Double Hydroxyl Salt as Smart Biocompatible pH-Responsive Carrier for 6-Mercaptopurine
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HDS Synthesis
2.3. Mercaptopurine Sorption
2.4. Characterization of HDS and Modified HDS Materials
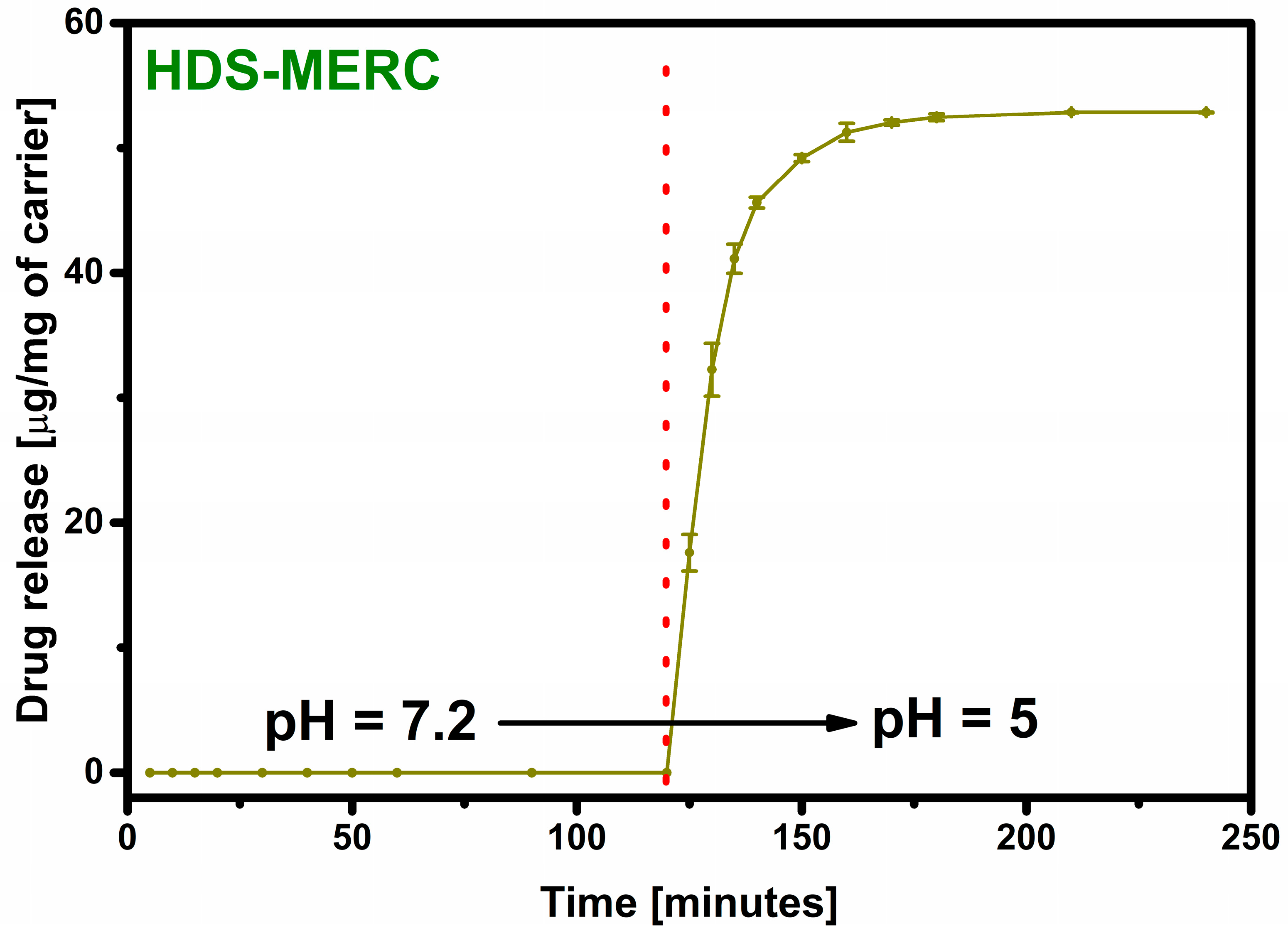
2.5. Drug Release
2.6. In Vitro Cytotoxicity
2.6.1. Cell Lines
2.6.2. Neutral Red Uptake Test
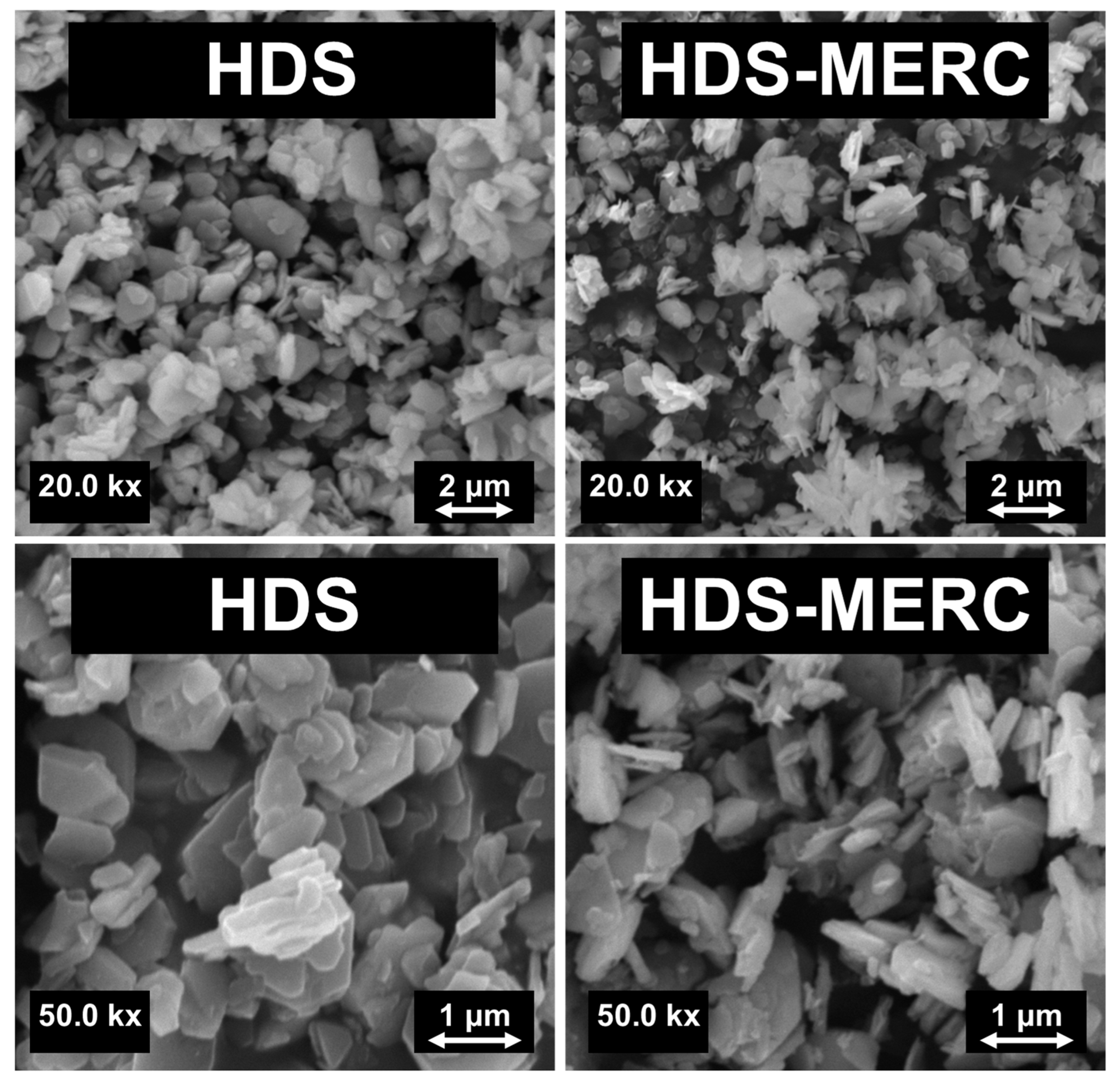
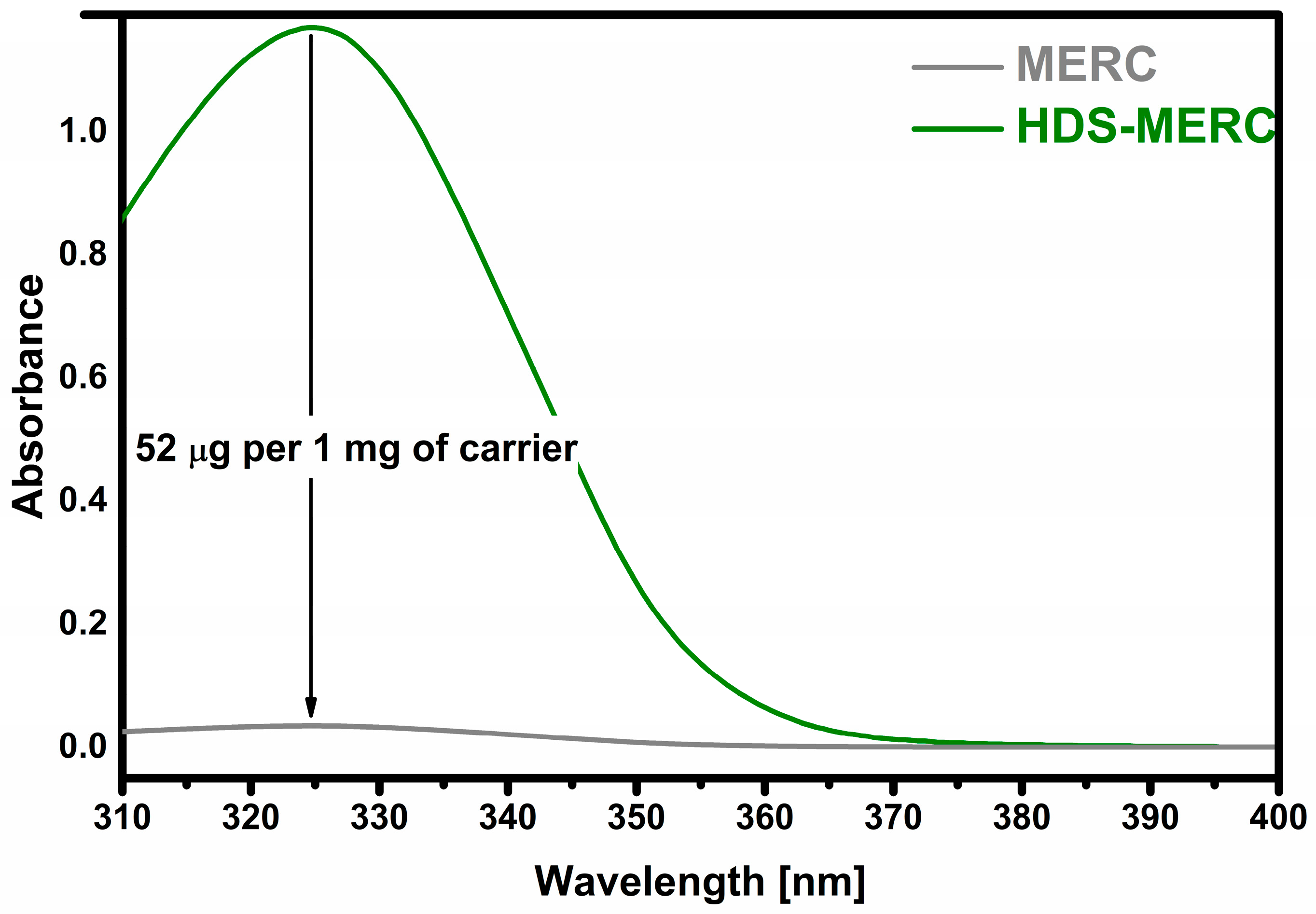
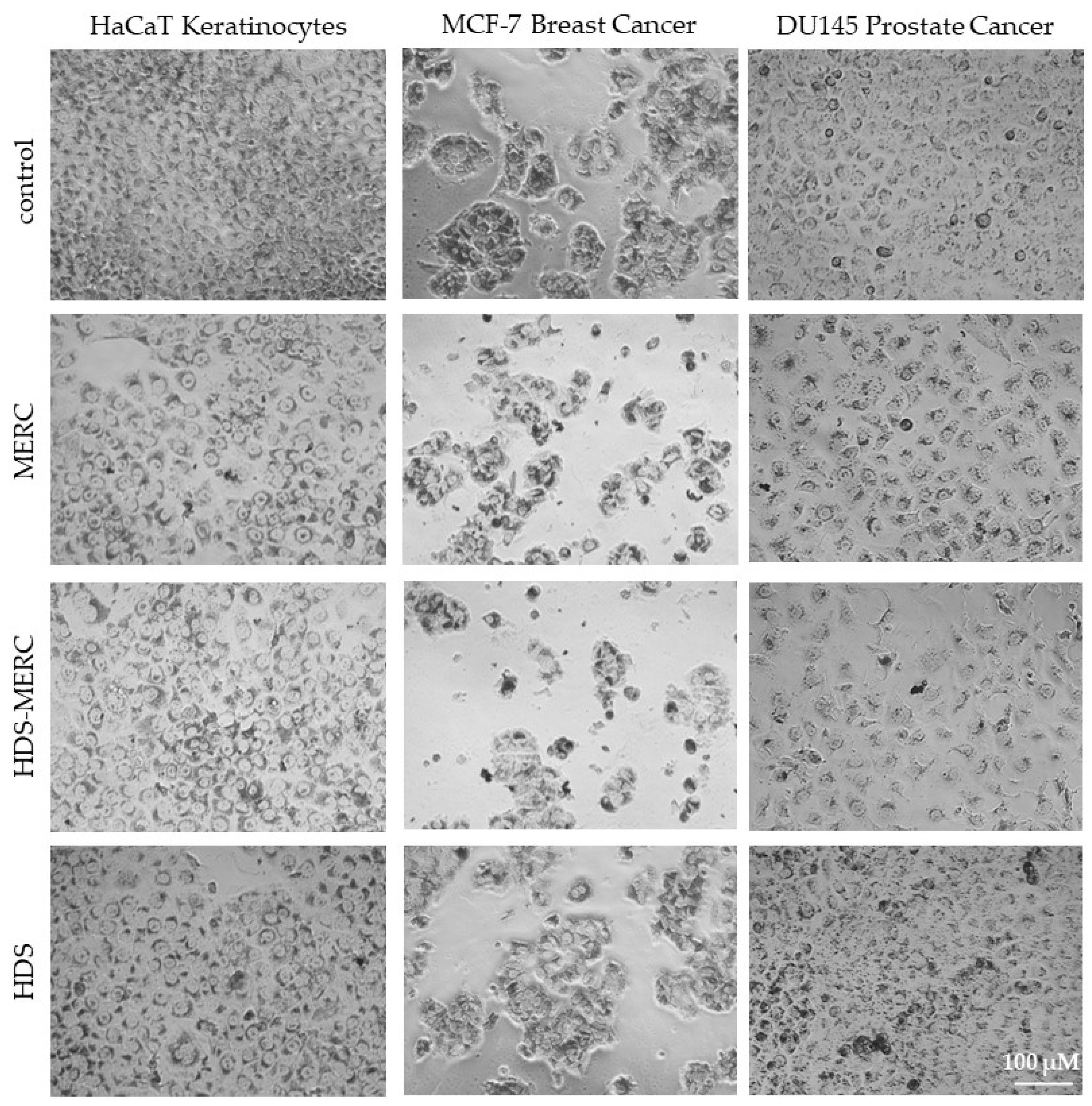
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Yuan, S.; Li, Y.; Ma, Y.; Zhang, C.; Zhang, M.; Mao, Q.; Luo, Y.; Zhao, J. Pre-programed hydroxy double salt templates for room-temperature controlled synthesis of mixed-metal zeolitic imidazolate frameworks. J. Mater. Chem. A 2021, 9, 18557–18563. [Google Scholar] [CrossRef]
- Kaassis, A.Y.A.; Wei, M.; Williams, G.R. New biocompatible hydroxy double salts and their drug delivery properties. J. Mater. Chem. B. 2016, 4, 5789–5793. [Google Scholar] [CrossRef]
- Li, A.; Mu, X.; Li, T.; Wen, H.; Li, W.; Li, Y.; Wang, B. Formation of porous Cu hydroxy double salt nanoflowers derived from metal–organic frameworks with efficient peroxidase-like activity for label-free detection of glucose. Nanoscale 2018, 10, 11948–11954. [Google Scholar] [CrossRef]
