Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics
Abstract
:Simple Summary
Abstract
1. Introduction
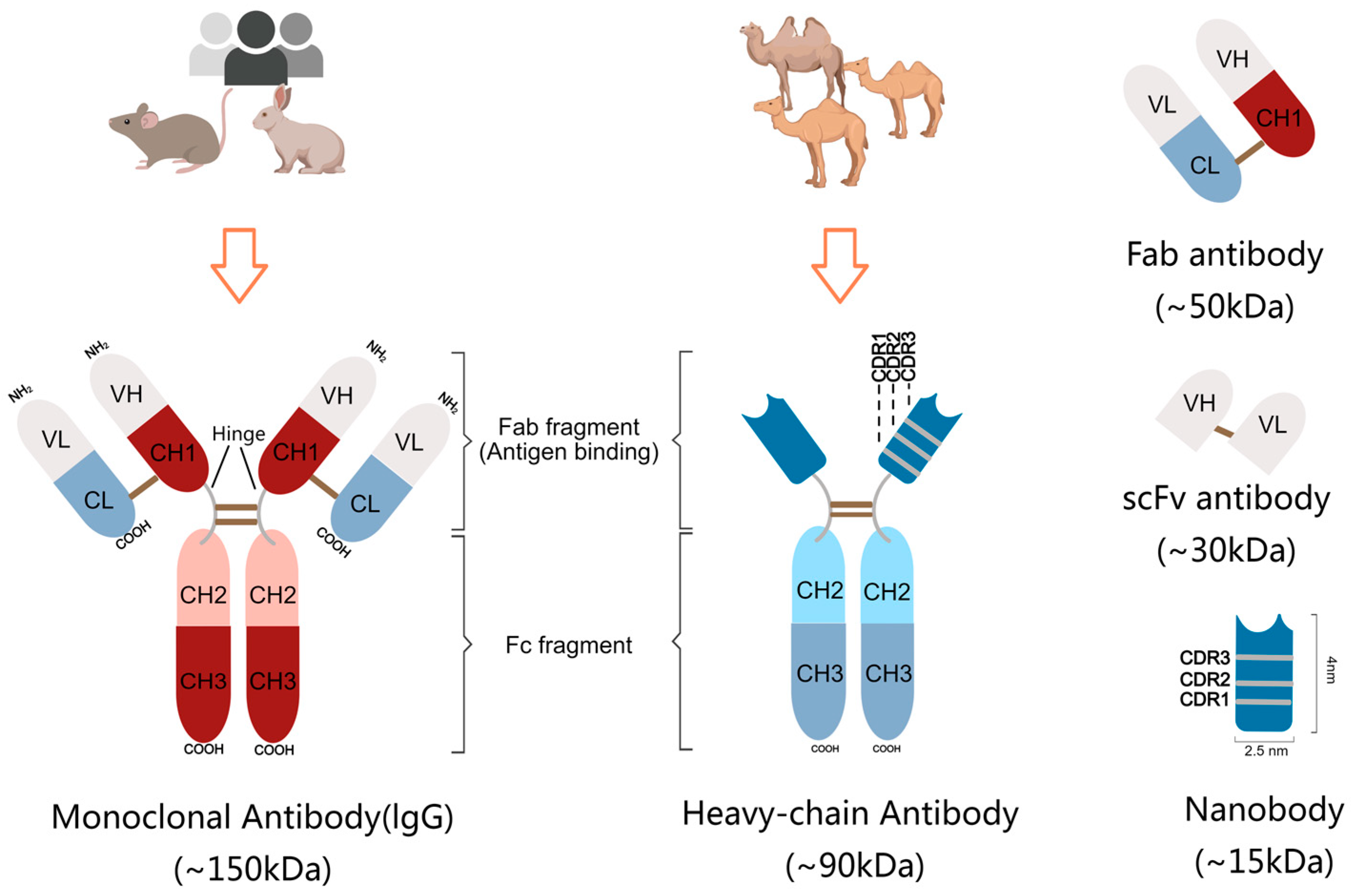
2. Structural Features of Nanobody
3. Desirable Properties of Nanobodies
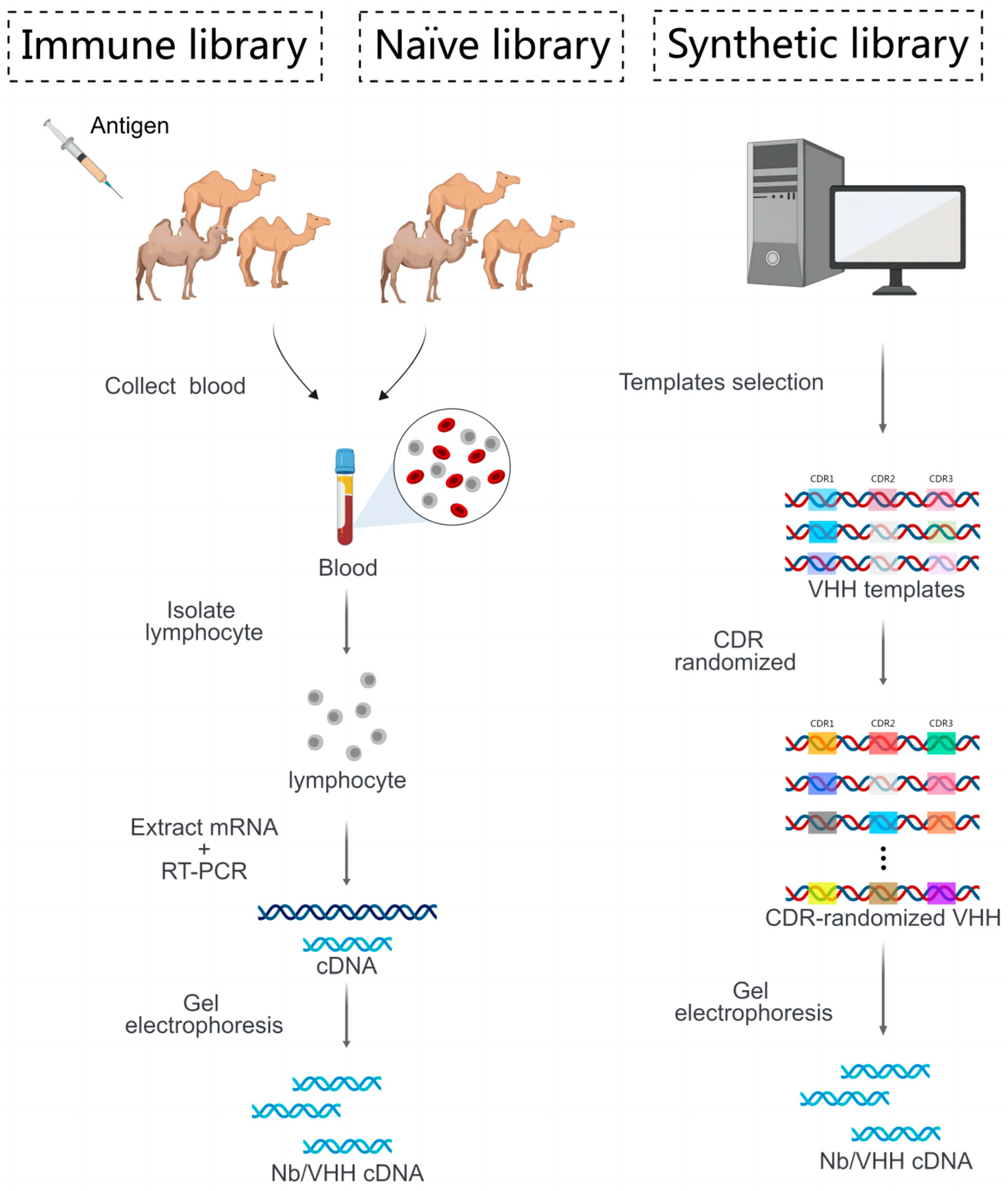
4. Strategy of Generate Desired Nanobody
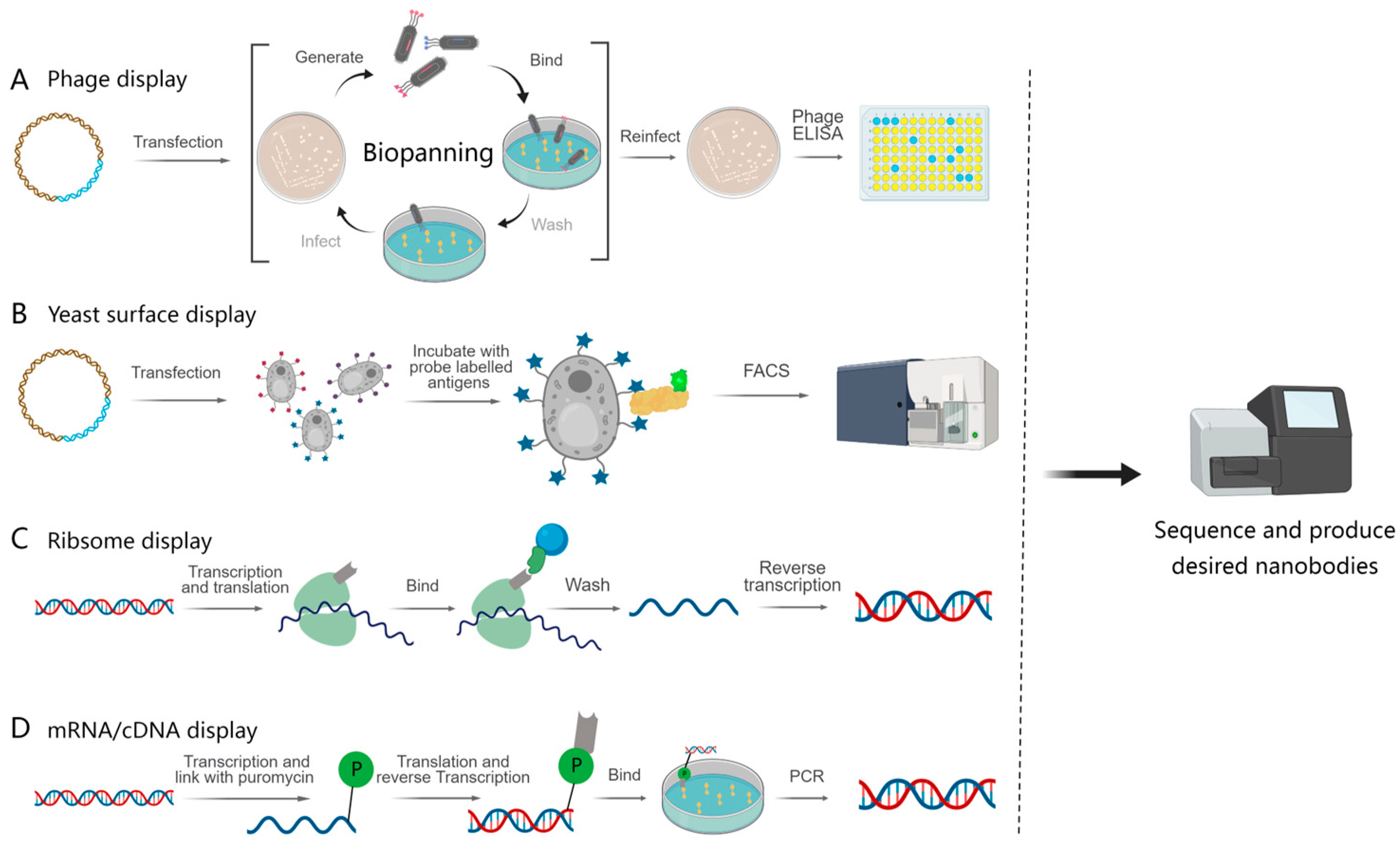
4.1. Cell-Based Display and Selection Method
4.1.1. Phage Display
4.1.2. Yeast Surface Display
4.2. Cell-Free Display and Selection Platform
4.2.1. Ribosome Display
4.2.2. mRNA/cDNA Display
5. Applications of Nanobodies in Tumor Diagnosis and Therapy
5.1. Nanobody-Based Immunopet/SPECT Applications for Cancers
5.2. Nanobody-Based Optical Imaging and Treatment for Malignancies
5.2.1. Nanobody-Based Near-Infrared (NIR) Optical Imaging
5.2.2. Combination of Nanobody and NIR in Photodynamic Therapy against Cancer
5.3. Nanobody-Based Ultrasound Molecular Imaging and Treatment
5.4. Nanobody-Based Anti-Tumor Therapies
5.4.1. Immune Checkpoint Blockade Therapy
5.4.2. T-Cell Immunotherapy
5.4.3. Nanobody-Based Targeting of Other Immune Cells
5.5. Nanobody-Based Delivery System
6. Clinical Applications of Nanobodies in Diverse Carcinomas
7. Conclusions and Respective
Author Contributions
Funding
Conflicts of Interest
References
- Köhler, G.; Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Bao, G.; Tang, M.; Zhao, J.; Zhu, X. Nanobody: A promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021, 11, 6. [Google Scholar] [CrossRef]
- Sun, S.; Ding, Z.; Yang, X.; Zhao, X.; Zhao, M.; Gao, L.; Chen, Q.; Xie, S.; Liu, A.; Yin, S.; et al. Nanobody: A Small Antibody with Big Implications for Tumor Therapeutic Strategy. Int. J. Nanomed. 2021, 16, 2337–2356. [Google Scholar] [CrossRef]
- Maeda, R.; Fujita, J.; Konishi, Y.; Kazuma, Y.; Yamazaki, H.; Anzai, I.; Watanabe, T.; Yamaguchi, K.; Kasai, K.; Nagata, K.; et al. A panel of nanobodies recognizing conserved hidden clefts of all SARS-CoV-2 spike variants including Omicron. Commun. Biol. 2022, 5, 669. [Google Scholar] [CrossRef]
- Li, T.; Huang, M.; Xiao, H.; Zhang, G.; Ding, J.; Wu, P.; Zhang, H.; Sheng, J.; Chen, C. Selection and characterization of specific nanobody against bovine virus diarrhea virus (BVDV) E2 protein. PLoS ONE 2017, 12, e0178469. [Google Scholar] [CrossRef]
- Rué, L.; Jaspers, T.; Degors, I.M.S.; Noppen, S.; Schols, D.; De Strooper, B.; Dewilde, M. Novel Human/Non-Human Primate Cross-Reactive Anti-Transferrin Receptor Nanobodies for Brain Delivery of Biologics. Pharmaceutics 2023, 15, 1748. [Google Scholar] [CrossRef]
- Erreni, M.; D’Autilia, F.; Avigni, R.; Bolli, E.; Arnouk, S.M.; Movahedi, K.; Debie, P.; Anselmo, A.; Parente, R.; Vincke, C.; et al. Size-advantage of monovalent nanobodies against the macrophage mannose receptor for deep tumor penetration and tumor-associated macrophage targeting. Theranostics 2023, 13, 355–373. [Google Scholar] [CrossRef]
- Qiu, H.; Hosking, C.; Rothzerg, E.; Samantha, A.; Chen, K.; Kuek, V.; Jin, H.; Zhu, S.; Vrielink, A.; Lim, K.; et al. ADR3, a next generation i-body to human RANKL, inhibits osteoclast formation and bone resorption. J. Biol. Chem. 2023, 299, 102889. [Google Scholar] [CrossRef]
- D’Huyvetter, M.; Vincke, C.; Xavier, C.; Aerts, A.; Impens, N.; Baatout, S.; De Raeve, H.; Muyldermans, S.; Caveliers, V.; Devoogdt, N.; et al. Targeted radionuclide therapy with A 177Lu-labeled anti-HER2 nanobody. Theranostics 2014, 4, 708–720. [Google Scholar] [CrossRef]
- Alirahimi, E.; Kazemi-Lomedasht, F.; Shahbazzadeh, D.; Habibi-Anbouhi, M.; Hosseininejad Chafi, M.; Sotoudeh, N.; Ghaderi, H.; Muyldermans, S.; Behdani, M. Nanobodies as novel therapeutic agents in envenomation. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2955–2965. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, G.; Zhao, S.; Tang, Y.; Tang, H.; Wang, K.; Liu, H.; Lan, K.; Bi, X.; Duan, S. Nanobodies targeting immune checkpoint molecules for tumor immunotherapy and immunoimaging (Review). Int. J. Mol. Med. 2021, 47, 444–454. [Google Scholar] [CrossRef]
- Meltzer, M.; Eliash, N.; Azoulay, Z.; Hadad, U.; Papo, N. In vitro inhibition of cancer angiogenesis and migration by a nanobody that targets the orphan receptor Tie1. Cell Mol. Life Sci. 2022, 79, 312. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lai, G.H.; Lin, T.Y.; Tseng, T.S.; Tsai, T.H.; Chen, W.C.; Lee, C.C.; Tsai, K.C. Development of anti-aflatoxin B1 nanobodies from a novel mutagenesis-derived synthetic library for traditional Chinese medicine and foods safety testing. J. Biol. Eng. 2023, 17, 30. [Google Scholar] [CrossRef]
- Payandeh, Z.; Rasooli, I.; Mousavi Gargari, S.L.; Rajabi Bazl, M.; Ebrahimizadeh, W. Immunoreaction of a recombinant nanobody from camelid single domain antibody fragment with Acinetobacter baumannii. Trans. R. Soc. Trop. Med. Hyg. 2014, 108, 92–98. [Google Scholar] [CrossRef]
