Effects of Physical Activity Interventions on Self-Perceived Health Status among Lung Cancer Patients: Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Intervention
2.3. Comparison
2.4. Outcomes
2.5. Search Strategy, Article Selection, and Data Extraction
- English: Quality of life AND lung cancer OR lung neoplasms OR lung tumour AND intervention AND exercise OR physical activity OR fitness OR aerobic training OR strength training OR cardiovascular training AND randomized controlled trials OR rct OR randomised control trials OR randomized clinical trial OR randomized OR randomised clinical trial.
- Spanish: calidad de vida Y cáncer de pulmón O neoplasias pulmonares O tumor pulmonar Y intervención Y ejercicio O actividad física O fitness O entrenamiento aeróbico O entrenamiento de fuerza O entrenamiento cardiovascular Y ensayo clínico aleatorizado O ensayo controlado aleatorizado O eca O aleatorizado O aleatorio.
2.6. Quality of Evidence and Risk of Bias
2.7. Statistical Analysis
3. Results
3.1. Description of Participants Characteristics
3.2. Intervention Characterisctics
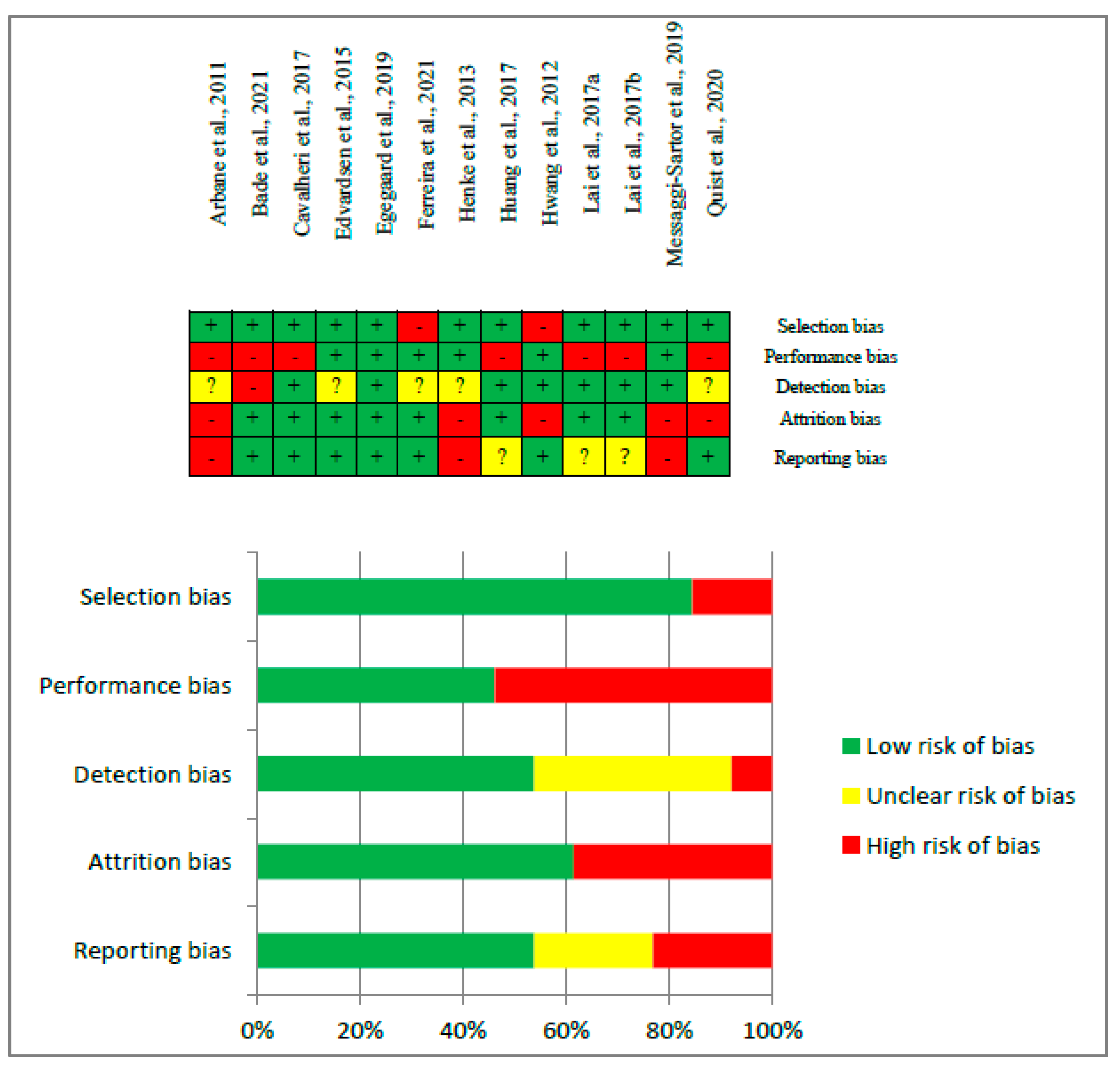
3.3. Risk of Bias and Quality of Evidence Assessments
3.4. Physical Activity Effects on Self-Perceived Quality of Life
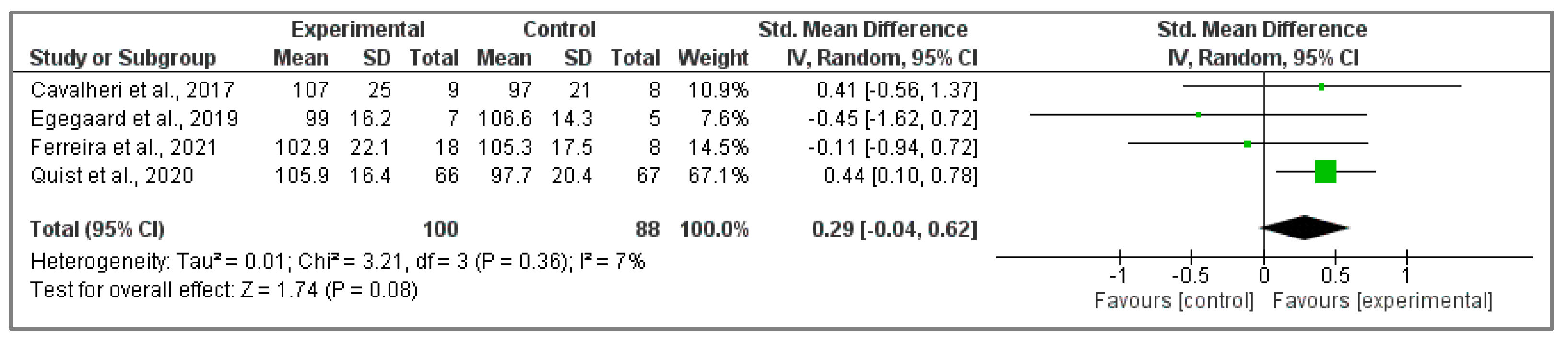
3.4.1. Analysis of EORTC QLQ-C30 Questionnaire
3.4.2. Assessment of FACT-L Questionnaire
3.5. Physical Activity Effects on Self-Perceived Wellbeing
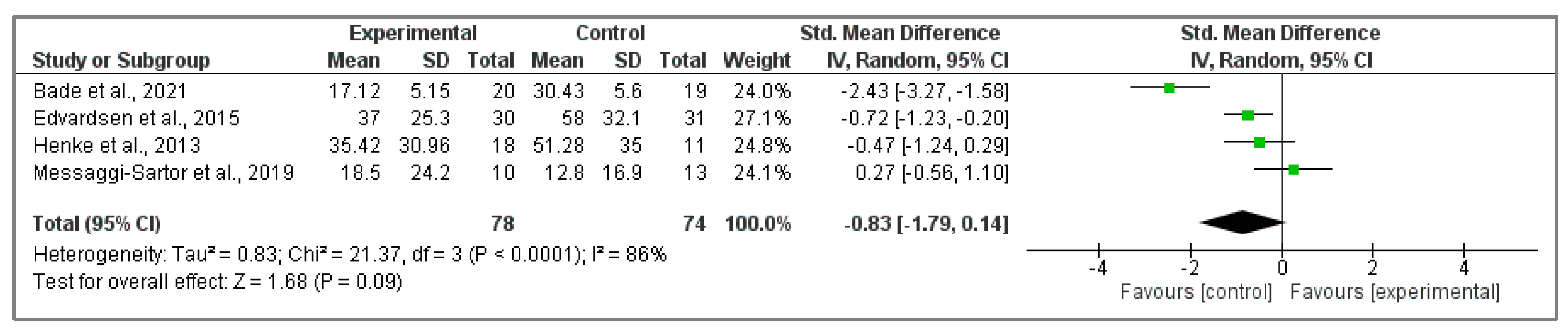
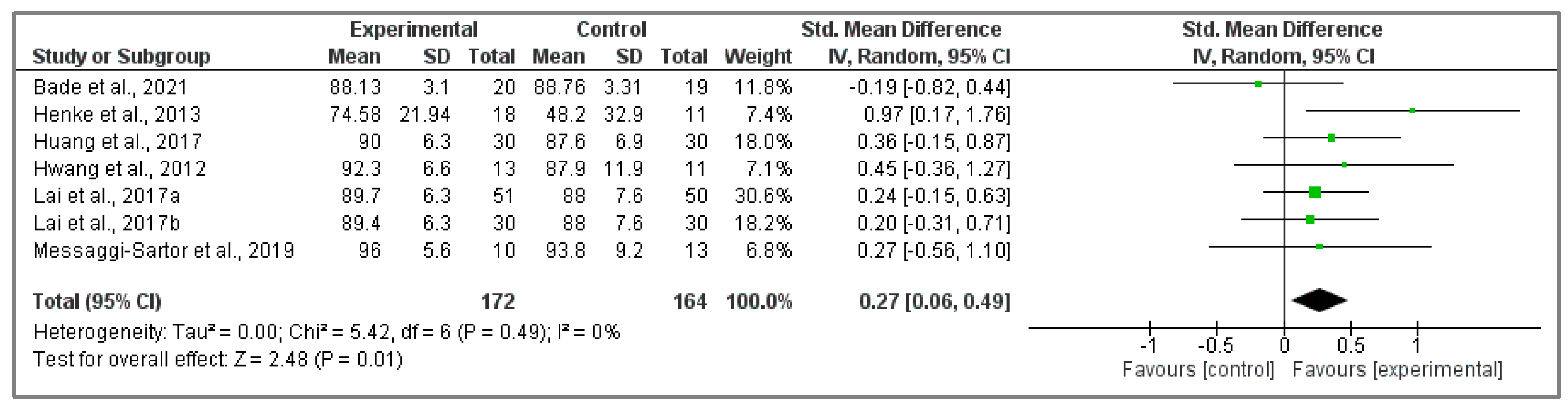
3.5.1. Analysis of EORTC QLQ-C30 Questionnaire
3.5.2. Assessment of FACT-L Questionnaire
3.6. Physical Activity Effects on Self-Perceived Dyspnea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jakobsen, E.; Rasmussen, T.R.; Green, A. Mortality and Survival of Lung Cancer in Denmark: Results from the Danish Lung Cancer Group 2000–2012. Acta Oncol. 2016, 55 (Suppl. S2), S2–S9. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. Cancer Statistics, 2011: The Impact of Eliminating Socioeconomic and Racial Disparities on Premature Cancer Deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; Hassan, O.U.I.; Yang, Y.W.; Buchanan, P. Lung Cancer: Biology and Treatment Options. Biochim. Biophys. Acta 2015, 1856, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Assaran Darban, R.; Javid, H.; Hashemy, S.I. The Therapeutic Potential of Aprepitant in Glioblastoma Cancer Cells through Redox Modification. BioMed Res. Int. 2022, 2022, 8540403. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.E.; Ahmed, O.M.; Zoheir, K.M.A.; El-Shahawy, A.A.G.; Tamur, S.; Shams, A.; Burcher, J.T.; Bishayee, A.; Abdel-Moneim, A. Naringin-Dextrin Nanocomposite Abates Diethylnitrosamine/Acetylaminofluorene-Induced Lung Carcinogenesis by Modulating Oxidative Stress, Inflammation, Apoptosis, and Cell Proliferation. Cancers 2023, 15, 5102. [Google Scholar] [CrossRef]
- Filippatos, K.; Koukourakis, I.M.; Anevlavis, S.; Giaktzidis, A.; Koukourakis, M.I. Ultra-Hypofractionated Re-Irradiation with Anti-PD-1 Immunotherapy for Locoregionally Recurrent (after Radical Chemo-Radiotherapy) Non-Small Cell Lung Cancer. Cancers 2023, 15, 5083. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Elshoeibi, A.M.; Elsayed, B.; Kaleem, M.Z.; Elhadary, M.R.; Abu-Haweeleh, M.N.; Haithm, Y.; Krzyslak, H.; Vranic, S.; Pedersen, S. Proteomic Profiling of Small-Cell Lung Cancer: A Systematic Review. Cancers 2023, 15, 5005. [Google Scholar] [CrossRef]
- Tanaka, K.; Akechi, T.; Okuyama, T.; Nishiwaki, Y.; Uchitomi, Y. Impact of Dyspnea, Pain, and Fatigue on Daily Life Activities in Ambulatory Patients with Advanced Lung Cancer. J. Pain. Symptom Manag. 2002, 23, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.L.; Parry, S.M.; Edbrooke, L.; Denehy, L. Deterioration in Physical Activity and Function Differs According to Treatment Type in Non-Small Cell Lung Cancer—Future Directions for Physiotherapy Management. Physiotherapy 2016, 102, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Hornsby, W.E.; Goetzinger, A.; Forbes, L.M.; Sherrard, E.L.; Quist, M.; Lane, A.T.; West, M.; Eves, N.D.; Gradison, M.; et al. Prognostic Significance of Functional Capacity and Exercise Behavior in Patients with Metastatic Non-Small Cell Lung Cancer. Lung Cancer 2012, 76, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Taylor-Stokes, G.; Roughley, A. Symptom Burden and Quality of Life in Advanced Non-Small Cell Lung Cancer Patients in France and Germany. Lung Cancer 2013, 81, 288–293. [Google Scholar] [CrossRef]