- Hara, T.; Fujita, N.; Ichikuni, N.; Wilson, K.; Lee, A.F.; Shimazu, S. Efficient 1,4-Addition of Enones and Boronic Acids Catalyzed by a Ni–Zn Hydroxyl Double Salt-Intercalated Anionic Rhodium(III) Complex. ACS Catal. 2014, 4, 4040–4046. [Google Scholar] [CrossRef]
- Lu, J.; Xu, C.; Tian, Z.; Lu, J.; Lin, Y.; Shi, Z. Green emission and Ag+ sensing of hydroxy double salt supported gold nanoclusters. Nanoscale 2016, 8, 5120–5125. [Google Scholar] [CrossRef]
- Majoni, S.; Hossenlopp, J.M. Controlled Release Kinetics in Hydroxy Double Salts: Effect of Host Anion Structure. Adv. Phys. Chem. 2014, 2014, e710487. [Google Scholar] [CrossRef]
- Kaassis, A.Y.A.; Al-Jamal, W.T.; Strimaite, M.; Severic, M.; Williams, G.R. Biocompatible hydroxy double salts as delivery matrices for non-steroidal anti-inflammatory and anti-epileptic drugs. Appl. Clay Sci. 2022, 221, 106456. [Google Scholar] [CrossRef]
- Richardson-Chong, S.S.D.; Patel, R.; Williams, G.R. Intercalation and Controlled Release of Bioactive Ions Using a Hydroxy Double Salt. Ind. Eng. Chem. Res. 2012, 51, 2913–2921. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Env. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Ceylan, M.N.; Akdas, S.; Yazihan, N. Is Zinc an Important Trace Element on Bone-Related Diseases and Complications? A Meta-analysis and Systematic Review from Serum Level, Dietary Intake, and Supplementation Aspects. Biol. Trace Elem. Res. 2021, 199, 535–549. [Google Scholar] [CrossRef]
- Consolo, L.Z.Z.; Melnikov, P.; Cônsolo, F.Z.; Nascimento, V.A.; Pontes, J.C.D.V. Zinc supplementation in children and adolescents with acute leukemia. Eur. J. Clin. Nutr. 2013, 67, 1056–1059. [Google Scholar] [CrossRef]
- Eby, G.A. Treatment of acute lymphocytic leukemia using zinc adjuvant with chemotherapy and radiation—A case history and hypothesis. Med. Hypotheses 2005, 64, 1124–1126. [Google Scholar] [CrossRef]
- Jakubowski, M.; Kucinska, M.; Ratajczak, M.; Pokora, M.; Murias, M.; Voelkel, A.; Sandomierski, M. Zinc forms of faujasite zeolites as a drug delivery system for 6-mercaptopurine. Microporous Mesoporous Mater. 2022, 343, 112194. [Google Scholar] [CrossRef]
- Polifka, J.E.; Friedman, J.M. Teratogen update: Azathioprine and 6-mercaptopurine. Teratology 2002, 65, 240–261. [Google Scholar] [CrossRef]