- Guo, Y.; Ouyang, Z.; He, W.; Zhang, J.; Qin, Q.; Jiao, M.; Muyldermans, S.; Zheng, F.; Wen, Y. Screening and epitope characterization of diagnostic nanobody against total and activated Bacteroides fragilis toxin. Front. Immunol. 2023, 14, 1065274. [Google Scholar] [CrossRef]
- Yu, X.L.; Long, Y.R.; Chen, B.F.; Tong, Y.L.; Shan, M.W.; Jia, X.M.; Hu, C.; Liu, M.; Zhou, J.; Tang, F.; et al. PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way. J. Immunother. Cancer 2022, 10, e004590. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Chen, T.; Wang, J.; Zhao, Q.; Zhou, E.M.; Liu, B. Development and Application of a Nanobody-Based Competitive ELISA for Detecting Antibodies against Hepatitis E Virus from Humans and Domestic Animals. Microbiol. Spectr. 2023, 11, e0360722. [Google Scholar] [CrossRef]
- Dhehibi, A.; Allaoui, A.; Raouafi, A.; Terrak, M.; Bouhaouala-Zahar, B.; Hammadi, M.; Raouafi, N.; Salhi, I. Nanobody-Based Sandwich Immunoassay for Pathogenic Escherichia coli F17 Strain Detection. Biosensors 2023, 13, 229. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Q.; Fan, H.; Liao, C.; Zhang, J.; Hu, H.; Yi, H.; Peng, Y.; Lu, J.; Chen, Z. A Multivalent and Thermostable Nanobody Neutralizing SARS-CoV-2 Omicron (B.1.1.529). Int. J. Nanomed. 2023, 18, 353–367. [Google Scholar] [CrossRef]
- Bacon, K.; Menegatti, S.; Rao, B.M. Isolation of Single-Domain Antibodies to Transmembrane Proteins Using Magnetized Yeast Cell Targets. In Single-Domain Antibodies: Methods and Protocols; Hussack, G., Henry, K.A., Eds.; Springer: New York, NY, USA, 2022; pp. 95–119. [Google Scholar]
- Lei, M.; Trivedi, V.D.; Nair, N.U.; Lee, K.; Van Deventer, J.A. Flow cytometric evaluation of yeast-bacterial cell-cell interactions. Biotechnol. Bioeng. 2023, 120, 399–408. [Google Scholar] [CrossRef]
- Kajiwara, K.; Aoki, W.; Koike, N.; Ueda, M. Development of a yeast cell surface display method using the SpyTag/SpyCatcher system. Sci. Rep. 2021, 11, 11059. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An ultrapotent synthetic nanobody neutralizes SARS-CoV-2 by stabilizing inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef]
- Cavallari, M. Rapid Antigen and Antibody-Like Molecule Discovery by Staphylococcal Surface Display. Methods Mol. Biol. 2020, 2070, 79–94. [Google Scholar]
- Ohoka, A.; Sarkar, C.A. Facile Display of Homomultivalent Proteins for In Vitro Selections. ACS Synth. Biol. 2023, 12, 634–638. [Google Scholar] [CrossRef]
- Li, R.; Kang, G.; Hu, M.; Huang, H. Ribosome Display: A Potent Display Technology used for Selecting and Evolving Specific Binders with Desired Properties. Mol. Biotechnol. 2019, 61, 60–71. [Google Scholar] [CrossRef]
- Kunamneni, A.; Ogaugwu, C.; Bradfute, S.; Durvasula, R. Ribosome Display Technology: Applications in Disease Diagnosis and Control. Antibodies 2020, 9, 28. [Google Scholar] [CrossRef]
- Chen, X.; Gentili, M.; Hacohen, N.; Regev, A. A cell-free nanobody engineering platform rapidly generates SARS-CoV-2 neutralizing nanobodies. Nat. Commun. 2021, 12, 5506. [Google Scholar] [CrossRef]
- Hu, M.; Kang, G.; Cheng, X.; Wang, J.; Li, R.; Bai, Z.; Yang, D.; Huang, H. In vitro affinity maturation to improve the efficacy of a hypoxia-inducible factor 1α single-domain intrabody. Biochem. Biophys. Res. Commun. 2020, 529, 936–942. [Google Scholar] [CrossRef]
- Haga, K.; Takai-Todaka, R.; Matsumura, Y.; Song, C.; Takano, T.; Tojo, T.; Nagami, A.; Ishida, Y.; Masaki, H.; Tsuchiya, M.; et al. Nasal delivery of single-domain antibody improves symptoms of SARS-CoV-2 infection in an animal model. PLoS Pathog. 2021, 17, e1009542. [Google Scholar] [CrossRef]
- Nemoto, N.; Kumachi, S.; Arai, H. In Vitro Selection of Single-Domain Antibody (VHH) Using cDNA Display. Methods Mol. Biol. 2018, 1827, 269–285. [Google Scholar]
- Jayathilake, C.; Kumachi, S.; Arai, H.; Motohashi, M.; Terai, T.; Murakami, A.; Nemoto, N. In vitro selection of anti-gliadin single-domain antibodies from a naïve library for cDNA-display mediated immuno-PCR. Anal. Biochem. 2020, 589, 113490. [Google Scholar] [CrossRef]
- Suzuki, T.; Mochizuki, Y.; Kimura, S.; Akazawa-Ogawa, Y.; Hagihara, Y.; Nemoto, N. Anti-survivin single-domain antibodies derived from an artificial library including three synthetic random regions by in vitro selection using cDNA display. Biochem. Biophys. Res. Commun. 2018, 503, 2054–2060. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Qin, L.; Ye, H.; Wang, Y.; Long, B.; Jiao, Z. Anti-EGFR Binding Nanobody Delivery System to Improve the Diagnosis and Treatment of Solid Tumours. Recent. Pat. Anticancer. Drug Discov. 2020, 15, 200–211. [Google Scholar] [CrossRef]
- Pothin, E.; Lesuisse, D.; Lafaye, P. Brain Delivery of Single-Domain Antibodies: A Focus on VHH and VNAR. Pharmaceutics 2020, 12, 937. [Google Scholar] [CrossRef]
