Magnetic Resonance Imaging Features Associated with a High and Low Expression of Tumor-Infiltrating Lymphocytes: A Stratified Analysis According to Molecular Subtypes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Acquisition
2.3. MRI Interpretation
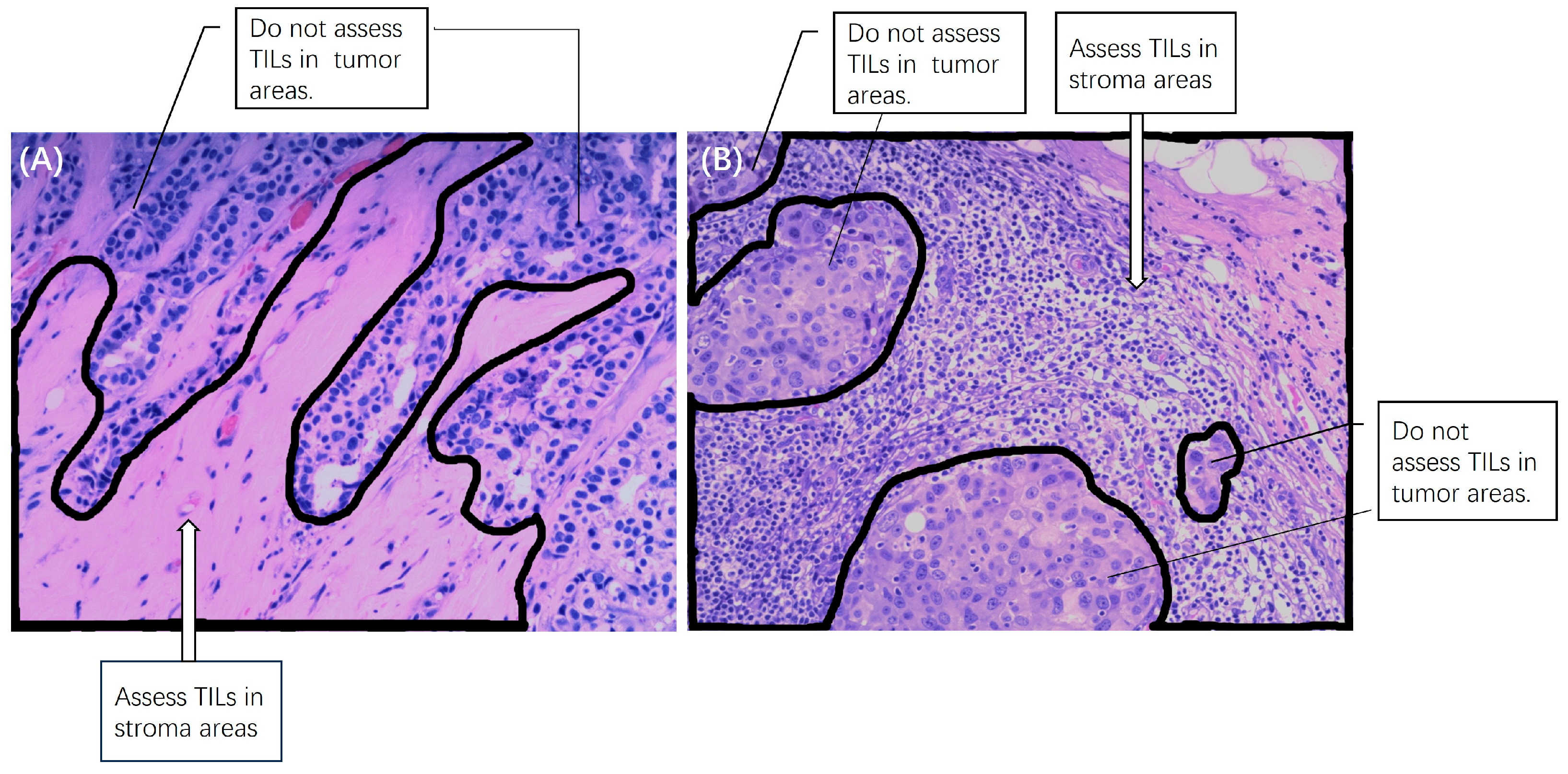
2.4. Pathology
2.5. Statistical Analysis
3. Results
3.1. Patients, Subtypes, and TILs Levels
3.2. Comparison of MRI Features among the Three Subtypes
3.3. Comparison of MRI Features between Cases with Low and High TILs in Each Subtype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the Incidence and Magnitude of Tumor-Infiltrating Lymphocytes in Breast Cancer Subtypes: A Systematic Review. JAMA Oncol. 2016, 2, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Luen, S.J.; Savas, P.; Fox, S.B.; Salgado, R.; Loi, S. Tumour-infiltrating lymphocytes and the emerging role of immunotherapy in breast cancer. Pathology 2017, 49, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Pruneri, G.; Vingiani, A.; Denkert, C. Tumor infiltrating lymphocytes in early breast cancer. Breast 2018, 37, 207–214. [Google Scholar] [CrossRef]
- Ravelli, A.; Roviello, G.; Cretella, D.; Cavazzoni, A.; Biondi, A.; Cappelletti, M.R.; Zanotti, L.; Ferrero, G.; Ungari, M.; Zanconati, F.; et al. Tumor-infiltrating lymphocytes and breast cancer: Beyond the prognostic and predictive utility. Tumor Biol. 2017, 39, 1010428317695023. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Drubay, D.; Adams, S.; Pruneri, G.; Francis, P.A.; Lacroix-Triki, M.; Joensuu, H.; Dieci, M.V.; Badve, S.; Demaria, S.; et al. Tumor-Infiltrating Lymphocytes and Prognosis A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J. Clin. Oncol. 2019, 37, 559–569. [Google Scholar] [CrossRef]
