Upfront or Deferred Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Triplet and Quadruplet Induction and Minimal Residual Disease/Risk-Adapted Therapy
Abstract
:Simple Summary
Abstract
1. Introduction
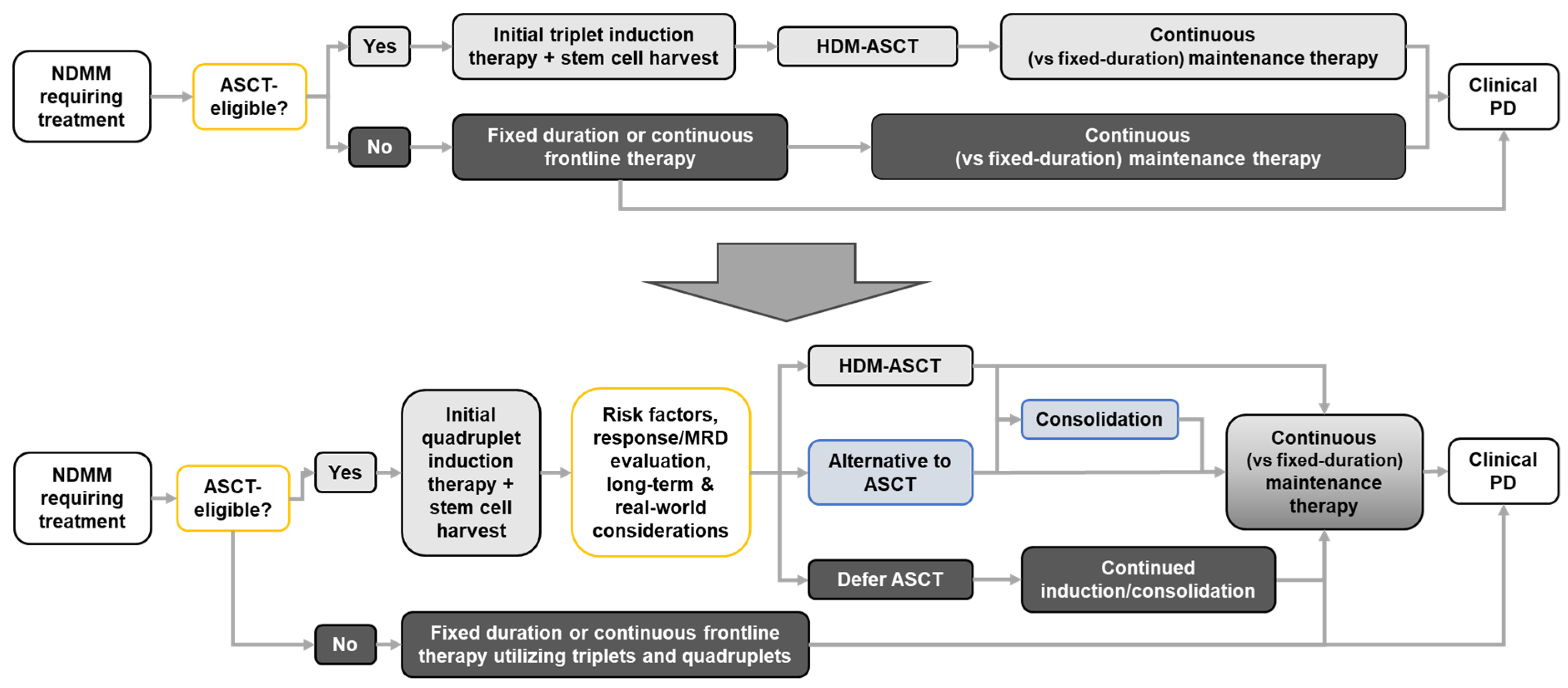
Current Treatment of Newly Diagnosed MM (NDMM) and the Role of HDM-ASCT
2. The Challenge of Comparing Upfront Versus Deferred HDM-ASCT
3. The Rationale for Deferring HDM-ASCT
3.1. Acute Adverse Impacts of High-Dose Melphalan
3.2. Long-Term Sequelae of HDM
3.3. Personalization of Treatment and Patient Preferences
3.4. Depth of Response and Rate of MRD Negativity with Quadruplet Induction
4. Discussion and Future Perspectives
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; KMiller, D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- NIH National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Myeloma. 2023. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 23 October 2023).