- Salvador, J.P.; Vilaplana, L.; Marco, M.P. Nanobody: Outstanding features for diagnostic and therapeutic applications. Anal. Bioanal. Chem. 2019, 411, 1703–1713. [Google Scholar] [CrossRef]
- Philpott, G.W.; Schwarz, S.W.; Anderson, C.J.; Dehdashti, F.; Connett, J.M.; Zinn, K.R.; Meares, C.F.; Cutler, P.D.; Welch, M.J.; Siegel, B.A. RadioimmunoPET: Detection of colorectal carcinoma with positron-emitting copper-64-labeled monoclonal antibody. J. Nucl. Med. 1995, 36, 1818–1824. [Google Scholar]
- Yang, E.R.; Liu, Q.F.; Huang, G.; Liu, J.J.; Wei, W.J. Engineering nanobodies for next-generation molecular imaging. Drug Discov. Today 2022, 27, 1622–1638. [Google Scholar] [CrossRef]
- Qin, S.B.; Yu, Y.; Guan, H.; Yang, Y.L.; Sun, F.H.; Sun, Y.; Zhu, J.X.; Xing, L.G.; Yu, J.M.; Sun, X.R. A preclinical study: Correlation between PD-L1 PET imaging and the prediction of therapy efficacy of MC38 tumor with Ga-68-labeled PD-L1 targeted nanobody. Aging 2021, 13, 13006–13022. [Google Scholar] [CrossRef]
- Rinne, S.S.; Orlova, A.; Tolmachev, V. PET and SPECT Imaging of the EGFR Family (RTK Class I) in Oncology. Int. J. Mol. Sci. 2021, 22, 3663. [Google Scholar] [CrossRef]
- Hynes, N.E.; Lane, H.A. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat. Rev. Cancer 2005, 5, 341–354. [Google Scholar] [CrossRef]
- van Oordt, C.W.M.; Gootjes, E.C.; Huisman, M.C.; Vugts, D.J.; Roth, C.; Luik, A.M.; Mulder, E.R.; Schuit, R.C.; Boellaard, R.; Hoekstra, O.S.; et al. Zr-89-cetuximab PET imaging in patients with advanced colorectal cancer. Oncotarget 2015, 6, 30384–30393. [Google Scholar] [CrossRef]
- van Helden, E.J.; Hoekstra, O.S.; Huisman, M.C.; Boon, E.; van Es, S.C.; van Dongen, G.A.M.S.; Vugts, D.J.; de Groot, D.J.; Boellaard, R.; van Herpen, C.M.L.; et al. Imaging tumor biology with Zr-89-cetuximab, O-15-H2O and F-18-FDG PET/CT in patients with advanced colorectal cancer treated with cetuximab monotherapy. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, S148. [Google Scholar]
- Tang, Y.; Hu, Y.J.; Liu, W.H.; Chen, L.; Zhao, Y.; Ma, H.; Yang, J.J.; Yang, Y.Y.; Liao, J.L.; Cai, J.M.; et al. A radiopharmaceutical [Zr-89]Zr-DFO-nimotuzumab for immunoPET with epidermal growth factor receptor expression in vivo. Nucl. Med. Biol. 2019, 70, 23–31. [Google Scholar] [CrossRef]
- Reilly, E.B.; Phillips, A.C.; Buchanan, F.G.; Kingsbury, G.; Zhang, Y.M.; Meulbroek, J.A.; Cole, T.B.; DeVries, P.J.; Falls, H.D.; Beam, C.; et al. Characterization of ABT-806, a Humanized Tumor-Specific Anti-EGFR Monoclonal Antibody. Mol. Cancer Ther. 2015, 14, 1141–1151. [Google Scholar] [CrossRef]
- Phillips, A.C.; Boghaert, E.R.; Vaidya, K.S.; Falls, H.D.; Mitten, M.J.; DeVries, P.J.; Benatuil, L.; Hsieh, C.M.; Meulbroek, J.A.; Panchal, S.C.; et al. Characterization of ABBV-221, a Tumor-Selective EGFR-Targeting Antibody Drug Conjugate. Mol. Cancer Ther. 2018, 17, 795–805. [Google Scholar] [CrossRef]
- Tintelnot, J.; Baum, N.; Schultheiss, C.; Braig, F.; Trentmann, M.; Finter, J.; Fumey, W.; Bannas, P.; Fehse, B.; Riecken, K.; et al. Nanobody Targeting of Epidermal Growth Factor Receptor (EGFR) Ectodomain Variants Overcomes Resistance to Therapeutic EGFR Antibodies. Mol. Cancer Ther. 2019, 18, 823–833. [Google Scholar] [CrossRef]
- Kruwel, T.; Nevoltris, D.; Bode, J.; Dullin, C.; Baty, D.; Chames, P.; Alves, F. In vivo detection of small tumour lesions by multi-pinhole SPECT applying a Tc-99m-labelled nanobody targeting the Epidermal Growth Factor Receptor. Sci. Rep. 2016, 6, 21834. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Walsum, M.S.V.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A.M.S. Improved tumor targeting of anti-epidermal growth factor receptor Nanobodies through albumin binding: Taking advantage of modular Nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef]
- Beltrán Hernandez, I.; Rompen, R.; Rossin, R.; Xenaki, K.T.; Katrukha, E.A.; Nicolay, K.; van Bergen En Henegouwen, P.; Gruell, H.; Oliveira, S. Imaging of Tumor Spheroids, Dual-Isotope SPECT, and Autoradiographic Analysis to Assess the Tumor Uptake and Distribution of Different Nanobodies. Mol. Imaging Biol. 2019, 21, 1079–1088. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; McDougald, D.; Devoogdt, N.; Zalutsky, M.R.; Vaidyanathan, G. Labeling Single Domain Antibody Fragments with Fluorine-18 Using 2,3,5,6-Tetrafluorophenyl 6-[F-18]Fluoronicotinate Resulting in High Tumor-to-Kidney Ratios. Mol. Pharm. 2019, 16, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.J.; Zhang, D.; Zhang, Y.; Li, L.H.; Jin, Y.C.; An, S.X.; Lv, C.; Zhao, H.T.; Wang, C.; Huang, Y.S.; et al. Development and comparison of 68Ga/18F/64Cu-labeled nanobody tracers probing Claudin18.2. Mol. Ther-Oncolytics 2022, 27, 305–314. [Google Scholar] [CrossRef] [PubMed]