- Salgado, R.; Loi, S. Tumour infiltrating lymphocytes in breast cancer: Increasing clinical relevance. Lancet Oncol. 2018, 19, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Dieci, M.V.; Radosevic-Robin, N.; Fineberg, S.; van den Eynden, G.; Ternes, N.; Penault-Llorca, F.; Pruneri, G.; D’Alfonso, T.M.; Demaria, S.; Castaneda, C.; et al. Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin. Cancer Biol. 2018, 52, 16–25. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Huang, J.; Chen, X.; Fei, X.; Huang, O.; Wu, J.; Zhu, L.; He, J.; Chen, W.; Li, Y.; Shen, K. Changes of Tumor Infiltrating Lymphocytes after Core Needle Biopsy and the Prognostic Implications in Early Stage Breast Cancer: A Retrospective Study. Cancer Res. Treat. 2019, 51, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Amgad, M.; Sarkar, A.; Srinivas, C.; Redman, R.; Ratra, S.; Bechert, C.J.; Calhoun, B.C.; Mrazeck, K.; Kurkure, U.; Cooper, L.A.; et al. Joint Region and Nucleus Segmentation for Characterization of Tumor Infiltrating Lymphocytes in Breast Cancer. Proc. SPIE Int. Soc. Opt. Eng. 2019, 10956, 129–136. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, X.; Tang, K.; Zhao, Y.; Lu, Z.; Feng, Q. DDTNet: A dense dual-task network for tumor-infiltrating lymphocyte detection and segmentation in histopathological images of breast cancer. Med. Image Anal. 2022, 78, 102415. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.M.; Cho, N.; Moy, L. Breast MRI: State of the Art. Radiology 2019, 292, 520–536. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.A.; Helbich, T.; Baltzer, P.; Pinker-Domenig, K. Multiparametric MRI of the breast: A review. J. Magn. Reson. Imaging 2018, 47, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Pinker, K.; Helbich, T.H.; Morris, E.A. The potential of multiparametric MRI of the breast. Br. J. Radiol. 2017, 90, 20160715. [Google Scholar] [CrossRef]
- Du, S.; Gao, S.; Zhang, L.; Yang, X.; Qi, X.; Li, S. Improved discrimination of molecular subtypes in invasive breast cancer: Comparison of multiple quantitative parameters from breast MRI. Magn. Reson. Imaging 2021, 77, 148–158. [Google Scholar] [CrossRef]