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International Staging System for Multiple Myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- van de Donk, N.W.C.; Pawlyn, C.; Yong, K.L. Multiple Myeloma. Lancet 2021, 397, 410–427. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric Assessment Predicts Survival and Toxicities in Elderly Myeloma Patients: An International Myeloma Working Group Report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Facon, T.; Dimopoulos, M.A.; Meuleman, N.; Belch, A.; Mohty, M.; Chen, W.M.; Kim, K.; Zamagni, E.; Rodriguez-Otero, P.; Renwick, W.; et al. A Simplified Frailty Scale Predicts Outcomes in Transplant-Ineligible Patients with Newly Diagnosed Multiple Myeloma Treated in the First (Mm-020) Trial. Leukemia 2020, 34, 224–233. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.; Terragna, C.; van Duin, M. Editorial: Risk Factors in Multiple Myeloma Identified before and During Treatment: Are We Ready to Personalize Treatment? Front. Oncol. 2023, 13, 1247808. [Google Scholar] [CrossRef]
- Bar, N.; Firestone, R.S.; Usmani, S.Z. Aiming for the Cure in Myeloma: Putting Our Best Foot Forward. Blood Rev. 2023, 101116. [Google Scholar] [CrossRef]
- Richardson, P.G.; Miguel, J.F.S.; Moreau, P.; Hajek, R.; Dimopoulos, M.A.; Laubach, J.P.; Palumbo, A.; Luptakova, K.; Romanus, D.; Skacel, T.; et al. Interpreting Clinical Trial Data in Multiple Myeloma: Translating Findings to the Real-World Setting. Blood Cancer J. 2018, 8, 109. [Google Scholar] [CrossRef]
- Joseph, N.S.; Kaufman, J.L.; Dhodapkar, M.V.; Hofmeister, C.C.; Almaula, D.K.; Heffner, L.T.; Gupta, V.A.; Boise, L.H.; Lonial, S.; Nooka, A.K. Long-Term Follow-up Results of Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy and Risk-Adapted Maintenance Approach in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2020, 38, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Callander, N.S.; Baljevic, M.; Adekola, K.; Anderson, L.D.; Campagnaro, E.; Castillo, J.J.; Costello, C.; Devarakonda, S.; Elsedawy, N.; Faiman, M.; et al. Nccn Guidelines(R) Insights: Multiple Myeloma, Version 3.2022. J. Natl. Compr. Cancer Netw. 2022, 20, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Baizer, L.; Callander, N.S.; Giralt, S.A.; Hillengass, J.; Freidlin, B.; Hoering, A.; Richardson, P.G.; Schwartz, E.I.; Reiman, A.; et al. Gaps and Opportunities in the Treatment of Relapsed-Refractory Multiple Myeloma: Consensus Recommendations of the Nci Multiple Myeloma Steering Committee. Blood Cancer J. 2022, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (Cartitude-1): A Phase 1b/2 Open-Label Study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2022, 387, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Lesokhin, A.M.; Tomasson, M.H.; Arnulf, B.; Bahlis, N.J.; Prince, H.M.; Niesvizky, R.; Rodriotaguez-Otero, P.; Martinez-Lopez, J.; Koehne, G.; Touzeau, C.; et al. Elranatamab in Relapsed or Refractory Multiple Myeloma: Phase 2 Magnetismm-3 Trial Results. Nat. Med. 2023, 29, 2259–2267. [Google Scholar] [CrossRef]
- Chari, A.; Minnema, M.C.; Berdeja, J.G.; Oriol, A.; van de Donk, N.; Rodriguez-Otero, P.; Askari, E.; Mateos, M.V.; Costa, L.J.; Caers, J.; et al. Talquetamab, a T-Cell-Redirecting Gprc5d Bispecific Antibody for Multiple Myeloma. N. Engl. J. Med. 2022, 387, 2232–2244. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple Myeloma: Eha-Esmo Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up(Dagger). Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Child, J.A.; Morgan, G.J.; Davies, F.E.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.T.; Selby, P.J.; Party Medical Research Council Adult Leukaemia Working. High-Dose Chemotherapy with Hematopoietic Stem-Cell Rescue for Multiple Myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef]
- Koreth, J.; Cutler, C.S.; Djulbegovic, B.; Behl, R.; Schlossman, R.L.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C.; Soiffer, R.J.; Alyea, E.P., 3rd. High-Dose Therapy with Single Autologous Transplantation Versus Chemotherapy for Newly Diagnosed Multiple Myeloma: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Biol. Blood Marrow Transpl. 2007, 13, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Cazaubiel, T.; Facon, T.; Caillot, D.; Clement-Filliatre, L.; Macro, M.; Decaux, O.; Belhadj, K.; Mohty, M.; et al. Early Versus Late Autologous Stem Cell Transplant in Newly Diagnosed Multiple Myeloma: Long-Term Follow-up Analysis of the Ifm 2009 Trial. Blood 2020, 136 (Suppl. S1), 39. [Google Scholar] [CrossRef]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Musto, P.; Rota-Scalabrini, D.; Bertamini, L.; Belotti, A.; Galli, M.; Offidani, M.; Zamagni, E.; Ledda, A.; Grasso, M.; et al. Carfilzomib with Cyclophosphamide and Dexamethasone or Lenalidomide and Dexamethasone Plus Autologous Transplantation or Carfilzomib Plus Lenalidomide and Dexamethasone, Followed by Maintenance with Carfilzomib Plus Lenalidomide or Lenalidomide Alone for Patients with Newly Diagnosed Multiple Myeloma (Forte): A Randomised, Open-Label, Phase 2 Trial. Lancet Oncol. 2021, 22, 1705–1720. [Google Scholar] [PubMed]