- D’Huyvetter, M.; De Vos, J.; Xavier, C.; Pruszynski, M.; Sterckx, Y.G.J.; Massa, S.; Raes, G.; Caveliers, V.; Zalutsky, M.R.; Lahoutte, T.; et al. I-131-labeled Anti-HER2 Camelid sdAb as a Theranostic Tool in Cancer Treatment. Clin. Cancer Res. 2017, 23, 6616–6628. [Google Scholar] [CrossRef]
- Ducharme, M.; Hall, L.; Eckenroad, W.; Cingoranelli, S.J.; Houson, H.A.; Jaskowski, L.; Hunter, C.; Larimer, B.M.; Lapi, S.E. Evaluation of [Zr-89]Zr-DFO-2Rs15d Nanobody for Imaging of HER2-Positive Breast Cancer. Mol. Pharm. 2023, 20, 4629–4639. [Google Scholar] [CrossRef] [PubMed]
- Warnders, F.J.; van Scheltinga, A.G.T.T.; Knuehl, C.; van Roy, M.; de Vries, E.F.J.; Kosterink, J.G.W.; de Vries, E.G.E.; Lub-de Hooge, M.N. Human Epidermal Growth Factor Receptor 3-Specific Tumor Uptake and Biodistribution of Zr-89-MSB0010853 Visualized by Real-Time and Noninvasive PET Imaging. J. Nucl. Med. 2017, 58, 1210–1215. [Google Scholar] [CrossRef]
- Rashidian, M.; Ingram, J.R.; Dougan, M.; Dongre, A.; Whang, K.A.; LeGall, C.; Cragnolini, J.J.; Bierie, B.; Gostissa, M.; Gorman, J.; et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 2017, 214, 2243–2255. [Google Scholar] [CrossRef]
- Zhao, H.T.; Wang, C.; Yang, Y.L.; Sun, Y.; Wei, W.J.; Wang, C.; Wan, L.R.; Zhu, C.; Li, L.H.; Huang, G.; et al. ImmunoPET imaging of human CD8(+) T cells with novel Ga-68-labeled nanobody companion diagnostic agents. J. Nanobiotechnol. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Davies, C.W.; Gill, H.; Kiefer, J.R.; Yin, J.P.; Ogasawara, A.; Urrutia, A.; Javinal, V.; Lin, Z.H.; Seshasayee, D.; et al. Development of an F-18-labeled anti-human CD8 VHH for same-day immunoPET imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 679–691. [Google Scholar] [CrossRef]
- Li, L.Q.; Lin, X.F.; Wang, L.; Ma, X.P.; Zeng, Z.Q.; Liu, F.T.; Jia, B.; Zhu, H.; Wu, A.W.; Yang, Z. Immuno-PET of colorectal cancer with a CEA-targeted [68 Ga]Ga-nanobody: From bench to bedside. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3735–3749. [Google Scholar] [CrossRef]
- Dewulf, J.; Flieswasser, T.; Delahaye, T.; Vangestel, C.; Miranda, A.; de Haard, H.; Jacobs, J.; Smits, E.; van den Wyngaert, T.; Elvas, F. Site-specific Ga-68-labeled nanobody for PET imaging of CD70 expression in preclinical tumor models. EJNMMI Radiopharm. Chem. 2023, 8, 8. [Google Scholar] [CrossRef]
- Rashidian, M.; Keliher, E.J.; Dougan, M.; Juras, P.K.; Cavallari, M.; Wojtkiewicz, G.R.; Jacobsen, J.T.; Edens, J.G.; Tas JM, J.; Victora, G.; et al. Use of 18F-2-fluorodeoxyglucose (FDG) to label antibody fragments for immuno-PET of pancreatic cancer. ACS Cent. Sci. 2015, 1, 142–147. [Google Scholar] [CrossRef]
- Li, D.; Cheng, S.Y.; Zou, S.J.; Zhu, D.L.; Zhu, T.H.; Wang, P.L.; Zhu, X.H. Immuno-PET Imaging of Zr-89 Labeled Anti-PD-L1 Domain Antibody. Mol. Pharm. 2018, 15, 1674–1681. [Google Scholar] [CrossRef]
- Liu, Q.Z.; Jiang, L.; Li, K.; Li, H.; Lv, G.C.; Lin, J.G.; Qiu, L. Immuno-PET imaging of Ga-68-labeled nanobody Nb109 for dynamic monitoring the PD-L1 expression in cancers. Cancer Immunol. Immun. 2021, 70, 1721–1733. [Google Scholar] [CrossRef]
- Bridoux, J.; Broos, K.; Lecocq, Q.; Debie, P.; Martin, C.; Ballet, S.; Raes, G.; Neyt, S.; Vanhove, C.; Breckpot, K.; et al. Anti-Human PD-L1 Nanobody for Immuno-PET Imaging: Validation of a Conjugation Strategy for Clinical Translation. Biomolecules 2020, 10, 1388. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.P.; Zhou, X.; Hu, B.; Li, X.D.; Yao, M.A.; Li, L.Q.; Qin, X.; Li, D.P.; Yao, Y.; Hou, X.G.; et al. Preclinical evaluation and pilot clinical study of [Ga-68]Ga-THP-APN09, a novel PD-L1 targeted nanobody radiotracer for rapid one-step radiolabeling and PET imaging. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 3838–3850. [Google Scholar] [CrossRef] [PubMed]
- Broos, K.; Lecocq, Q.; Raes, G.; Devoogdt, N.; Keyaerts, M.; Breckpot, K. Noninvasive imaging of the PD-1:PD-L1 immune checkpoint: Embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics 2018, 8, 3559–3570. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Chand, G.; Liu, C.C.; Cook, G.J.R.; O’Doherty, J.; Zhao, L.Z.; Wong, N.C.L.; Meszaros, L.K.; Ting, H.H.; Zhao, J.H. Early Phase I Study of a Tc-99m-Labeled Anti-Programmed Death Ligand-1 (PD-L1) Single-Domain Antibody in SPECT/CT Assessment of PD-L1 Expression in Non-Small Cell Lung Cancer. J. Nucl. Med. 2019, 60, 1213–1220. [Google Scholar] [CrossRef]
- Lv, G.C.; Sun, X.R.; Qiu, L.; Sun, Y.; Li, K.; Liu, Q.Z.; Zhao, Q.; Qin, S.B.; Lin, J.G. PET Imaging of Tumor PD-L1 Expression with a Highly Specific Nonblocking Single-Domain Antibody. J. Nucl. Med. 2020, 61, 117–122. [Google Scholar] [CrossRef]