- Shin, S.U.; Cho, N.; Lee, H.B.; Kim, S.Y.; Yi, A.; Kim, S.Y.; Lee, S.H.; Chang, J.M.; Moon, W.K. Neoadjuvant Chemotherapy and Surgery for Breast Cancer: Preoperative MRI Features Associated with Local Recurrence. Radiology 2018, 289, 30–38. [Google Scholar] [CrossRef]
- Gu, Y.L.; Pan, S.M.; Ren, J.; Yang, Z.X.; Jiang, G.Q. Role of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: A Meta-analysis. Clin. Breast Cancer 2017, 17, 245–255. [Google Scholar] [CrossRef]
- Fogante, M.; Tagliati, C.; De Lisa, M.; Berardi, R.; Giuseppetti, G.M.; Giovagnoni, A. Correlation between apparent diffusion coefficient of magnetic resonance imaging and tumor-infiltrating lymphocytes in breast cancer. Radiol. Med. 2019, 124, 581–587. [Google Scholar] [CrossRef]
- Ku, Y.J.; Kim, H.H.; Cha, J.H.; Shin, H.J.; Baek, S.H.; Lee, H.J.; Gong, G. Correlation Between MRI and the Level of Tumor-Infiltrating Lymphocytes in Patients With Triple-Negative Breast Cancer. AJR Am. J. Roentgenol. 2016, 207, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Kim, Y.; Cha, J.H.; Shin, H.J.; Chae, E.Y.; Yoon, G.Y.; Kim, H.H. Correlation between magnetic resonance imaging and the level of tumor-infiltrating lymphocytes in patients with estrogen receptor-negative HER2-positive breast cancer. Acta Radiol. 2020, 61, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Wu, Z.; Lin, Q.; Mao, Y.; Wang, H.; Chen, J.; Chen, Q.; Fu, G.; Cui, C.; Su, X. Evaluating Tumor-Infiltrating Lymphocytes in Breast Cancer Using Preoperative MRI-Based Radiomics. J. Magn. Reson. Imaging 2022, 55, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Su, G.H.; Xiao, Y.; Jiang, L.; Zheng, R.C.; Wang, H.; Chen, Y.; Gu, Y.J.; You, C.; Shao, Z.M. Radiomics features for assessing tumor-infiltrating lymphocytes correlate with molecular traits of triple-negative breast cancer. J. Transl. Med. 2022, 20, 471. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhou, J.; He, X.; Ye, S.; Miao, H.; Liu, H.; Chen, Z.; Zhao, Y.; Pan, Z.; Wang, M. Radiomics Model for Evaluating the Level of Tumor-Infiltrating Lymphocytes in Breast Cancer Based on Dynamic Contrast-Enhanced MRI. Clin. Breast Cancer 2021, 21, 440–449.e441. [Google Scholar] [CrossRef]
- Kwon, M.J. Emerging immune gene signatures as prognostic or predictive biomarkers in breast cancer. Arch. Pharm. Res. 2019, 42, 947–961. [Google Scholar] [CrossRef]
- Denkert, C.; von Minckwitz, G.; Darb-Esfahani, S.; Lederer, B.; Heppner, B.I.; Weber, K.E.; Budczies, J.; Huober, J.; Klauschen, F.; Furlanetto, J.; et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018, 19, 40–50. [Google Scholar] [CrossRef]
- Negrao, E.M.S.; Souza, J.A.; Marques, E.F.; Bitencourt, A.G.V. Breast cancer phenotype influences MRI response evaluation after neoadjuvant chemotherapy. Eur. J. Radiol. 2019, 120, 108701. [Google Scholar] [CrossRef]
- Tang, W.J.; Jin, Z.; Zhang, Y.L.; Liang, Y.S.; Cheng, Z.X.; Chen, L.X.; Liang, Y.Y.; Wei, X.H.; Kong, Q.C.; Guo, Y.; et al. Whole-Lesion Histogram Analysis of the Apparent Diffusion Coefficient as a Quantitative Imaging Biomarker for Assessing the Level of Tumor-Infiltrating Lymphocytes: Value in Molecular Subtypes of Breast Cancer. Front. Oncol. 2020, 10, 611571. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.E.; Jeong, W.G.; Ki, S.Y.; Park, M.H.; Lee, J.S.; Nah, Y.K.; Lim, H.S. HER2-Positive Breast Cancer: Association of MRI and Clinicopathologic Features with Tumor-Infiltrating Lymphocytes. AJR Am. J. Roentgenol. 2022, 218, 258–269. [Google Scholar] [CrossRef]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef]