- Cavo, M.; Gay, F.; Beksac, M.; Pantani, L.; Petrucci, M.T.; Dimopoulos, M.A.; Dozza, L.; van der Holt, B.; Zweegman, S.; Oliva, S.; et al. Autologous Haematopoietic Stem-Cell Transplantation Versus Bortezomib-Melphalan-Prednisone, with or without Bortezomib-Lenalidomide-Dexamethasone Consolidation Therapy, and Lenalidomide Maintenance for Newly Diagnosed Multiple Myeloma (Emn02/Ho95): A Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Haematol. 2020, 7, e456–e468. [Google Scholar] [PubMed]
- Yong, K.; Wilson, W.; de Tute, R.M.; Camilleri, M.; Ramasamy, K.; Streetly, M.; Sive, J.; Bygrave, C.A.; Benjamin, R.; Chapman, M.; et al. Upfront Autologous Haematopoietic Stem-Cell Transplantation Versus Carfilzomib-Cyclophosphamide-Dexamethasone Consolidation with Carfilzomib Maintenance in Patients with Newly Diagnosed Multiple Myeloma in England and Wales (Cardamon): A Randomised, Phase 2, Non-Inferiority Trial. Lancet Haematol. 2023, 10, e93–e106. [Google Scholar] [PubMed]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Dholaria, B.R.; Schmidt, T.M.; Godby, K.N.; Silbermann, R.; Dhakal, B.; Bal, S.; Giri, S.; et al. Daratumumab, Carfilzomib, Lenalidomide, and Dexamethasone with Minimal Residual Disease Response-Adapted Therapy in Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2022, 40, 2901–2912. [Google Scholar] [CrossRef]
- Landgren, O.; Hultcrantz, M.; Diamond, B.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.A.; Lu, S.X.; Salcedo, M.; et al. Safety and Effectiveness of Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab Combination Therapy for Patients with Newly Diagnosed Multiple Myeloma: The Manhattan Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 862–868. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Bene, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, Thalidomide, and Dexamethasone with or without Daratumumab before and after Autologous Stem-Cell Transplantation for Newly Diagnosed Multiple Myeloma (Cassiopeia): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, Lenalidomide, Bortezomib, and Dexamethasone for Transplant-Eligible Newly Diagnosed Multiple Myeloma: The Griffin Trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Maura, F.; Weinhold, N.; Diamond, B.; Kazandjian, D.; Rasche, L.; Morgan, G.; Landgren, O. The Mutagenic Impact of Melphalan in Multiple Myeloma. Leukemia 2021, 35, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Samur, M.K.; Roncador, M.; Samur, A.A.; Fulciniti, M.; Bazarbachi, A.H.; Szalat, R.; Shammas, M.A.; Sperling, A.S.; Richardson, P.G.; Magrangeas, F.; et al. High-Dose Melphalan Treatment Significantly Increases Mutational Burden at Relapse in Multiple Myeloma. Blood 2023, 141, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Rustad, E.H.; Yellapantula, V.; Leongamornlert, D.; Bolli, N.; Ledergor, G.; Nadeu, F.; Angelopoulos, N.; Dawson, K.J.; Mitchell, T.J.; Osborne, R.J.; et al. Timing the Initiation of Multiple Myeloma. Nat. Commun. 2020, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Radivoyevitch, T.; Dean, R.M.; Shaw, B.E.; Brazauskas, R.; Tecca, H.R.; Molenaar, R.J.; Battiwalla, M.; Savani, B.N.; Flowers, M.E.D.; Cooke, K.R.; et al. Risk of Acute Myeloid Leukemia and Myelodysplastic Syndrome after Autotransplants for Lymphomas and Plasma Cell Myeloma. Leuk. Res. 2018, 74, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Straka, C.; Schaefer-Eckart, K.; Hertenstein, B.; Bassermann, F.; Salwender, H.; Langer, C.; Krönke, J.; Kull, M.; Schilling, G.; Schieferdecker, A.; et al. Long-Term Outcome of a Prospective Randomized Trial Comparing Continuous Lenalidomide/Dexamethasone with Lenalidomide/Dexamethasone Induction, Mel140 with Autologous Blood Stem Cell Transplantation and Single Agent Lenalidomide Maintenance in Patients of Age 60-75 Years with Newly Diagnosed Multiple Myeloma. Blood 2022, 140 (Suppl. S1), 287–288. [Google Scholar]
- Sive, J.; Cuthill, K.; Hunter, H.; Kazmi, M.; Pratt, G.; Smith, D.; British Society of Haematology. Guidelines on the Diagnosis, Investigation and Initial Treatment of Myeloma: A British Society for Haematology/UK Myeloma Forum Guideline. Br. J. Haematol. 2021, 193, 245–268. [Google Scholar] [CrossRef]
- Dhakal, B.; Shah, N.; Kansagra, A.; Kumar, A.; Lonial, S.; Garfall, A.; Cowan, A.; Poudyal, B.S.; Costello, C.; Gay, F.; et al. Astct Clinical Practice Recommendations for Transplantation and Cellular Therapies in Multiple Myeloma. Transpl. Cell Ther. 2022, 28, 284–293. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple Myeloma: 2022 Update on Diagnosis, Risk Stratification, and Management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- mSMART. Treatment Guidelines: Multiple Myeloma. 2023. Available online: https://www.msmart.org/mm-treatment-guidelines (accessed on 23 October 2023).