- Weidemann, S.; Gagelmann, P.; Gorbokon, N.; Lennartz, M.; Menz, A.; Luebke, A.M.; Kluth, M.; Hube-Magg, C.; Blessin, N.C.; Fraune, C.; et al. Mesothelin Expression in Human Tumors: A Tissue Microarray Study on 12,679 Tumors. Biomedicines 2021, 9, 397. [Google Scholar] [CrossRef]
- Faust, J.R.; Hamill, D.; Kolb, E.A.; Gopalakrishnapillai, A.; Barwe, S.P. Mesothelin: An Immunotherapeutic Target beyond Solid Tumors. Cancers 2022, 14, 1550. [Google Scholar] [CrossRef]
- Benloucif, A.; Meyer, D.; Balasse, L.; Goubard, A.; Danner, L.; Bouhlel, A.; Castellano, R.; Guillet, B.; Chames, P.; Kerfelec, B. Rapid nanobody-based imaging of mesothelin expressing malignancies compatible with blocking therapeutic antibodies. Front. Immunol. 2023, 14, 1200652. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.Y.; Jones, C.; Baek, Y.; Park, G.K.; Kashiwagi, S.; Choi, H.S. Near-infrared fluorescence imaging in immunotherapy. Adv. Drug Deliv. Rev. 2020, 167, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; van Dongen, G.A.M.S.; Stigter-van Walsum, M.; Roovers, R.C.; Stam, J.C.; Mali, W.; van Diest, P.J.; van Bergen en Henegouwen, P.M.P. Rapid Visualization of Human Tumor Xenografts through Optical Imaging with a Near-infrared Fluorescent Anti-Epidermal Growth Factor Receptor Nanobody. Mol. Imaging 2012, 11, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.B.; Wu, H.F.; Hu, W.Q.; Jin, Y.J.; Kong, M.Y.; Wang, Y.L.; Chen, B.F.; Li, Q.X.; Huang, K.K.; Yang, Z.L.; et al. An antigen-strengthened dye-modified fully-human-nanobody-based immunoprobe for second near infrared bioimaging of metastatic tumors. Biomaterials 2022, 287, 121637. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Li, C.J.; Jia, X.H.; Qu, Y.W.; Li, M.M.; Cao, C.G.; Zhang, Z.Y.; Qu, Q.J.; Luo, S.L.; Tang, J.Q.; et al. NIR-II fluorescence imaging-guided colorectal cancer surgery targeting CEACAM5 by a nanobody. Ebiomedicine 2023, 89, 104476. [Google Scholar] [CrossRef]
- Xiao, Y.T.; Mei, C.M.; Xu, D.; Yang, F.; Yang, M.L.; Bi, L.; Mao, J.J.; Pang, P.F.; Li, D. Identification of a CEACAM5 targeted nanobody for positron emission tomography imaging and near-infrared fluorescence imaging of colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2305–2318. [Google Scholar] [CrossRef]
- Lwin, T.M.; Turner, M.A.; Nishino, H.; Amirfakhri, S.; Hernot, S.; Hoffman, R.M.; Bouvet, M. Fluorescent Anti-CEA Nanobody for Rapid Tumor-Targeting and Imaging in Mouse Models of Pancreatic Cancer. Biomolecules 2022, 12, 711. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.M.; Hernot, S.; Hollandsworth, H.; Amirfakhri, S.; Filemoni, F.; Debie, P.; Hoffman, R.M.; Bouvet, M. Tumor-specific near-infrared nanobody probe rapidly labels tumors in an orthotopic mouse model of pancreatic cancer. Surgery 2020, 168, 85–91. [Google Scholar] [CrossRef]
- Debie, P.; Vanhoeij, M.; Poortmans, N.; Puttemans, J.; Gillis, K.; Devoogdt, N.; Lahoutte, T.; Hernot, S. Improved Debulking of Peritoneal Tumor Implants by Near-Infrared Fluorescent Nanobody Image Guidance in an Experimental Mouse Model. Mol. Imaging Biol. 2018, 20, 361–367. [Google Scholar] [CrossRef]
- van Driel, P.B.A.A.; van der Vorst, J.R.; Verbeek, F.P.R.; Oliveira, S.; Snoeks, T.J.A.; Keereweer, S.; Chan, B.; Boonstra, M.C.; Frangioni, J.V.; van Bergen en Henegouwen, P.M.P.; et al. Intraoperative fluorescence delineation of head and neck cancer with a fluorescent Anti- epidermal growth factor receptor nanobody. Int. J. Cancer 2014, 134, 2663–2673. [Google Scholar] [CrossRef]
- Hou, Y.J.; Yang, X.X.; Liu, R.Q.; Zhao, D.; Guo, C.X.; Zhu, A.C.; Wen, M.N.; Liu, Z.; Qu, G.F.; Meng, H.X. Pathological Mechanism of Photodynamic Therapy and Photothermal Therapy Based on Nanoparticles. Int. J. Nanomed. 2020, 15, 6827–6838. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.Z.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef] [PubMed]
- St Denis, T.G.; Hamblin, M.R. Synthesis, bioanalysis and biodistribution of photosensitizer conjugates for photodynamic therapy. Bioanalysis 2013, 5, 1099–1114. [Google Scholar] [CrossRef]
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.B.A.A.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; Henegouwen, P.M.P.V.E.; et al. EGFR targeted nanobody-photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, H.S.; Mashayekhi, V.; Schreurs, T.J.L.; van Driel, P.B.A.A.; Strijkers, G.J.; van Diest, P.J.; Lowik, C.W.G.M.; Seynhaeve, A.L.B.; ten Hagen, T.L.M.; Prompers, J.J.; et al. Acute cellular and vascular responses to photodynamic therapy using EGFR-targeted nanobody-photosensitizer conjugates studied with intravital optical imaging and magnetic resonance imaging. Theranostics 2020, 10, 2436–2452. [Google Scholar] [CrossRef]
- Mashayekhi, V.; Xenaki, K.T.; Henegouwen, P.M.P.V.E.; Oliveira, S. Dual Targeting of Endothelial and Cancer Cells Potentiates In Vitro Nanobody-Targeted Photodynamic Therapy. Cancers 2020, 12, 2732. [Google Scholar] [CrossRef]
