 2019 | 10 | 8324 | 2013–2019 | OS, PFS, AE | N/A | N/A |
| Sathianathen N | 2020 | DMA | ADT vs. Abiraterone | 15 May 2020 | 9 | 2201 | 2013–2019 | OS, AE, FACT-P | 67.0 (33.0–92.0) | 53.5 (19.0–165.0) |
| Landre T | 2019 | DMA | ADT vs. Docetaxel, ADT vs. Abiraterone | Jan 2018 | 4 | 2264 | 2015–2017 | OS, PFS | N/A | N/A |
| Vale C | 2018 | NMA | ADT vs. Docetaxel vs. Abiraterone | 30 September 30th | 5 | 6204 | 2016–2017 | OS, PFS | N/A | N/A |
| Feyerabend S | 2018 | NMA | ADT vs. Docetaxel vs. Abiraterone | July 2017 | 10 | 4804 | 2015–2018 | OS, PFS, FACT-P | N/A | N/A |
| Wallis C | 2018 | NMA | ADT vs. Docetaxel vs. Abiraterone | 4 August 2017 | 5 | 6067 | 2015–2017 | OS, AE | 65.0 (33.0–92.0) | 51.0 (5.0–165.0) |
| Kassem L | 2018 | NMA | ADT vs. Docetaxel vs. Abiraterone | June 2017 | 7 | 7469 | 2013–2017 | OS, PFS, AE | 64.5 | N/A |
| Tan P | 2018 | NMA | ADT vs. Docetaxel vs. Abiraterone * | 26 August 2017 | 6 | 3877 | 2013–2017 | OS, PFS, AE | N/A | N/A |
| Aoun F | 2017 | NMA | ADT vs. Docetaxel vs. Abiraterone | not mentioned | 5 | 4827 | 2015–2017 | OS, PFS, AE | N/A | N/A |
| Rydzewska L | 2017 | DMA | ADT vs. Abiraterone | May 2017 | 2 | 2201 | 2017 | OS, PFS, AE | 67.0 (33.0–92.0) | N/A |
| Ramos-Esquivela A | 2016 | DMA | ADT vs. Docetaxel * | 1 October 2015 | 3 | 2261 | 2013–2016 | OS, PFS, AE | N/A | 53.5 (5.0–181.0) |
| Botrel T | 2016 | DMA | ADT vs. Docetaxel | not mentioned | 7 | 2264 | 2013–2016 | OS, PFS, AE | 63.0 | 38.0 |
| Hosseini S | 2016 | DMA | GnRH agonists vs. GnRH antagonists | up to 2014 | 6 | 2296 | 2008–2013 | AE | 72.0 (50.0–98.0) | 19.8 (8.2–68.0) |
| Lei J | 2016 | DMA | ADT vs. Docetaxel * | August 2014 | 2 | 1175 | 2013–2014 | OS in OR | N/A | N/A |
| Tucci M | 2016 | DMA | ADT vs. Docetaxel | August 2015 | 4 | 2951 | 2013–2015 | OS, PFS | 64.0 (39.0–91.0) | 39.0 (5.0–127.0) |
| Vale C | 2016 | DMA | ADT vs. Docetaxel | 30 September 2015 | 4 | 2992 | 2013–2016 | OS, PFS | 64.0 (36.0–91.0) | N/A |
| Sciarra A | 2016 | DMA | GnRH agonists vs. GnRH antagonists | 30 July 2015 | 7 | 1719 | 2008–2015 | AE | 71.9 (51.0–98.0) | 19.1 (0.01–12,861.0) |
| Kunath F | 2015 | DMA | GnRH agonists vs. GnRH antagonists | March 2015 | 17 ** | 3641 ** | 2001–2014 | AE | N/A | N/A |
| Zhu X | 2019 | DMA | ADT vs. Enzalutamide | July 2019 | 7 | 7347 | 2012–2019 | AE | N/A | N/A |
| Zhu J | 2017 | DMA | ADT vs. Abiraterone vs. Enzalutamide | June 2017 | 10 | 9520 | 2011–2017 | AE | N/A | N/A |
| Scailteux L | 2016 | NMA | Bilateral orchiectomy vs. GnRH agonists vs. GnRH antagonists | July 2014 | 57 ** | 31,037 ** | 1985–2013 | AE | N/A | N/A |
| Author | Year | 1. Study Eligibility Criteria | 2. Identification and Selection of Studies | 3. Data Collection and Study Appraisal | 4. Synthesis and Findings | 5. Risk of Bias in the Review |
|---|---|---|---|---|---|---|
| 1. Ferro M | 2021 | ☹ | ☺ | ☹ | ☹ | ☹ |
| 2. Wang L | 2021 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 3. Mohammad A | 2021 | ☹ | ☺ | ☺ | ☺ | ☺ |
| 4. Sathianathen N | 2020 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 5. Chen J | 2020 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 6. Wang Y | 2020 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 7. Nunno VD | 2020 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 8. Sathianathen N | 2020 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 9. Landre T | 2019 | ☺ | ☹ | ☹ | ☺ | ☹ |
| 10. Vale CL | 2018 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 11. Feyerabend S | 2018 | ☺ | ☺ | ☺ | ☹ | ☺ |
| 12. Wallis CJD | 2018 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 13. Kassem L | 2018 | ☺ | ☺ | ☹ | ☹ | ☺ |
| 14. Tan PS | 2018 | ☹ | ☹ | ☹ | ☹ | ☹ |
| 15. Aoun F | 2017 | ☹ | ☹ | ☹ | ☹ | ☹ |
| 16. Rydzewska LHM | 2017 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 17. Ramos-Esquivela A | 2016 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 18. Botrel TEA | 2016 | ☹ | ☺ | ☺ | ☺ | ☺ |
| 19. Hosseini SA | 2016 | ☹ | ☺ | ☺ | ☹ | ☹ |
| 20. Lei J | 2016 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 21. Tucci M | 2016 | ☺ | ☺ | ☹ | ☺ | ☹ |
| 22. Vale CL | 2016 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 23. Sciarra A | 2016 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 24. Kunath F | 2015 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 25. Zhu X | 2019 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 26. Zhu J | 2017 | ☺ | ☺ | ☺ | ☺ | ☺ |
| 27. Scailteux L | 2016 | ☺ | ☺ | ☺ | ☺ | ☺ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirisreetreerux, P.; Poprom, N.; Numthavaj, P.; Rattanasiri, S.; Thakkinstian, A. Efficacy of Treatment for Metastatic Hormone-Sensitive Prostate Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses. Cancers 2023, 15, 5714. https://doi.org/10.3390/cancers15245714
Sirisreetreerux P, Poprom N, Numthavaj P, Rattanasiri S, Thakkinstian A. Efficacy of Treatment for Metastatic Hormone-Sensitive Prostate Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses. Cancers. 2023; 15(24):5714. https://doi.org/10.3390/cancers15245714
Chicago/Turabian StyleSirisreetreerux, Pokket, Napaphat Poprom, Pawin Numthavaj, Sasivimol Rattanasiri, and Ammarin Thakkinstian. 2023. "Efficacy of Treatment for Metastatic Hormone-Sensitive Prostate Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses" Cancers 15, no. 24: 5714. https://doi.org/10.3390/cancers15245714
APA StyleSirisreetreerux, P., Poprom, N., Numthavaj, P., Rattanasiri, S., & Thakkinstian, A. (2023). Efficacy of Treatment for Metastatic Hormone-Sensitive Prostate Cancer: An Umbrella Review of Systematic Reviews and Meta-Analyses. Cancers, 15(24), 5714. https://doi.org/10.3390/cancers15245714