- Bhatia, S.; Landier, W.; Shangguan, M.; Hageman, L.; Schaible, A.N.; Carter, A.R.; Hanby, C.L.; Leisenring, W.; Yasui, Y.; Kornegay, N.M.; et al. Nonadherence to Oral Mercaptopurine and Risk of Relapse in Hispanic and Non-Hispanic White Children with Acute Lymphoblastic Leukemia: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2094–2101. [Google Scholar] [CrossRef]
- Bökkerink, J.P.M.; Stet, E.H.; De Abreu, R.A.; Damen, F.J.M.; Hulscher, T.W.; Bakker, M.A.H.; Van Baal, J.A. 6-Mercaptopurine: Cytotoxicity and biochemical pharmacology in human malignant T-lymphoblasts. Biochem. Pharmacol. 1993, 45, 1455–1463. [Google Scholar] [CrossRef]
- Zacchigna, M.; Cateni, F.; Di Luca, G.; Drioli, S. A simple method for the preparation of PEG-6-mercaptopurine for oral administration. Bioorg. Med. Chem. Lett. 2007, 17, 6607–6609. [Google Scholar] [CrossRef]
- Podsiadlo, P.; Sinani, V.A.; Bahng, J.H.; Kam, N.W.S.; Lee, J.; Kotov, N.A. Gold Nanoparticles Enhance the Anti-Leukemia Action of a 6-Mercaptopurine Chemotherapeutic Agent. Langmuir 2008, 24, 568–574. [Google Scholar] [CrossRef]
- Wang, J.-R.; Yu, X.; Zhou, C.; Lin, Y.; Chen, C.; Pan, G.; Mei, X. Improving the dissolution and bioavailability of 6-mercaptopurine via co-crystallization with isonicotinamide. Bioorg. Med. Chem. Lett. 2015, 25, 1036–1039. [Google Scholar] [CrossRef]