- Gandolfi, S.; Vekstein, C.; Laubach, J.P.; O’Brien, A.; Masone, K.; Munshi, N.C.; Anderson, K.C.; Richardson, P.G. The Evolving Role of Transplantation in Multiple Myeloma: The Need for a Heterogeneous Approach to a Heterogeneous Disease. Clin. Adv. Hematol. Oncol. 2018, 16, 564–574. [Google Scholar]
- Dunavin, N.C.; Wei, L.; Elder, P.; Phillips, G.S.; Benson, D.M., Jr.; Hofmeister, C.C.; Penza, S.; Greenfield, C.; Rose, K.S.; Rieser, G.; et al. Early Versus Delayed Autologous Stem Cell Transplant in Patients Receiving Novel Therapies for Multiple Myeloma. Leuk. Lymphoma 2013, 54, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Remenyi, P.; Varga, G.; Mikala, G.; Reti, M.; Gopcsa, L.; Batai, A.; Csukly, Z.; Lengyel, L.; Torbagyi, E.; Barta, A.; et al. Early Versus Delayed Autologous Stem Cell Transplantation and Interferon Maintenance in Multiple Myeloma: Single-Center Experience of 18 Years. Transpl. Proc. 2016, 48, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, C.; Muffly, L.S.; Iberri, D.J.; Craig, J.K.; Johnston, L.J.; Lowsky, R.; Shiraz, P.; Rezvani, A.R.; Frank, M.J.; Weng, W.K.; et al. Outcomes after Delayed and Second Autologous Stem Cell Transplant in Patients with Relapsed Multiple Myeloma. Bone Marrow Transpl. 2021, 56, 2664–2671. [Google Scholar] [CrossRef] [PubMed]
- Van Oekelen, O.; Nath, K.; Mouhieddine, T.H.; Farzana, T.; Aleman, A.; Melnekoff, D.T.; Ghodke-Puranik, Y.; Shah, G.L.; Lesokhin, A.; Giralt, S.; et al. Interventions and Outcomes of Patients with Multiple Myeloma Receiving Salvage Therapy after Bcma-Directed Car T Therapy. Blood 2023, 141, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.; Szabo, A.; Chhabra, S.; Hamadani, M.; D’Souza, A.; Usmani, S.Z.; Sieracki, R.; Gyawali, B.; Jackson, J.L.; Asimakopoulos, F.; et al. Autologous Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Novel Agent Induction: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 343–350. [Google Scholar] [CrossRef]
- Cho, Y.K.; Irby, D.J.; Li, J.; Sborov, D.W.; Mould, D.R.; Badawi, M.; Dauki, A.; Lamprecht, M.; Rosko, A.E.; Fernandez, S.; et al. Pharmacokinetic-Pharmacodynamic Model of Neutropenia in Patients with Myeloma Receiving High-Dose Melphalan for Autologous Stem Cell Transplant. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Gordillo, C.A.; Parmar, S.; Blanco, M.; Delille, E.M.; Assal, A.; Mapara, M.; Reshef, R. Gastrointestinal Toxicity of High-Dose Melphalan in Autologous Hematopoietic Stem Cell Transplantation: Identification of Risk Factors and a Benchmark for Experimental Therapies. Ann. Hematol. 2021, 100, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Cavallo, F.; Gay, F.; Di Raimondo, F.; Yehuda, D.B.; Petrucci, M.T.; Pezzatti, S.; Caravita, T.; Cerrato, C.; Ribakovsky, E.; et al. Autologous Transplantation and Maintenance Therapy in Multiple Myeloma. N. Engl. J. Med. 2014, 371, 895–905. [Google Scholar] [CrossRef]
- Gay, F.; Oliva, S.; Petrucci, M.T.; Conticello, C.; Catalano, L.; Corradini, P.; Siniscalchi, A.; Magarotto, V.; Pour, L.; Carella, A.; et al. Chemotherapy Plus Lenalidomide Versus Autologous Transplantation, Followed by Lenalidomide Plus Prednisone Versus Lenalidomide Maintenance, in Patients with Multiple Myeloma: A Randomised, Multicentre, Phase 3 Trial. Lancet Oncol. 2015, 16, 1617–1629. [Google Scholar] [CrossRef]
- Grazziutti, M.L.; Dong, L.; Miceli, M.H.; Krishna, S.G.; Kiwan, E.; Syed, N.; Fassas, A.; van Rhee, F.; Klaus, H.; Barlogie, B.; et al. Oral Mucositis in Myeloma Patients Undergoing Melphalan-Based Autologous Stem Cell Transplantation: Incidence, Risk Factors and a Severity Predictive Model. Bone Marrow Transpl. 2006, 38, 501–506. [Google Scholar] [CrossRef]