- Felce, D.; Perry, J. Quality of Life: Its Definition and Measurement. Res. Dev. Disabil. 1995, 16, 51–74. [Google Scholar] [CrossRef]
- Avery, K.N.L.; Blazeby, J.M.; Chalmers, K.A.; Batchelor, T.J.P.; Casali, G.; Internullo, E.; Krishnadas, R.; Evans, C.; West, D. Impact on Health-Related Quality of Life of Video-Assisted Thoracoscopic Surgery for Lung Cancer. Ann. Surg. Oncol. 2020, 27, 1259–1271. [Google Scholar] [CrossRef]
- Nugent, S.M.; Golden, S.E.; Hooker, E.R.; Sullivan, D.R.; Thomas, C.R.; Deffebach, M.E.; Sukumar, M.S.; Schipper, P.H.; Tieu, B.H.; Moghanaki, D.; et al. Longitudinal Health-Related Quality of Life among Individuals Considering Treatment for Stage I Non-Small-Cell Lung Cancer. Ann. Am. Thorac. Soc. 2020, 17, 988–997. [Google Scholar] [CrossRef]
- Akin, S.; Can, G.; Aydiner, A.; Ozdilli, K.; Durna, Z. Quality of Life, Symptom Experience and Distress of Lung Cancer Patients Undergoing Chemotherapy. Eur. J. Oncol. Nurs. 2010, 14, 400–409. [Google Scholar] [CrossRef]
- Ferreira, V.; Lawson, C.; Ekmekjian, T.; Carli, F.; Scheede-Bergdahl, C.; Chevalier, S. Effects of Preoperative Nutrition and Multimodal Prehabilitation on Functional Capacity and Postoperative Complications in Surgical Lung Cancer Patients: A Systematic Review. Support. Care Cancer 2021, 29, 5597–5610. [Google Scholar] [CrossRef]
- Paramanandam, V.S.; Dunn, V. Exercise for the Management of Cancer-Related Fatigue in Lung Cancer: A Systematic Review. Eur. J. Cancer Care Engl. 2015, 24, 4–14. [Google Scholar] [CrossRef]
- Chen, H.M.; Tsai, C.M.; Wu, Y.C.; Lin, K.C.; Lin, C.C. Randomised Controlled Trial on the Effectiveness of Home-Based Walking Exercise on Anxiety, Depression and Cancer-Related Symptoms in Patients with Lung Cancer. Br. J. Cancer 2015, 112, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Glattki, G.P.; Manika, K.; Sichletidis, L.; Alexe, G.; Brenke, R.; Spyratos, D. Pulmonary Rehabilitation in Non-Small Cell Lung Cancer Patients after Completion of Treatment. Am. J. Clin. Oncol. 