- Driehuis, E.; Spelier, S.; Hernandez, I.B.; de Bree, R.; Willems, S.M.; Clevers, H.; Oliveira, S. Patient-Derived Head and Neck Cancer Organoids Recapitulate EGFR Expression Levels of Respective Tissues and Are Responsive to EGFR-Targeted Photodynamic Therapy. J. Clin. Med. 2019, 8, 1880. [Google Scholar] [CrossRef]
- Renard, E.; Camps, E.C.; Canovas, C.; Kip, A.; Gotthardt, M.; Rijpkema, M.; Denat, F.; Goncalves, V.; van Lith, S.A.M. Site-Specific Dual-Labeling of a VHH with a Chelator and a Photosensitizer for Nuclear Imaging and Targeted Photodynamic Therapy of EGFR-Positive Tumors. Cancers 2021, 13, 428. [Google Scholar] [CrossRef]
- Xiong, T.; Peng, Q.; Chen, Y.C.; Li, M.; Du, J.J.; Fan, J.L.; Jia, L.Y.; Peng, X.J. A Novel Nanobody-Photosensitizer Conjugate for Hypoxia Resistant Photoimmunotherapy. Adv. Funct. Mater. 2021, 31, 2103629. [Google Scholar] [CrossRef]
- Liu, Y.N.; Scrivano, L.; Peterson, J.D.; Fens, M.H.A.M.; Hernandez, I.B.; Mesquita, B.; Torano, J.S.; Hennink, W.E.; van Nostrum, C.F.; Oliveira, S. EGFR-Targeted Nanobody Functionalized Polymeric Micelles Loaded with mTHPC for Selective Photodynamic Therapy. Mol. Pharm. 2020, 17, 1276–1292. [Google Scholar] [CrossRef]
- O’Connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Mann, F.A.; Herrmann, N.; Meyer, D.; Kruss, S. Tuning Selectivity of Fluorescent Carbon Nanotube-Based Neurotransmitter Sensors. Sensor 2017, 17, 1521. [Google Scholar] [CrossRef]
- Mann, F.A.; Lv, Z.Y.; Grosshans, J.; Opazo, F.; Kruss, S. Nanobody-Conjugated Nanotubes for Targeted Near-Infrared In Vivo Imaging and Sensing. Angew. Chem. Int. Ed. 2019, 58, 11469–11473. [Google Scholar] [CrossRef]
- Otani, K.; Yamahara, K. Development of Antibody-Carrying Microbubbles Based on Clinically Available Ultrasound Contrast Agent for Targeted Molecular Imaging: A Preliminary Chemical Study. Mol. Imaging Biol. 2011, 13, 250–256. [Google Scholar] [CrossRef]
- Ma, J.J.; Wang, Y.; Xi, X.H.; Tang, J.J.; Wang, L.P.; Wang, L.K.; Wang, D.; Liang, X.L.; Zhang, B. Contrast-enhanced ultrasound combined targeted microbubbles for diagnosis of highly aggressive papillary thyroid carcinoma. Front. Endocrinol. 2023, 14, 1052862. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Chen, Y.Q.; Liu, R.Q.; Duan, S.B.; Chen, L.J.; Sun, J.; Zhang, L.Z. Study on Contrast-Enhanced Ultrasound Imaging and Anti-Tumor Effects of Drug-Loaded Nanodroplets with Tumor Targeting and Ultrasound Sensitivity. Front. Biosci. 2023, 28, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.J.; Li, Z.F.; Feng, X.Q.; Yang, D.Z.; Lin, M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 11–26. [Google Scholar] [CrossRef]
- Ghahroudi, M.A.; Desmyter, A.; Wyns, L.; Hamers, R.; Muyldermans, S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997, 414, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Rothbauer, U.; Zolghadr, K.; Tillib, S.; Nowak, D.; Schermelleh, L.; Gahl, A.; Backmann, N.; Conrath, K.; Muyldermans, S.; Cardoso, M.C.; et al. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nat. Methods 2006, 3, 887–889. [Google Scholar] [CrossRef]
- Conrath, K.E.; Lauwereys, M.; Wyns, L.; Muyldermans, S. Camel single-domain antibodies as modular building units in bispecific and bivalent antibody constructs. J. Biol. Chem. 2001, 276, 7346–7350. [Google Scholar] [CrossRef] [PubMed]
- Hernot, S.; Unnikrishnan, S.; Du, Z.; Cosyns, B.; Broisat, A.; Muyldermans, S.; Lahoutte, T.; Klibanov, A.L.; Devoogdt, N. Nanobody-coupled microbubbles as novel molecular tracer. Eur. Heart J. 2012, 33, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.S.; Natarajan, A.; Massoud, T.F.; Paulmurugan, R. FN3 linked nanobubbles as a targeted contrast agent for US imaging of cancer-associated human PD-L1. J. Control. Release 2022, 346, 317–327. [Google Scholar] [CrossRef]
- van den Bijgaart, R.J.E.; Eikelenboom, D.C.; Hoogenboom, M.; Futterer, J.J.; den Brok, M.H.; Adema, G.J. Thermal and mechanical high-intensity focused ultrasound: Perspectives on tumor ablation, immune effects and combination strategies. Cancer Immunol. Immun. 2017, 66, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Narbona, J.; Hernandez-Baraza, L.; Gordo, R.G.; Sanz, L.; Lacadena, J. Nanobody-Based EGFR-Targeting Immunotoxins for Colorectal Cancer Treatment. Biomolecules 2023, 13, 1042. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Xu, S.Y.; Li, L.Z.; Li, Y.X.; Zhao, M.Q.; Chen, J.S.; Zhu, S.Y.; Xie, Y.Q.; Jiang, H.; Zhu, J.W.; et al. Tumour inhibitory activity on pancreatic cancer by bispecific nanobody targeting PD-L1 and CXCR4. BMC Cancer 2022, 22, 1092. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int. Immunol. 2016, 28, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, M.E.; Bargou, R. Blinatumomab: A CD19/CD3 bispecific T cell engager (BiTE) with unique anti-tumor efficacy. Leuk. Lymphoma 2016, 57, 1021–1032. [Google Scholar] [CrossRef]