- Qiu, J.; Cheng, R.; Zhang, J.; Sun, H.; Deng, C.; Meng, F.; Zhong, Z. Glutathione-Sensitive Hyaluronic Acid-Mercaptopurine Prodrug Linked via Carbonyl Vinyl Sulfide: A Robust and CD44-Targeted Nanomedicine for Leukemia. Biomacromolecules 2017, 18, 3207–3214. [Google Scholar] [CrossRef]
- Gong, M.; Yang, J.; Li, Y.; Gu, J. Glutathione-responsive nanoscale MOFs for effective intracellular delivery of the anticancer drug 6-mercaptopurine. Chem. Commun. 2020, 56, 6448–6451. [Google Scholar] [CrossRef]
- Talib, A.B.; Mohammed, M.H. Preparation, characterization and preliminary cytotoxic evaluation of 6-mercaptopurine-coated biotinylated carbon dots nanoparticles as a drug delivery system. Mater. Today Proc. 2021, 80, 2327–2333. [Google Scholar] [CrossRef]
- Mosavi, S.H.; Zare-Dorabei, R. Synthesis of NMOF-5 Using Microwave and Coating with Chitosan: A Smart Biocompatible pH-Responsive Nanocarrier for 6-Mercaptopurine Release on MCF-7 Cell Lines. ACS Biomater. Sci. Eng. 2022, 8, 2477–2488. [Google Scholar] [CrossRef]
- Kaur, H.; Mohanta, G.C.; Gupta, V.; Kukkar, D.; Tyagi, S. Synthesis and characterization of ZIF-8 nanoparticles for controlled release of 6-mercaptopurine drug. J. Drug Deliv. Sci. Technol. 2017, 41, 106–112. [Google Scholar] [CrossRef]
- Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Voelkel, A. Zeolitic Imidazolate Framework-8 (ZIF-8) modified titanium alloy for controlled release of drugs for osteoporosis. Sci. Rep. 2022, 12, 9103. [Google Scholar] [CrossRef]
- Solutions, S. Common Buffers, Media, and Stock Solutions; John and Wiley and Sons: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Jin, M.; Li, W.; Spillane, D.E.M.; Geraldes, C.F.G.C.; Williams, G.R.; Bligh, S.W.A. Hydroxy double salts intercalated with Mn(II) complexes as potential contrast agents. Solid State Sci. 2016, 53, 9–16. [Google Scholar] [CrossRef]
- Viudez, A.J.; Madueño, R.; Pineda, T.; Blázquez, M. Stabilization of Gold Nanoparticles by 6-Mercaptopurine Monolayers. Effects of the Solvent Properties. J. Phys. Chem. B 2006, 110, 17840–17847. [Google Scholar] [CrossRef]
- Kumar, G.P.; Sanganal, J.S.; Phani, A.R.; Manohara, C.; Tripathi, S.M.; Raghavendra, H.L.; Janardhana, P.B.; Amaresha, S.; Swamy, K.B.; Prasad, R.G.S.V. Anti-cancerous efficacy and pharmacokinetics of 6-mercaptopurine loaded chitosan nanoparticles. Pharmacol. Res. 2015, 100, 47–57. [Google Scholar] [CrossRef]