- Chakraborty, R.; Hamilton, B.K.; Hashmi, S.K.; Kumar, S.K.; Majhail, N.S. Health-Related Quality of Life after Autologous Stem Cell Transplantation for Multiple Myeloma. Biol. Blood Marrow Transpl. 2018, 24, 1546–1553. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Hebraud, B.; Hulin, C.; Perrot, A.; Caillot, D.; Stoppa, A.M.; Macro, M.; Escoffre, M.; Arnulf, B.; Belhadj, K.; et al. Health-Related Quality of Life Results from the Ifm 2009 Trial: Treatment with Lenalidomide, Bortezomib, and Dexamethasone in Transplant-Eligible Patients with Newly Diagnosed Multiple Myeloma. Leuk. Lymphoma 2020, 61, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Ziccheddu, B.; Biancon, G.; Bagnoli, F.; De Philippis, C.; Maura, F.; Rustad, E.H.; Dugo, M.; Devecchi, A.; De Cecco, L.; Sensi, M.; et al. Integrative Analysis of the Genomic and Transcriptomic Landscape of Double-Refractory Multiple Myeloma. Blood Adv. 2020, 4, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Landau, H.J.; Yellapantula, V.; Diamond, B.T.; Rustad, E.H.; Maclachlan, K.H.; Gundem, G.; Medina-Martinez, J.; Ossa, J.A.; Levine, M.F.; Zhou, Y.; et al. Accelerated Single Cell Seeding in Relapsed Multiple Myeloma. Nat. Commun. 2020, 11, 3617. [Google Scholar] [CrossRef] [PubMed]
- Misund, K.; Bruinink, D.H.O.; Coward, E.; Hoogenboezem, R.M.; Rustad, E.H.; Sanders, M.A.; Rye, M.; Sponaas, A.M.; van der Holt, B.; Zweegman, S.; et al. Clonal Evolution after Treatment Pressure in Multiple Myeloma: Heterogenous Genomic Aberrations and Transcriptomic Convergence. Leukemia 2022, 36, 1887–1897. [Google Scholar] [CrossRef]
- Ragon, B.K.; Shah, M.V.; D’Souza, A.; Estrada-Merly, N.; Gowda, L.; George, G.; DeLima, M.; Hashmi, S.; Kharfan-Dabaja, M.A.; Majhail, N.S.; et al. Impact of Second Primary Malignancy Post-Autologous Transplantation on Outcomes of Multiple Myeloma: A Cibmtr Analysis. Blood Adv. 2023, 7, 2746–2757. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Minnema, M.C.; Visser, O.; Levin, M.D.; Posthuma, E.; Broijl, A.; Sonneveld, P.; van der Klift, M.; Roeloffzen, W.W.H.; Westerman, M.; et al. Increased Mortality Risk in Multiple-Myeloma Patients with Subsequent Malignancies: A Population-Based Study in The Netherlands. Blood Cancer J. 2022, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Thibaud, S.; Mia, B.; Van Oekelen, O.; Mouhieddine, T.H.; Schaniel, C.; Ghodke-Puranik, Y.; Aleman, A.; Upadhyaya, B.; Lancman, G.; Lagana, A.; et al. Comprehensive Characterization of Prolonged Unexplained Cytopenias in Relapsed/Refractory Multiple Myeloma Patients Following Bcma-Directed Car-T Cell Therapy. Blood 2022, 140 (Suppl. S1), 614–616. [Google Scholar] [CrossRef]
- Arora, M.; Chen, Y.; Hageman, L.; Wu, J.; Landier, W.; Francisco, L.; Kung, M.; Ness, E.; Bosworth, A.; Pamukcuoglu, M.; et al. Morbidity Burden in Survivors of Multiple Myeloma Who Underwent Autologous Transplantation: A Bone Marrow Transplantation Survivor Study. Cancer 2020, 126, 3322–3329. [Google Scholar] [CrossRef]
- Chakraborty, R.; Yi, J.; Rybicki, L.; Preussler, J.; Deol, A.; Loren, A.; Savani, B.; Jim, H.S.L.; Cerny, J.; Reynolds, J.; et al. Patient-Reported Outcomes in Long-Term Survivors of Autologous Hematopoietic Cell Transplantation in Multiple Myeloma. Transpl. Cell Ther. 2023, 29, 388.e1–388.e6. [Google Scholar] [CrossRef]
- D’Souza, A.; Lonial, S. What the Princess Bride Teaches Us About Outcomes in Multiple Myeloma. J. Clin. Oncol. 2021, 39, 2423–2425. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, H.; Jacobus, S.J.; Richardson, P.G.; Zonder, J.A.; Voorhees, P.M.; Kaufman, J.L.; Yee, A.J.; Scott, E.C.; Torka, P.; Libby, E.; et al. Multivariable Analyses of Prognostic Factors for Progression-Free Survival (Pfs) and Complete Response (Cr) with Lenalidomide, Bortezomib, and Dexamethasone (Rvd) Alone Versus Rvd Plus Autologous Stem Cell Transplantation (Asct) in Patients (Pts) with Newly Diagnosed Multiple Myeloma (Ndmm) in the Determination Phase 3 Trial. Blood 2022, 140 (Suppl. S1), 4834–4838. [Google Scholar]
- Anderson, K.C.; Auclair, D.; Adam, S.J.; Agarwal, A.; Anderson, M.; Avet-Loiseau, H.; Bustoros, M.; Chapman, J.; Connors, D.E.; Dash, A.; et al. Minimal Residual Disease in Myeloma: Application for Clinical Care and New Drug Registration. Clin. Cancer Res. 2021, 27, 5195–5212. [Google Scholar] [CrossRef] [PubMed]