- Zhai, T.H.; Wang, C.; Xu, Y.F.; Huang, W.F.; Yuan, Z.J.; Wang, T.; Dai, S.; Peng, S.G.; Pang, T.L.; Jiang, W.C.; et al. Generation of a safe and efficacious llama single-domain antibody fragment (vHH) targeting the membrane-proximal region of 4-1BB for engineering therapeutic bispecific antibodies for cancer. J. Immunother. Cancer 2021, 9, e002131. [Google Scholar] [CrossRef]
- Bachmann, M. The UniCAR system: A modular CAR T cell approach to improve the safety of CAR T cells. Immunol. Lett. 2019, 211, 13–22. [Google Scholar] [CrossRef]
- Maali, A.; Gholizadeh, M.; Feghhi-Najafabadi, S.; Noei, A.; Seyed-Motahari, S.S.; Mansoori, S.; Sharifzadeh, Z. Nanobodies in cell-mediated immunotherapy: On the road to fight cancer. Front. Immunol. 2023, 14, 1012841. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Zhong, D.N.; Luo, H.G.; Shi, W.; Xie, S.X.; Qiang, H.B.; Zhu, L.C.; Gao, L.; Liu, J.; Sun, S.Y.; et al. Nanobody-based CAR T cells targeting intracellular tumor antigens. Biomed. Pharmacother. 2022, 156, 113919. [Google Scholar] [CrossRef]
- Castellarin, M.; Watanabe, K.; June, C.H.; Kloss, C.C.; Posey, A.D. Driving cars to the clinic for solid tumors. Gene Ther. 2018, 25, 165–175. [Google Scholar] [CrossRef]
- Du, H.W.; Hirabayashi, K.; Ahn, S.; Kren, N.P.; Montgomery, S.A.; Wang, X.H.; Tiruthani, K.; Mirlekar, B.; Michaud, D.; Greene, K.; et al. Antitumor Responses in the Absence of Toxicity in Solid Tumors by Targeting B7-H3 via Chimeric Antigen Receptor T Cells. Cancer Cell 2019, 35, 221–237.e8. [Google Scholar] [CrossRef]
- Nikkhoi, S.K.; Li, G.; Eleya, S.; Yang, G.; Vandavasi, V.G.; Hatefi, A. Bispecific killer cell engager with high affinity and specificity toward CD16a on NK cells for cancer immunotherapy. Front. Immunol. 2023, 13, 1039969. [Google Scholar] [CrossRef] [PubMed]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef]
- Kumari, N.; Choi, S.H. Tumor-associated macrophages in cancer: Recent advancements in cancer nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022, 41, 68. [Google Scholar] [CrossRef]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular vesicles: Masters of intercellular communication and potential clinical interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.Q.; Wei, L.K.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.H.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.T.; Liang, X.L.; Li, Y.; Feng, Q.Q.; Cheng, K.M.; Ma, N.N.; Zhu, F.; Guo, X.J.; Yue, Y.L.; Liu, G.N.; et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat. Commun. 2023, 14, 1606. [Google Scholar] [CrossRef] [PubMed]
- Erreni, M.; Schorn, T.; D’Autilia, F.; Doni, A. Nanobodies as Versatile Tool for Multiscale Imaging Modalities. Biomolecules 2020, 10, 1695. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of Ga-68-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef]
- Zhao, L.Z.; Liu, C.C.; Xing, Y.; He, J.; O’Doherty, J.; Huang, W.H.; Zhao, J.H. Development of a Tc-99m-Labeled Single-Domain Antibody for SPECT/CT Assessment of HER2 Expression in Breast Cancer. Mol. Pharm. 2021, 18, 3616–3622. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kawanishi, M.; Nakanishi, M.; Yamasaki, H.; Takeuchi, T. Efficacy and safety of the anti-TNF multivalent NANOBODY (R) compound ozoralizumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: A 52-week result of a Phase II/III study (OHZORA trial). Mod. Rheumatol. 2023, 33, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kawanishi, M.; Nakanishi, M.; Yamasaki, H.; Tanaka, Y. Phase II/III Results of a Trial of Anti-Tumor Necrosis Factor Multivalent NANOBODY Compound Ozoralizumab in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2022, 74, 1776–1785. [Google Scholar] [CrossRef]
- Martin, T.; Lin, Y.; Agha, M.; Cohen, A.D.; Htut, M.; Stewart, A.K.; Hari, P.; Berdeja, J.G.; Usmani, S.Z.; Yeh, T.M.; et al. Health-related quality of life in patients given ciltacabtagene autoleucel for relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b-2, open-label study. Lancet Haematol. 2022, 9, E897–E905. [Google Scholar] [CrossRef]
- Li, J.; Deng, Y.H.; Zhang, W.J.; Zhou, A.P.; Guo, W.J.; Yang, J.W.; Yuan, Y.; Zhu, L.J.; Qin, S.K.; Xiang, S.L.; et al. Subcutaneous envafolimab monotherapy in patients with advanced defective mismatch repair/microsatellite instability high solid tumors. J. Hematol. Oncol. 2021, 14, 95. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.Y.; Lu, H.L.; Lu, N.; He, Y.; Xu, T.; Dong, R.P.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, E1514–E1525. [Google Scholar] [CrossRef]