2012, 35, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Ostroff, J.S.; Krebs, P.; Coups, E.J.; Burkhalter, J.E.; Feinstein, M.B.; Steingart, R.M.; Logue, A.E.; Park, B.J. Health-Related Quality of Life among Early-Stage, Non-Small Cell, Lung Cancer Survivors. Lung Cancer 2011, 71, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Toohey, K.; Chapman, M.; Rushby, A.M.; Urban, K.; Ingham, G.; Singh, B. The Effects of Physical Exercise in the Palliative Care Phase for People with Advanced Cancer: A Systematic Review with Meta-Analysis. J. Cancer Surviv. 2023, 17. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Estarli, M.; Barrera, E.S.A.; et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 2015, 4, 148–160. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; Haes, J.C.J.M.D.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A Quality-of-Life Instrument for Use in International Clinical Trials in Oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef]
- Maringwa, J.T.; Quinten, C.; King, M.; Ringash, J.; Osoba, D.; Coens, C.; Martinelli, F.; Vercauteren, J.; Cleeland, C.S.; Flechtner, H.; et al. Minimal Important Differences for Interpreting Health-Related Quality of Life Scores from the EORTC QLQ-C30 in Lung Cancer Patients Participating in Randomized Controlled Trials. Support. Care Cancer 2011, 19, 1753–1760. [Google Scholar] [CrossRef]
- Cella, D.F.; Bonomi, A.E.; Lloyd, S.R.; Tulsky, D.S.; Kaplan, E.; Bonomi, P. Reliability and Validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) Quality of Life Instrument. Lung Cancer 1995, 12, 199–220. [Google Scholar] [CrossRef]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An Emerging Consensus on Rating Quality of Evidence and Strength of Recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Assessing Risk of Bias in a Randomized Trial. In Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Chichester, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust Variance Estimation in Meta-Regression with Dependent Effect Size Estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [CrossRef]