- Avet-Loiseau, H.; Ludwig, H.; Landgren, O.; Paiva, B.; Morris, C.; Yang, H.; Zhou, K.; Ro, S.; Mateos, M.V. Minimal Residual Disease Status as a Surrogate Endpoint for Progression-Free Survival in Newly Diagnosed Multiple Myeloma Studies: A Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e30–e37. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A Large Meta-Analysis Establishes the Role of Mrd Negativity in Long-Term Survival Outcomes in Patients with Multiple Myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Mohty, M.; Avet-Loiseau, H.; Harousseau, J.L. Potential Future Direction of Measurable Residual Disease Evaluation in Multiple Myeloma. Blood 2023, 142, 1509–1517. [Google Scholar] [CrossRef]
- Ficek, J.; Kalaitzaki, E.; Yuan, S.S.; Tosolini, A.; Du, L.; Kremer, B.E.; Davy, K.; Zhou, H.; Chen, T.T. Association of Minimal Residual Disease Negativity Rates with Progression Free Survival in Frontline Therapy Trials for Newly Diagnosed Multiple Myeloma: A Meta-Analysis. Clin. Lymphoma Myeloma Leuk. 2023, 23, e213–e221. [Google Scholar] [CrossRef]
- Coffey, D.G.; Maura, F.; Gonzalez-Kozlova, E.; Diaz-Mejia, J.J.; Luo, P.; Zhang, Y.; Xu, Y.; Warren, E.H.; Dawson, T.; Lee, B.; et al. Immunophenotypic Correlates of Sustained Mrd Negativity in Patients with Multiple Myeloma. Nat. Commun. 2023, 14, 5335. [Google Scholar] [CrossRef]
- Leypoldt, L.B.; Tichy, D.; Besemer, B.; Hanel, M.; Raab, M.S.; Mann, C.; Munder, M.; Reinhardt, H.C.; Nogai, A.; Gorner, M.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone for the Treatment of High-Risk Newly Diagnosed Multiple Myeloma. J. Clin. Oncol. 2023. Online ahead of print. [Google Scholar] [CrossRef]
- Belotti, A.; Ribolla, R.; Crippa, C.; Chiarini, M.; Giustini, V.; Ferrari, S.; Peli, A.; Cattaneo, C.; Roccaro, A.; Frittoli, B.; et al. Predictive Role of Sustained Imaging Mrd Negativity Assessed by Diffusion-Weighted Whole-Body Mri in Multiple Myeloma. Am. J. Hematol. 2023, 98, E230–E232. [Google Scholar] [CrossRef]
- Fonseca, R.; Arribas, M.; Wiedmeier-Nutor, J.E.; Kusne, Y.N.; Velez, M.G.; Kosiorek, H.E.; Butterfield, R.D.J.; Kirsch, I.R.; Mikhael, J.R.; Stewart, A.K.; et al. Integrated Analysis of Next Generation Sequencing Minimal Residual Disease (Mrd) and Pet Scan in Transplant Eligible Myeloma Patients. Blood Cancer J. 2023, 13, 32. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Genuardi, E.; Paris, L.; D’Agostino, M.; Rogers, J.; Rota-Scalabrini, D.; Jacob, A.P.; Patriarca, F.; Luppi, M.; Bertazzoni, P.; et al. Prospective Evaluation of Minimal Residual Disease in the Phase Ii Forte Trial: A Head-to-Head Comparison between Multiparameter Flow Cytometry and Next-Generation Sequencing. EClinicalMedicine 2023, 60, 102016. [Google Scholar] [CrossRef]
- Martinez-Lopez, J.; Wong, S.W.; Shah, N.; Bahri, N.; Zhou, K.; Sheng, Y.; Huang, C.Y.; Martin, T.; Wolf, J. Clinical Value of Measurable Residual Disease Testing for Assessing Depth, Duration, and Direction of Response in Multiple Myeloma. Blood Adv. 2020, 4, 3295–3301. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.J.; Chhabra, S.; Medvedova, E.; Schmidt, T.M.; Dholaria, B.; Godby, K.N.; Silbermann, R.; Bal, S.; D’Souza, A.; Giri, S.; et al. Outcomes of Mrd-Adapted Treatment Modulation in Patients with Newly Diagnosed Multiple Myeloma Receiving Daratumumab, Carfilzomib, Lenalidomide and Dexamethasone (Dara-Krd) and Autologous Transplantation: Extended Follow up of the Master Trial. Blood 2022, 140 (Suppl. S1), 7275–7277. [Google Scholar] [CrossRef]
- Sborov, D.W.; Laubach, J.; Kaufman, J.L.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; Nathwani, N.; et al. Daratumumab (Dara) + Lenalidomide, Bortezomib, and Dexamethasone (Rvd) in Patients with Transplant-Eligible Newly Diagnosed Multiple Myeloma (Ndmm): Final Analysis of Griffin. In Proceedings of the 19th International Myeloma Society (IMS) Annual Meeting (2022), Los Angeles, CA, USA, 25–27 August 2022. Abstract OAB-057. [Google Scholar]