- Sirivat, A.; Paradee, N. Facile synthesis of gelatin-coated Fe3O4 nanoparticle: Effect of pH in single-step co-precipitation for cancer drug loading. Mater. Des. 2019, 181, 107942. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Liao, J.; Peng, H.; Wei, X.; Song, Y.; Liu, C.; Li, D.; Yin, Y.; Xiong, X.; Zheng, H.; Wang, Q. A bio-responsive 6-mercaptopurine/doxorubicin based “Click Chemistry” polymeric prodrug for cancer therapy. Mater. Sci. Eng. C 2020, 108, 110461. [Google Scholar] [CrossRef]
- Laera, L.; Guaragnella, N.; Giannattasio, S.; Moro, L. 6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner. Cancers 2019, 11, 945. [Google Scholar] [CrossRef]




| Order | Reagent | Amount |
|---|---|---|
| 1 | NaCl | 8.035 g |
| 2 | NaHCO3 | 0.355 g |
| 3 | KCl | 0.225 g |
| 4 | K2HPO4·3H2O | 0.231 g |
| 5 | Na2SO4 | 0.072 g |
| 6 | TRIS | 0.6112 g |
| 7 | HCl | 0–5 mL |
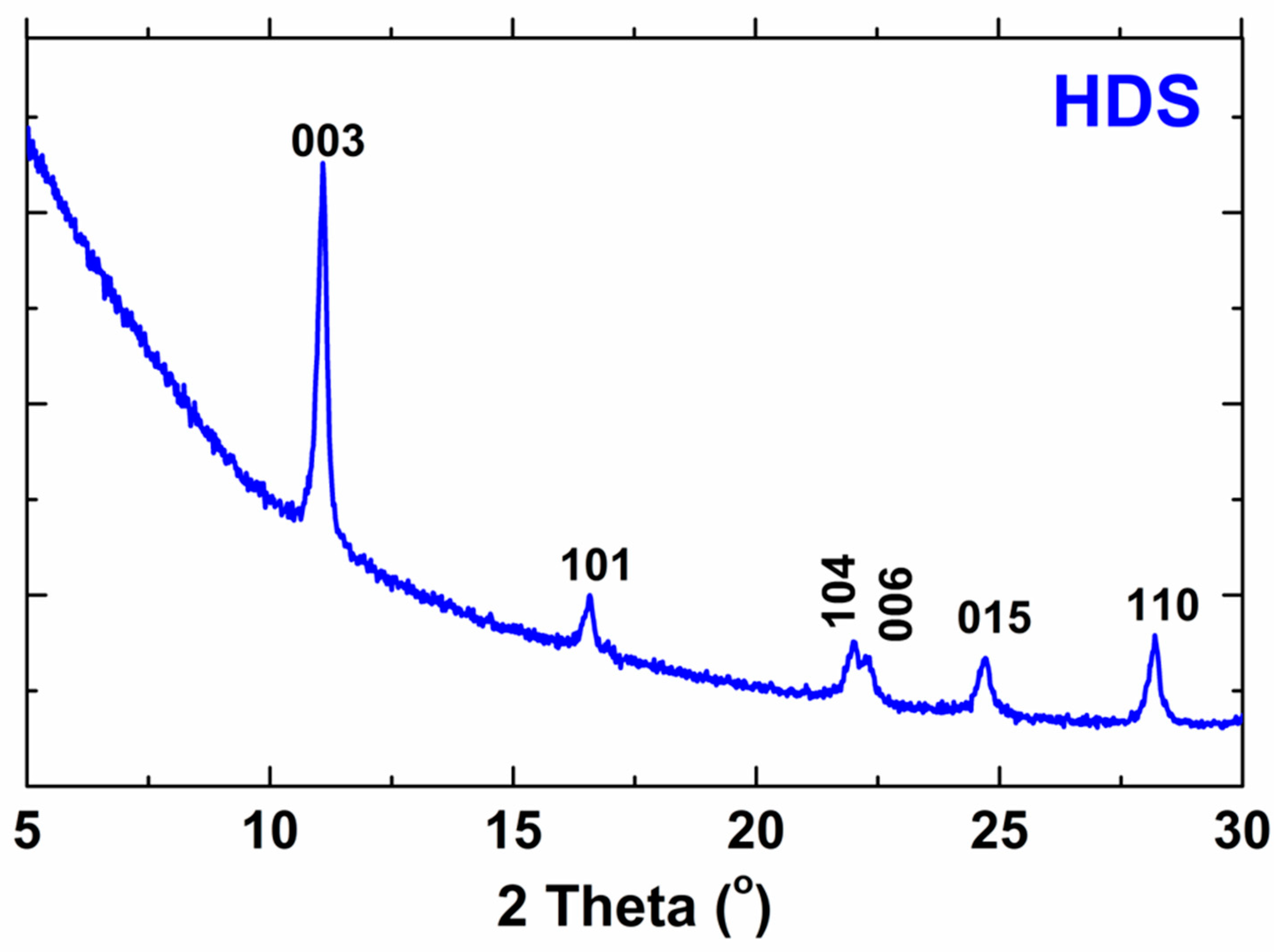
| h | k | l | 2 Theta This Work | 2 Theta Reference [2] | Difference |
|---|---|---|---|---|---|
| 0 | 0 | 3 | 11.0838 | 11.0238 | 0.0600 |
| 1 | 0 | 1 | 16.5966 | 16.5760 | 0.0206 |
| 1 | 0 | 4 | 22.0110 | 22.0506 | −0.0396 |
| 0 | 0 | 6 | 22.3260 | 22.3360 | −0.0100 |