- Nelson, M.A.; Ngamcherdtrakul, W.; Luoh, S.W.; Yantasee, W. Prognostic and therapeutic role of tumor-infiltrating lymphocyte subtypes in breast cancer. Cancer Metastasis Rev. 2021, 40, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.Y.; Hui, S.W.; Ni, Y.B.; Chan, S.K.; Yamaguchi, R.; Kwong, A.; Law, B.K.; Tse, G.M. Lymphocytic infiltrate is associated with favorable biomarkers profile in HER2-overexpressing breast cancers and adverse biomarker profile in ER-positive breast cancers. Breast Cancer Res. Treat. 2014, 143, 1–9. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, H.J.; Lee, S.B.; Ahn, J.H.; Kim, J.E.; Jung, K.H.; Gong, G.; Son, B.H.; Ahn, S.H.; Kim, S.B. Intrinsic Prognostic Impact of Tumor-infiltrating Lymphocytes in Systemically Untreated Patients With Early-stage Triple-negative Breast Cancer. Anticancer Res. 2019, 39, 3111–3119. [Google Scholar] [CrossRef]
- Yuan, C.; Jin, F.; Guo, X.; Zhao, S.; Li, W.; Guo, H. Correlation Analysis of Breast Cancer DWI Combined with DCE-MRI Imaging Features with Molecular Subtypes and Prognostic Factors. J. Med. Syst. 2019, 43, 83. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, Y.; Burnside, E.S.; Huang, E.; Drukker, K.; Hoadley, K.A.; Fan, C.; Conzen, S.D.; Zuley, M.; Net, J.M.; et al. Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer 2016, 2, 16012. [Google Scholar] [CrossRef]
- Panzironi, G.; Moffa, G.; Galati, F.; Marzocca, F.; Rizzo, V.; Pediconi, F. Peritumoral edema as a biomarker of the aggressiveness of breast cancer: Results of a retrospective study on a 3 T scanner. Breast Cancer Res. Treat. 2020, 181, 53–60. [Google Scholar] [CrossRef]
- Koh, J.; Park, A.Y.; Ko, K.H.; Jung, H.K. Can enhancement types on preoperative MRI reflect prognostic factors and surgical outcomes in invasive breast cancer? Eur. Radiol. 2019, 29, 7000–7008. [Google Scholar] [CrossRef]
- Wu, J.; Li, X.; Teng, X.; Rubin, D.L.; Napel, S.; Daniel, B.L.; Li, R. Magnetic resonance imaging and molecular features associated with tumor-infiltrating lymphocytes in breast cancer. Breast Cancer Res. 2018, 20, 101. [Google Scholar] [CrossRef]