- Goldschmidt, H.; Mai, E.K.; Bertsch, U.; Fenk, R.; Nievergall, E.; Tichy, D.; Besemer, B.; Durig, J.; Schroers, R.; von Metzler, I.; et al. Addition of Isatuximab to Lenalidomide, Bortezomib, and Dexamethasone as Induction Therapy for Newly Diagnosed, Transplantation-Eligible Patients with Multiple Myeloma (Gmmg-Hd7): Part 1 of an Open-Label, Multicentre, Randomised, Active-Controlled, Phase 3 Trial. Lancet Haematol. 2022, 9, e810–e821. [Google Scholar]
- Perrot, A.; Lauwers-Cances, V.; Touzeau, C.; Decaux, O.; Hulin, C.; Macro, M.; Stoppa, A.-M.; Chretien, M.L.; Karlin, L.; Mariette, C.; et al. Daratumumab Plus Ixazomib, Lenalidomide, and Dexamethasone as Extended Induction and Consolidation Followed by Lenalidomide Maintenance in Standard-Risk Transplant-Eligible Newly Diagnosed Multiple Myeloma (Ndmm) Patients (Ifm 2018-01): A Phase Ii Study of the Intergroupe Francophone Du Myélome (Ifm). Blood 2021, 138 (Suppl. S1), 464. [Google Scholar]
- Touzeau, C.; Perrot, A.; Hulin, C.; Manier, S.; Macro, M.; Caillot, D.; Karlin, L.; Decaux, O.; Jacquet, C.; Tiab, M.; et al. Daratumumab Carfilzomib Lenalidomide and Dexamethasone as Induction Therapy in High-Risk, Transplant-Eligible Patients with Newly Diagnosed Myeloma: Results of the Phase 2 Study Ifm 2018-04. J. Clin. Oncol. 2022, 40 (Suppl. S16), 8002. [Google Scholar] [CrossRef]
- Weisel, K.; Besemer, B.; Haenel, M.; Lutz, R.; Mann, C.; Munder, M.; Goerner, M.; Reinhardt, H.C.; Nogai, A.; Ko, Y.-D.; et al. Isatuximab, Carfilzomib, Lenalidomide, and Dexamethasone (Isa-Krd) in Patients with High-Risk Newly Diagnosed Multiple Myeloma: Planned Interim Analysis of the Gmmg-Concept Trial. Blood 2022, 140 (Suppl. S1), 1836–1838. [Google Scholar] [CrossRef]
- Derman, B.A.; Kansagra, A.; Zonder, J.; Stefka, A.T.; Grinblatt, D.L.; Anderson, L.D., Jr.; Gurbuxani, S.; Narula, S.; Rayani, S.; Major, A.; et al. Elotuzumab and Weekly Carfilzomib, Lenalidomide, and Dexamethasone in Patients with Newly Diagnosed Multiple Myeloma without Transplant Intent: A Phase 2 Measurable Residual Disease-Adapted Study. JAMA Oncol. 2022, 8, 1278–1286. [Google Scholar] [CrossRef]
- O’Donnell, E.K.; Mo, C.C.; Nadeem, O.; Yee, A.J.; Branagan, A.R.; Laubach, J.; Gammon, M.T.; Lively, K.J.; Packer, L.; Harrington, C.C.; et al. A Phase II Study of Once Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Isatuximab in Newly Diagnosed, Transplant-Eligible Multiple Myeloma (the Skylark Trial). Blood 2022, 140 (Suppl. S1), 7282–7283. [Google Scholar] [CrossRef]
- Boccadoro, M.; San-Miguel, J.; Suzuki, K.; Van De Donk, N.W.C.J.; Cook, G.; Jakubowiak, A.; Madduri, D.; Afifi, S.; Stevens, A.-S.; Schecter, J.M.; et al. Dvrd Followed by Ciltacabtagene Autoleucel Versus Dvrd Followed by Asct in Patients with Newly Diagnosed Multiple Myeloma Who Are Transplant Eligible: A Randomized Phase 3 Study (Emagine/Cartitude-6). Blood 2022, 140 (Suppl. S1), 4630–4632. [Google Scholar] [CrossRef]
| Publication | Year Published | Early HDM-ASCT | Deferred HDM-ASCT |
|---|---|---|---|
| EHA-ESMO Clinical Practice Guidelines [20] | 2021 | “For patients <70 years without comorbidities, induction therapy followed by HDM and ASCT is the recommended treatment” | Not included |
| BSH/Myeloma UK guidelines [38] | 2021 | “Recommended for younger, fitter patients” | “Lack of OS benefit … likely to be largely due to the use of delayed ASCT … supports the use of deferred ASCT as a clinical option… [the fact that] patients in the non-ASCT arm of the IFM 2009 study were unable to receive ASCT at relapse due to disease refractoriness reinforces the benefit of upfront ASCT where feasible” |