- Shimizu, T.; Nakajima, T.E.; Yamamoto, N.; Yonemori, K.; Koyama, T.; Kondo, S.; Sunakawa, Y.; Izawa, N.; Horie, Y.; Xiang, S.L.; et al. Phase I study of envafolimab (KN035), a novel subcutaneous single-domain anti-PD-L1 monoclonal antibody, in Japanese patients with advanced solid tumors. Investig. New Drugs 2022, 40, 1021–1031. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Chen, D.; Fu, X.R.; Meng, H.M.; Nan, F.F.; Sun, Z.C.; Yu, H.; Zhang, L.; Li, L.; Li, X.; et al. Autologous Nanobody-Derived Fratricide-Resistant CD7-CAR T-cell Therapy for Patients with Relapsed and Refractory T-cell Acute Lymphoblastic Leukemia/Lymphoma. Clin. Cancer Res. 2022, 28, 2830–2843. [Google Scholar] [CrossRef]
- Le Tourneau, C.; Becker, H.; Claus, R.; Elez, E.; Ricci, F.; Fritsch, R.; Silber, Y.; Hennequin, A.; Tabernero, J.; Jayadeva, G.; et al. Two phase I studies of BI 836880, a vascular endothelial growth factor/angiopoietin-2 inhibitor, administered once every 3 weeks or once weekly in patients with advanced solid tumors. Esmo Open 2022, 7, 100576. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Koyama, T.; Shimizu, T.; Todaka, A.; Kawakami, T.; Erzen, D.; Sarashina, A.; Li, B.; Hou, J.R.; Yamazaki, K. Phase I study of the VEGF/Ang-2 inhibitor BI 836880 alone or combined with the anti-programmed cell death protein-1 antibody ezabenlimab in Japanese patients with advanced solid tumors. Cancer Chemoth. Pharmcol. 2023, 91, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Butler, M.; O’Cearbhaill, R.E.; Oh, D.Y.; Johnson, M.; Zikaras, K.; Smalley, M.; Ross, M.; Tanyi, J.L.; Ghafoor, A.; et al. Mesothelin-targeting T cell receptor fusion construct cell therapy in refractory solid tumors: Phase 1/2 trial interim results. Nat. Med. 2023, 29, 2099–2109. [Google Scholar] [CrossRef] [PubMed]
- Gondry, O.; Xavier, C.; Raes, L.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Breckpot, K.; Lecocq, Q.; Decoster, L.; Fontaine, C.; et al. Phase I Study of [68Ga]Ga-Anti-CD206-sdAb for PET/CT Assessment of Protumorigenic Macrophage Presence in Solid Tumors (MMR Phase I). J. Nucl. Med. 2023, 64, 1378–1384. [Google Scholar] [CrossRef]
- Li, W.; Schafer, A.; Kulkarni, S.S.; Liu, X.L.; Martinez, D.R.; Chen, C.; Sun, Z.H.; Leist, S.R.; Drelich, A.; Zhang, L.Y.; et al. High Potency of a Bivalent Human V-H Domain in SARS-CoV-2 Animal Models. Cell 2020, 183, 429–441. [Google Scholar] [CrossRef]

| Name | Applications | Cancers | Phage | References |
|---|---|---|---|---|
| Ciltacabtagene autoleucel (cilta-cel) | CAR-T cell therapy expressing two BCMA-targeting single-domain antibodies and a CD3-ζ signaling domain with a 4-1BB costimulatory domain | Relapsed or refractory multiple myeloma | 1b/2 | [72,129] |
| Envafolimab | Anti-PD-L1 antibody fused to a human immunoglobulin Fc fragment | Advanced or metastatic malignant dMMR/MSI-H solid tumors | 2 | [130,131,132] |
| CD7 nanobody VHH6 | CD7-CAR T cells with humanized CD7 VHH joined to CD8α transmembrane domain | CD7-positive T-lymphoblastic leukemia/lymphoma | 1 | [133] |
| Ablynx | 68Ga-HER2 nanobody for SPECT and PET imaging | Primary or metastatic breast carcinoma | 1 | [125] |
| NM-01 | Nanobody against PD-L1 radiolabeled with 99mTc for SPECT imaging | Non-small cell lung cancer | 1 | [68] |
| NM-02 | Using sdAb (NM-02) to develop a 99mTc-labeled anti-HER2 sdAb (99mTc-NM-02) for SPECT/CT | Breast cancer | 1 | [126] |
| BI 836880 | A humanized bispecific nanobody that inhibits vascular endothelial growth factor and angiopoietin-2 | Locally advanced or metastatic solid tumors | 1 | [134,135] |
| Gavocabtagene autoleucel (gavo-cel; TC-210) | T cell receptor fusion construct from the fusion of single-domain anti-mesothelin antibody MH1 to a 15-amino acid flexible glycine/serine spacer (+G4S)3 and the human CD3ε subunit | Malignant pleural or peritoneal mesothelioma (MPM), non-small cell lung cancer (NSCLC), ovarian cancer or cholangiocarcinoma | 1/2 | [136] |
| [68Ga] Ga-NOTA-anti-CD206 single-domain antibody | Compared to NOTA-anti-CD206-sdAb | Solid tumor | 1 | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhang, N.; Xiao, H.; Wang, C.; He, L. Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics. Cancers 2023, 15, 5639. https://doi.org/10.3390/cancers15235639
Zhang Q, Zhang N, Xiao H, Wang C, He L. Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics. Cancers. 2023; 15(23):5639. https://doi.org/10.3390/cancers15235639
Chicago/Turabian StyleZhang, Qian, Nan Zhang, Han Xiao, Chen Wang, and Lian He. 2023. "Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics" Cancers 15, no. 23: 5639. https://doi.org/10.3390/cancers15235639
APA StyleZhang, Q., Zhang, N., Xiao, H., Wang, C., & He, L. (2023). Small Antibodies with Big Applications: Nanobody-Based Cancer Diagnostics and Therapeutics. Cancers, 15(23), 5639. https://doi.org/10.3390/cancers15235639