- Chen, S.; Sui, Y.; Ding, S.; Chen, C.; Liu, C.; Zhong, Z.; Liang, Y.; Kong, Q.; Tang, W.; Guo, Y. A simple and convenient model combining multiparametric MRI and clinical features to predict tumour-infiltrating lymphocytes in breast cancer. Clin. Radiol. 2023, 78, e1065–e1074. [Google Scholar] [CrossRef] [PubMed]

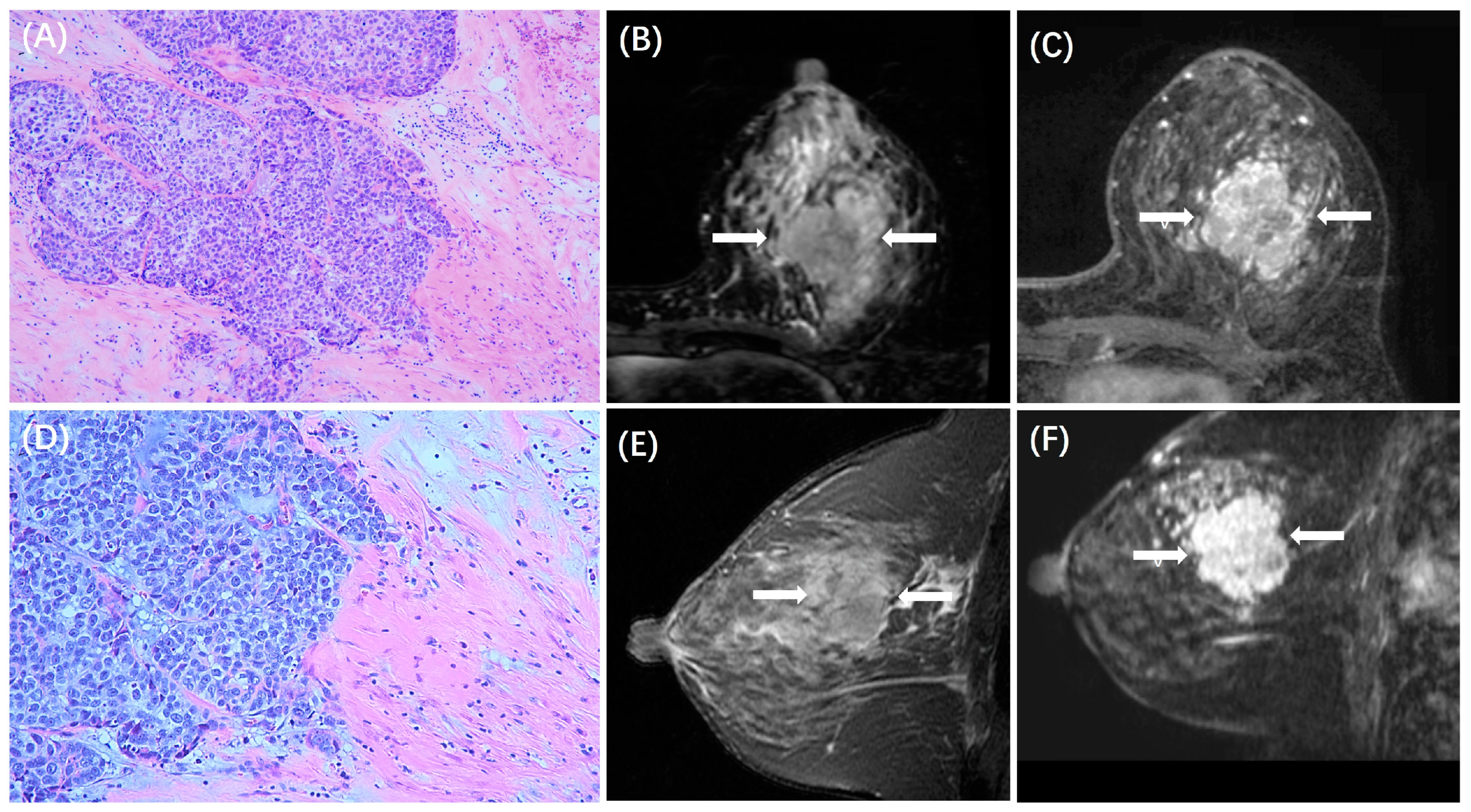
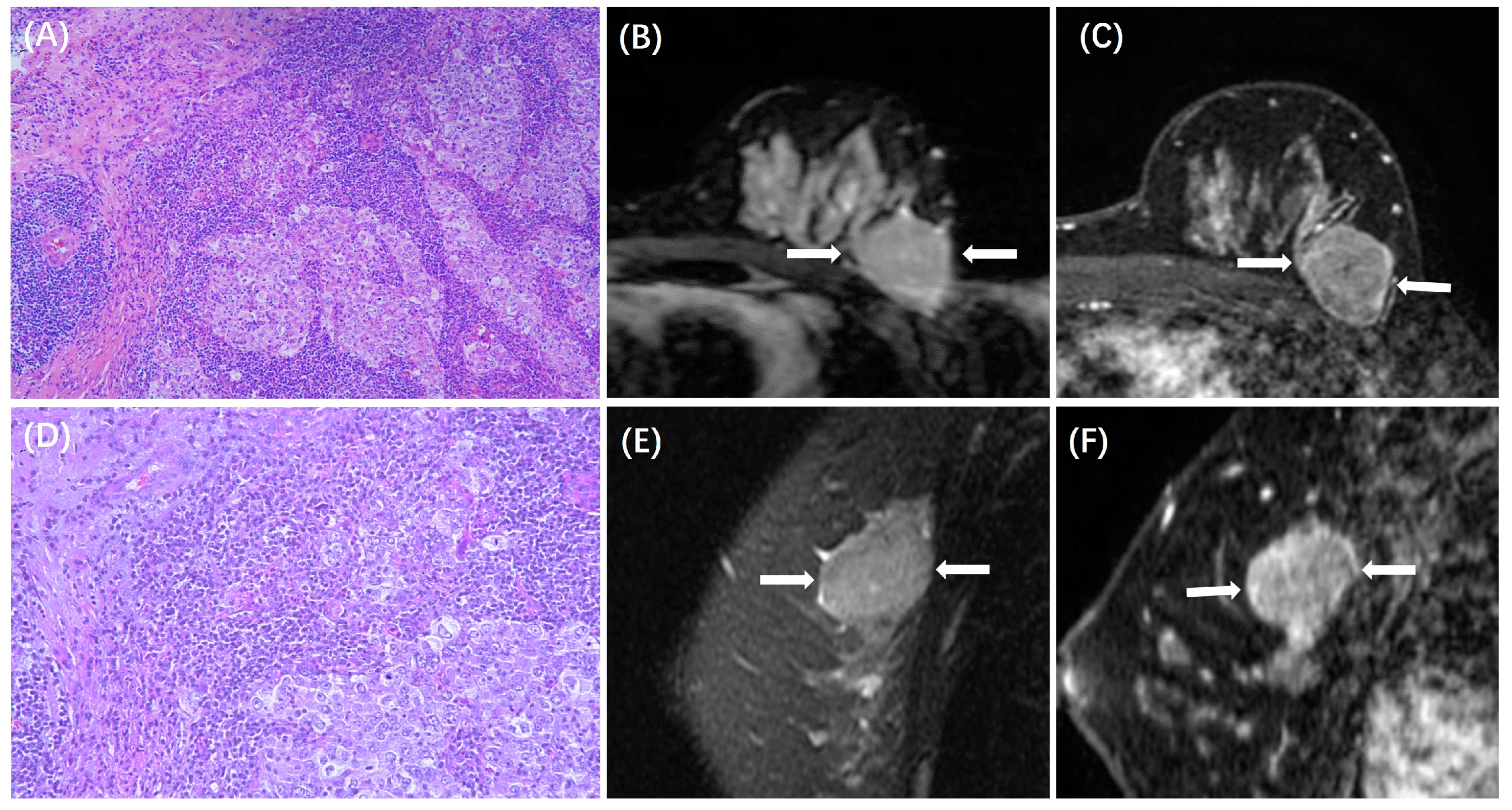
| Low TILs (n = 327) | High TILs (n = 130) | p | |
|---|---|---|---|
| Mean Age, years | 50.5 ± 9.9 | 50.7 ± 8.9 | 0.876 |
| Age, years | 0.023 | ||
| >50 | 177 (54%) | 55 (42%) | |
| ≤50 | 150 (46%) | 75 (58%) | |
| Postmenopausal | 0.249 | ||
| >Yes | 154 (47%) | 69 (53%) | |
| >No | 173 (53%) | 61 (47%) | |
| Histological grade | <0.001 | ||
| 1 | 36 (11%) | 1 (1%) | |
| 2 | 223 (68%) | 88 (68%) | |
| 3 | 68 (21%) | 41 (31%) | |
| Clinical T stage | 0.061 | ||
| 1 | 202 (62%) | 64 (49%) | |
| 2 | 111 (34%) | 60 (46%) | |
| 3 | 9 (3%) | 5 (4%) | |
| 4 | 5 (1%) | 1 (1%) | |
| N stage | 0.036 | ||
| N0 | 193 (59%) | 68 (52%) | |
| N1 | 90 (27%) | 32 (25%) | |
| ≥N2 | 44 (14%) | 20 (23%) |
| HR+/HER2− (n = 241) | HER2+ (n = 134) | TN (n = 82) | p | |
|---|---|---|---|---|
| TILs | <0.001 | |||
| Low (<10%) | 197 (82%) | 84 (63%) | 46 (56%) | |
| High (>10%) | 44 (18%) | 50 (37%) | 36 (44%) | |
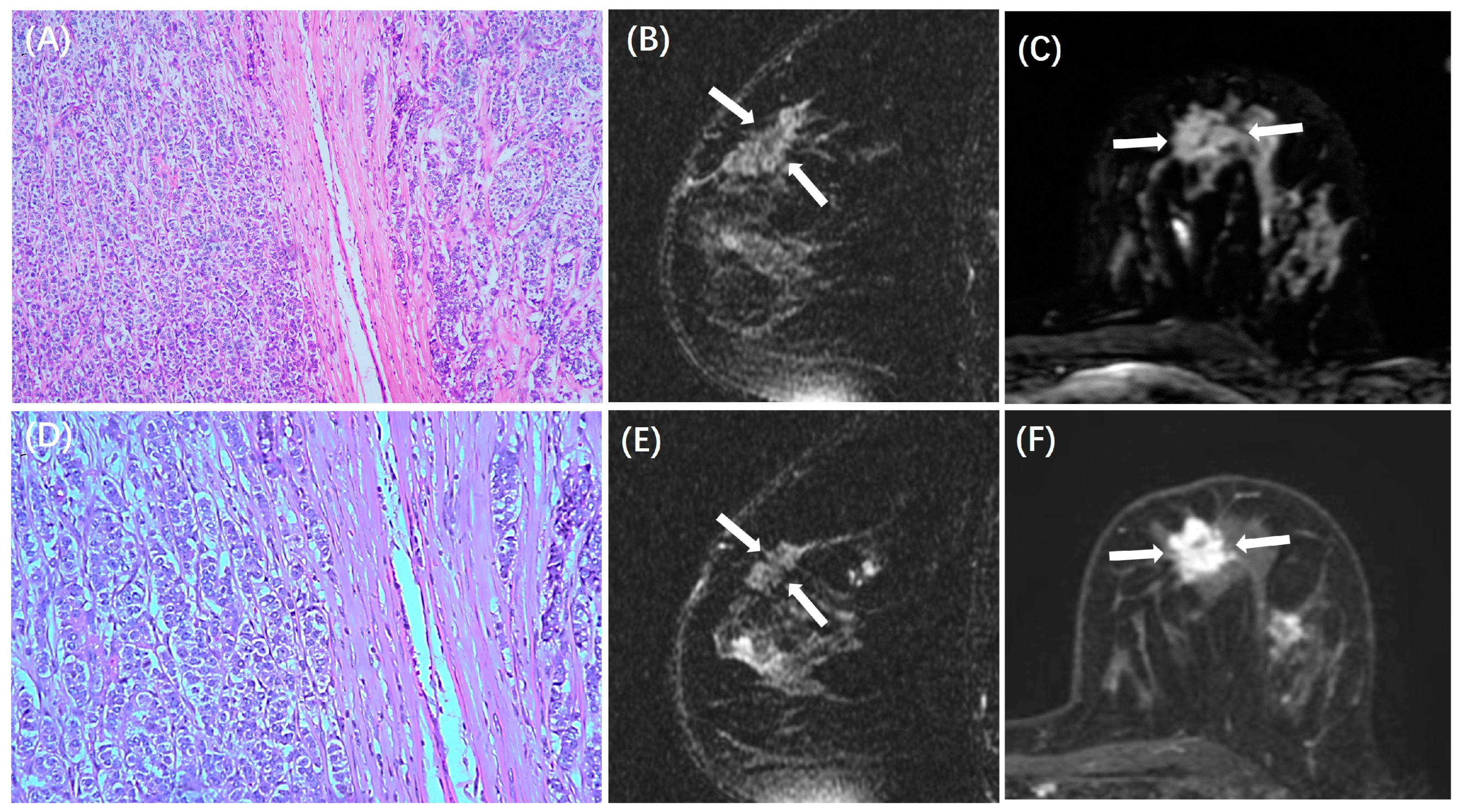
| MRI Tumor size (cm) | 1.7 ± 1.3 | 2.4 ± 1.5 | 2.2 ± 1.2 | <0.001 |
| ADC (×10–3 mm2/s) | 0.90 ± 0.22 | 0.95 ± 0.19 | 0.93 ± 0.19 | 0.091 |
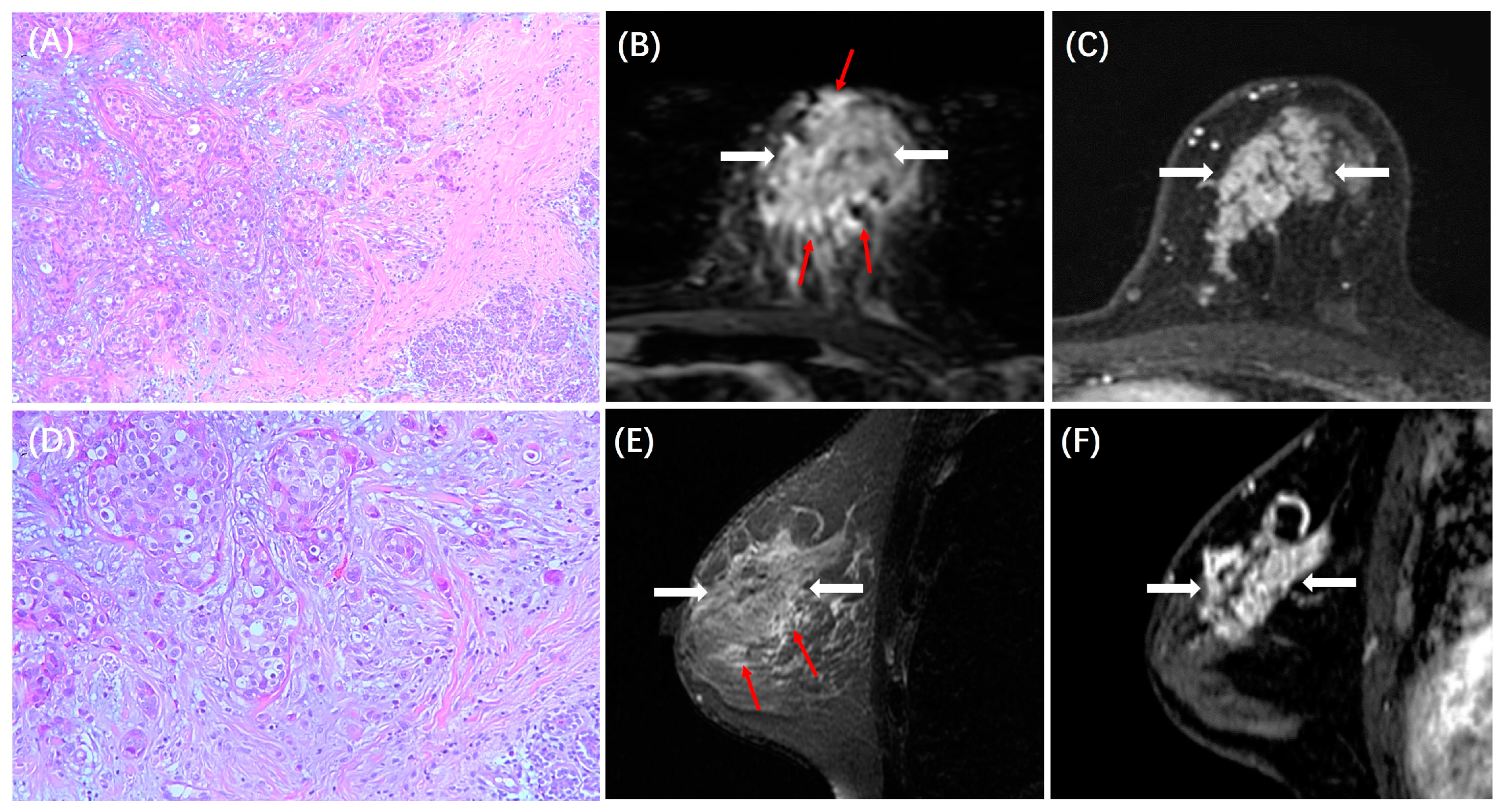
| Lesion Morphology | 0.031 | |||
| NME | 66 (27%) | 52 (39%) | 20 (24%) | |
| Mass | 175 (73%) | 82 (61%) | 62 (76%) | |
| Shape | 0.247 | |||
| Regular | 63 (26%) | 25 (19%) | 18 (22%) | |
| Irregular | 178 (74%) | 109 (81%) | 64 (78%) | |
| Margin | 0.192 | |||
| Circumscribed | 18 (7%) | 5 (4%) | 8 (10%) | |
| Non-circumscribed | 223 (93%) | 129 (96%) | 74 (90%) | |