| 0 | 1 | 5 | 24.6689 | 24.6976 | −0.0290 |
| 1 | 1 | 0 | 28.1932 | 28.1701 | 0.0231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandomierski, M.; Jakubowski, M.; Ratajczak, M.; Patalas, A.; Gaweł-Bęben, K.; Lechwar, P.; Voelkel, A. Double Hydroxyl Salt as Smart Biocompatible pH-Responsive Carrier for 6-Mercaptopurine. Cancers 2023, 15, 5670. https://doi.org/10.3390/cancers15235670
Sandomierski M, Jakubowski M, Ratajczak M, Patalas A, Gaweł-Bęben K, Lechwar P, Voelkel A. Double Hydroxyl Salt as Smart Biocompatible pH-Responsive Carrier for 6-Mercaptopurine. Cancers. 2023; 15(23):5670. https://doi.org/10.3390/cancers15235670
Chicago/Turabian StyleSandomierski, Mariusz, Marcel Jakubowski, Maria Ratajczak, Adam Patalas, Katarzyna Gaweł-Bęben, Paulina Lechwar, and Adam Voelkel. 2023. "Double Hydroxyl Salt as Smart Biocompatible pH-Responsive Carrier for 6-Mercaptopurine" Cancers 15, no. 23: 5670. https://doi.org/10.3390/cancers15235670
APA StyleSandomierski, M., Jakubowski, M., Ratajczak, M., Patalas, A., Gaweł-Bęben, K., Lechwar, P., & Voelkel, A. (2023). Double Hydroxyl Salt as Smart Biocompatible pH-Responsive Carrier for 6-Mercaptopurine. Cancers, 15(23), 5670. https://doi.org/10.3390/cancers15235670