- Higgins, J.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley and Sons: Chichester, UK, 2009; Volume 5. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Ferreira González, I.; Urrútia, G.; Alonso-Coello, P. Systematic Reviews and Meta-Analysis: Scientific Rationale and Interpretation. Rev. Esp. Cardiol. 2011, 64, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Arbane, G.; Tropman, D.; Jackson, D.; Garrod, R. Evaluation of an Early Exercise Intervention after Thoracotomy for Non-Small Cell Lung Cancer (NSCLC), Effects on Quality of Life, Muscle Strength and Exercise Tolerance: Randomised Controlled Trial. Lung Cancer 2011, 71, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bade, B.C.; Gan, G.; Li, F.; Lu, L.; Tanoue, L.; Silvestri, G.A.; Irwin, M.L. Randomized Trial of Physical Activity on Quality of Life and Lung Cancer Biomarkers in Patients with Advanced Stage Lung Cancer: A Pilot Study. BMC Cancer 2021, 21, 352. [Google Scholar] [CrossRef]
- Cavalheri, V.; Jenkins, S.; Cecins, N.; Gain, K.; Phillips, M.J.; Sanders, L.H.; Hill, K. Exercise Training for People Following Curative Intent Treatment for Non-Small Cell Lung Cancer: A Randomized Controlled Trial. Braz. J. Phys. Ther. 2017, 21, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Edvardsen, E.; Skjønsberg, O.H.; Holme, I.; Nordsletten, L.; Borchsenius, F.; Anderssen, S.A. High-Intensity Training Following Lung Cancer Surgery: A Randomised Controlled Trial. Thorax 2015, 70, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Egegaard, T.; Rohold, J.; Lillelund, C.; Persson, G.; Quist, M. Pre-Radiotherapy Daily Exercise Training in Non-Small Cell Lung Cancer: A Feasibility Study. Rep. Pract. Oncol. Radiother. 2019, 24, 375–382. [Google Scholar] [CrossRef]
- Ferreira, V.; Lawson, C.; Carli, F.; Scheede-Bergdahl, C.; Chevalier, S. Feasibility of a Novel Mixed-Nutrient Supplement in a Multimodal Prehabilitation Intervention for Lung Cancer Patients Awaiting Surgery: A Randomized Controlled Pilot Trial. Int. J. Surg. 2021, 93. [Google Scholar] [CrossRef]
- Henke, C.C.; Cabri, J.; Fricke, L.; Pankow, W.; Kandilakis, G.; Feyer, P.C.; De Wit, M. Strength and Endurance Training in the Treatment of Lung Cancer Patients in Stages IIIA/IIIB/IV. Support. Care Cancer 2014, 22, 95–101. [Google Scholar] [CrossRef]