| Internal Enhancement | <0.001 | |||
| Homogeneous | 35 (15%) | 4 (3%) | 2 (2%) | |
| Heterogeneous | 206 (85%) | 130 (97%) | 80 (98%) | |
| DCE Kinetic Pattern | 0.283 | |||
| Wash-in | 1 (0) | 0 (0) | 0 (0) | |
| Platform | 50 (21%) | 17 (13%) | 13 (16%) | |
| Wash-out | 190 (79%) | 117 (87%) | 69 (84%) | |
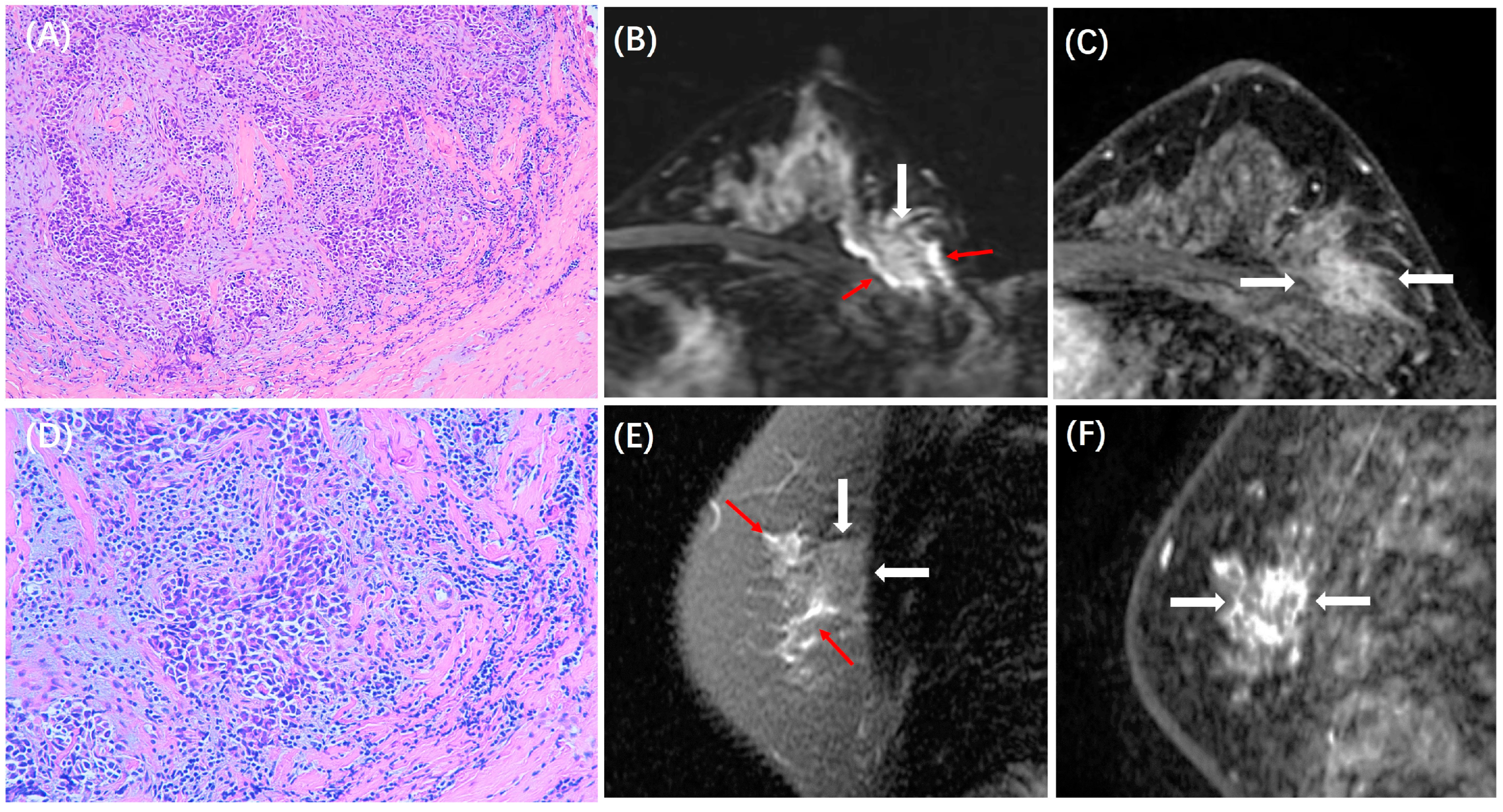
| Peritumoral Edema | <0.001 | |||
| Present | 109 (45%) | 95 (71%) | 66 (80%) | |
| Absent | 132 (55%) | 39 (29%) | 16 (20%) |
| HR+/HER2− Subtype (n = 241) | HER2+ Subtype (n = 134) | TN subtype (n = 82) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Low TILs (n = 197) | High TILs (n = 44) | p | Low TILs (n = 84) | High TILs (n = 50) | p | Low TILs (n = 46) | High TILs (n = 36) | p | |
| Largest size on MRI (cm) | 1.8 ± 1.3 | 2.1 ± 1.5 | 0.052 | 2.7 ± 2.6 | 2.6 ± 1.5 | 0.622 | 2.3 ± 1.1 | 2.4 ± 1.1 | 0.450 |
| ADC (×10–3 mm2/s) | 0.93 ± 0.18 | 0.84 ± 0.14 | <0.001 | 0.94 ± 0.16 | 0.97 ± 0.16 | 0.268 | 0.97 ± 0.14 | 0.92 ± 0.16 | 0.095 |
| Lesion Morphology Type | 0.271 | 0.883 | 0.910 | ||||||
| NME | 51 (26%) | 15 (34%) | 33 (39%) | 19 (38%) | 11 (24%) | 9 (25%) | |||
| Mass | 146 (74%) | 29 (66%) | 51 (61%) | 31 (62%) | 35 (76%) | 27 (75%) | |||
| Shape | 0.571 | 0.128 | 0.029 | ||||||
| Regular (Round/Oval) | 50 (25%) | 13 (30%) | 19 (23%) | 6 (12%) | 6 (13%) | 12 (33%) | |||
| Irregular | 147 (75%) | 31 (70%) | 65 (77%) | 44 (88%) | 40 (87%) | 24 (67%) | |||
| Margin | 0.856 | 0.900 | 0.009 | ||||||
| Circumscribed | 15 (8%) | 3 (7%) | 3 (4%) | 2 (4%) | 1 (2%) | 7 (19%) | |||
| Non-circumscribed | 182 (92%) | 31 (93%) | 81 (96%) | 48 (96%) | 45 (98%) | 29 (81%) | |||
| Internal Enhancements | 0.218 | 0.600 | 0.108 | ||||||
| Homogeneous | 26 (13%) | 9 (20%) | 2 (2%) | 2 (4%) | 0 (0%) | 2 (6%) | |||
| Heterogeneous | 171 (87%) | 35 (80%) | 82 (98%) | 48 (96%) | 46 (100%) | 34 (94%) | |||
| DCE Kinetic Pattern | 0.601 | 0.854 | 0.859 | ||||||