| ASTCT Clinical Practice Recommendations [39] | 2022 | “The panel recommends early autologous transplantation as a consolidation therapy in eligible, newly diagnosed myeloma patients after 4–6 cycles of induction” | “The panel recommends mobilization and storage of peripheral blood stem cells in newly diagnosed myeloma patients not undergoing autologous transplantation after first line of therapy for future use as a treatment at first relapse” |
| Rajkumar, update on diagnosis, risk-stratification and management [40] | 2022 | “ASCT should be considered in all eligible patients” | “In standard-risk patients responding well to therapy, ASCT can be delayed until first relapse provided stem cells are harvested early in the disease course” |
| mSMART guidelines [41] | 2023 | Preferred for standard-risk patients [t (11;14), t (6;14), trisomies], recommended for high-risk patients | An option for standard-risk patients |
| Study | Induction/Consolidation | MRD-Negative Rate | Outcomes |
|---|---|---|---|
| GMMG-HD7 [78] | Induction: 3 × Isa-RVd 6-week cycles | Post-induction: 50% | NR |
| IFM 2018-01 [79] | Induction: 6 × Dara-IRd (3-week cycles) ASCT Consolidation: 4 × Dara-IRd (4-week cycles) | 10−5/10−6 sensitivity Post-induction: 28%/6% Post-ASCT: 34%/29% Post-consolidation: 51%/40% | 2-year PFS: 95.2% |
| IFM 2018-04 [80] Patients with high-risk cytogenetics | Induction: 6 × Dara-KRd ASCT Consolidation: 4 × Dara-KRd (4-week cycles) | Post-induction: 62% (10−5) | 18-month PFS: 92% 18-month OS: 96% |
| GMMG-CONCEPT [81] High-risk MM | Induction: 6 (TE)/8 (TIE) × Isa-KRd ASCT (TE) Consolidation: 4 × Isa-KRd (4-week cycles) | Post-consolidation (10−5): TE: 68%; TIE: 54% | NR |
| CASSIOPEIA [31] | Induction: 4 × Dara-VTd ASCT Consolidation: 2 × Dara-VTd (4-week cycles) | 100 days post-ASCT (10−5): 64% | 18-month PFS: 93% |
| GRIFFIN [77] | Induction: 4 × Dara-RVd ASCT Consolidation: 2 × Dara-RVd Maintenance: DR | 10−5/10−6 sensitivity Post-induction: 22%/1% Post-consolidation: 50%/11% Post-1-year-maintenance: 59%/21% End of study: 64%/36% | 4-year PFS: 87.2% 4-year OS: 92.7% |
| MANHATTAN [30] | Induction: 8 × Dara-KRd (4-week cycles) No ASCT | 10−5: 71% | 1-year PFS: 98% 1-year OS: 100% |
| MASTER [76] | Induction: 4 × Dara-KRd (4-week cycles) ASCT Consolidation: 0, 4, or 8 × Dara-KRd | Post-consolidation (10−5/10−6): 81%/71% | 0/1/2 HRCA 3-year PFS: 91%/87%/51% 3-year OS: 96%/91%/75% |
| NCT02969837 [82] | Induction: 12—24 × Elo-KRd (4-week cycles) No ASCT | 10−5/10−6 sensitivity After 8 cycles: 63%/51% Best response: 70%/60% | 3-year PFS: 72% 3-year OS: 78% |
| SKylaRk [83] | Induction: 4 × Isa-KRd (4-week cycles) Optional ASCT If ASCT deferred: 4 × Isa-KRd (4-week cycles) | 10−5/10−6 sensitivity Post-cycle 4 (n = 28): 43%/32% | 1-year PFS: 97.9% 1-year OS: 97.9% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mo, C.C.; Hartley-Brown, M.A.; Midha, S.; Richardson, P.G. Upfront or Deferred Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Triplet and Quadruplet Induction and Minimal Residual Disease/Risk-Adapted Therapy. Cancers 2023, 15, 5709. https://doi.org/10.3390/cancers15245709
Mo CC, Hartley-Brown MA, Midha S, Richardson PG. Upfront or Deferred Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Triplet and Quadruplet Induction and Minimal Residual Disease/Risk-Adapted Therapy. Cancers. 2023; 15(24):5709. https://doi.org/10.3390/cancers15245709
Chicago/Turabian StyleMo, Clifton C., Monique A. Hartley-Brown, Shonali Midha, and Paul G. Richardson. 2023. "Upfront or Deferred Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Triplet and Quadruplet Induction and Minimal Residual Disease/Risk-Adapted Therapy" Cancers 15, no. 24: 5709. https://doi.org/10.3390/cancers15245709
APA StyleMo, C. C., Hartley-Brown, M. A., Midha, S., & Richardson, P. G. (2023). Upfront or Deferred Autologous Stem Cell Transplantation for Newly Diagnosed Multiple Myeloma in the Era of Triplet and Quadruplet Induction and Minimal Residual Disease/Risk-Adapted Therapy. Cancers, 15(24), 5709. https://doi.org/10.3390/cancers15245709