- Huang, J.; Lai, Y.; Zhou, X.; Li, S.; Su, J.; Yang, M.; Che, G. Short-Term High-Intensity Rehabilitation in Radically Treated Lung Cancer: A Three-Armed Randomized Controlled Trial. J. Thorac. Dis. 2017, 9, 1919–1929. [Google Scholar] [CrossRef]
- Hwang, C.L.; Yu, C.J.; Shih, J.Y.; Yang, P.C.; Wu, Y.T. Effects of Exercise Training on Exercise Capacity in Patients with Non-Small Cell Lung Cancer Receiving Targeted Therapy. Support. Care Cancer 2012, 20, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Su, J.; Qiu, P.; Wang, M.; Zhou, K.; Tang, Y.; Che, G. Systematic Short-Term Pulmonary Rehabilitation before Lung Cancer Lobectomy: A Randomized Trial. Interact. Cardiovasc. Thorac. Surg. 2017, 25, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Huang, J.; Yang, M.; Su, J.; Liu, J.; Che, G. Seven-Day Intensive Preoperative Rehabilitation for Elderly Patients with Lung Cancer: A Randomized Controlled Trial. J. Surg. Res. 2017, 209, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Messaggi-Sartor, M.; Marco, E.; Martínez-Téllez, E.; Rodriguez-Fuster, A.; Palomares, C.; Chiarella, S.; Muniesa, J.M.; Orozco-Levi, M.; Barreiro, E.; Güell, M.R. Combined Aerobic Exercise and High-Intensity Respiratory Muscle Training in Patients Surgically Treated for Non-Small Cell Lung Cancer: A Pilot Randomized Clinical Trial. Eur. J. Phys. Rehabil. Med. 2019, 55, 113–122. [Google Scholar] [CrossRef]
- Quist, M.; Langer, S.W.; Lillelund, C.; Winther, L.; Laursen, J.H.; Christensen, K.B.; Rørth, M.; Adamsen, L. Effects of an Exercise Intervention for Patients with Advanced Inoperable Lung Cancer Undergoing Chemotherapy: A Randomized Clinical Trial. Lung Cancer 2020, 145, 76–82. [Google Scholar] [CrossRef]
- Pompili, C.; Brunelli, A.; Xiumé, F.; Refai, M.; Salati, M.; Socci, L.; Di Nunzio, L.; Sabbatini, A. Prospective External Convergence Evaluation of Two Different Quality-of-Life Instruments in Lung Resection Patients. Eur. J. Cardiothorac. Surg. 2011, 40, 99–105. [Google Scholar] [CrossRef]
- Machado, P.; Pimenta, S.; Oliveiros, B.; Ferreira, J.P.; Martins, R.A.; Cruz, J. Effect of Exercise Training on Quality of Life after Colorectal and Lung Cancer Surgery: A Meta-Analysis. Cancers 2021, 13, 4975. [Google Scholar] [CrossRef]