| Wash-in | 1 (0) | 0 (0) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| Platform | 43 (22%) | 7 (16%) | 11 (13%) | 6 (12%) | 7 (15%) | 6 (17%) | |||
| Wash-out | 153 (78%) | 37 (84%) | 73 (87%) | 44 (88%) | 39 (85%) | 30 (83%) | |||
| Peritumoral Edema | <0.001 | 0.164 | 0.586 | ||||||
| Present | 78 (40%) | 31(70%) | 56 (67%) | 39 (78%) | 38 (83%) | 28 (78%) | |||
| Absent | 119 (60%) | 13 (30%) | 28 (33%) | 11 (22%) | 8 (17%) | 8 (22%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Jin, Y.; Miao, H.; Lu, S.; Liu, X.; He, Y.; Liu, H.; Zhao, Y.; Zhang, Y.; Liu, Y.-L.; et al. Magnetic Resonance Imaging Features Associated with a High and Low Expression of Tumor-Infiltrating Lymphocytes: A Stratified Analysis According to Molecular Subtypes. Cancers 2023, 15, 5672. https://doi.org/10.3390/cancers15235672
Zhou J, Jin Y, Miao H, Lu S, Liu X, He Y, Liu H, Zhao Y, Zhang Y, Liu Y-L, et al. Magnetic Resonance Imaging Features Associated with a High and Low Expression of Tumor-Infiltrating Lymphocytes: A Stratified Analysis According to Molecular Subtypes. Cancers. 2023; 15(23):5672. https://doi.org/10.3390/cancers15235672
Chicago/Turabian StyleZhou, Jiejie, Yi Jin, Haiwei Miao, Shanshan Lu, Xinmiao Liu, Yun He, Huiru Liu, Youfan Zhao, Yang Zhang, Yan-Lin Liu, and et al. 2023. "Magnetic Resonance Imaging Features Associated with a High and Low Expression of Tumor-Infiltrating Lymphocytes: A Stratified Analysis According to Molecular Subtypes" Cancers 15, no. 23: 5672. https://doi.org/10.3390/cancers15235672
APA StyleZhou, J., Jin, Y., Miao, H., Lu, S., Liu, X., He, Y., Liu, H., Zhao, Y., Zhang, Y., Liu, Y.-L., Pan, Z., Chen, J.-H., Wang, M., & Su, M.-Y. (2023). Magnetic Resonance Imaging Features Associated with a High and Low Expression of Tumor-Infiltrating Lymphocytes: A Stratified Analysis According to Molecular Subtypes. Cancers, 15(23), 5672. https://doi.org/10.3390/cancers15235672