- Sommer, M.S.; Staerkind, M.E.B.; Christensen, J.; Vibe-Petersen, J.; Larsen, K.R.; Pedersen, J.H.; Langberg, H. Effect of Postsurgical Rehabilitation Programmes in Patients Operated for Lung Cancer: A Systematic Review and Meta-Analysis. J. Rehabil. Med. 2018, 50, 236–245. [Google Scholar] [CrossRef]
- Peddle-McIntyre, C.J.; Singh, F.; Thomas, R.; Newton, R.U.; Galvao, D.A.; Cavalheri, V. Exercise Training for Advanced Lung Cancer. Cochrane Database Syst. Rev. 2019, 2, CD012685. [Google Scholar] [CrossRef]
- Blanchon, F.; Grivaux, M.; Asselain, B.; Lebas, F.X.; Orlando, J.P.; Piquet, J.; Zureik, M. 4-Year Mortality in Patients with Non-Small-Cell Lung Cancer: Development and Validation of a Prognostic Index. Lancet Oncol. 2006, 7, 829–836. [Google Scholar] [CrossRef]
- Steffens, D.; Beckenkamp, P.R.; Young, J.; Solomon, M.; da Silva, T.M.; Hancock, M.J. Is Preoperative Physical Activity Level of Patients Undergoing Cancer Surgery Associated with Postoperative Outcomes? A Systematic Review and Meta-Analysis. Eur. J. Surg. Oncol. 2019, 45, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Salakari, M.R.J.; Surakka, T.; Nurminen, R.; Pylkkänen, L. Effects of Rehabilitation among Patients with Advances Cancer: A Systematic Review. Acta Oncol. 2015, 54, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Gralla, R.J.; Hollen, P.J.; Msaouel, P.; Davis, B.V.; Petersen, J. An Evidence-Based Determination of Issues Affecting Quality of Life and Patient-Reported Outcomes in Lung Cancer: Results of a Survey of 660 Patients. J. Thorac. Oncol. 2014, 9, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Cavalheri, V.; Burtin, C.; Formico, V.R.; Nonoyama, M.L.; Jenkins, S.; Spruit, M.A.; Hill, K. Exercise Training Undertaken by People within 12 Months of Lung Resection for Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2019, 6, CD009955. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Sartipy, U. Predictors of Postoperative Quality of Life after Surgery for Lung Cancer. J. Thorac. Oncol. 2012, 7, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.W.; Eves, N.D.; Peterson, B.L.; Garst, J.; Crawford, J.; West, M.J.; Mabe, S.; Harpole, D.; Kraus, W.E.; Douglas, P.S. Safety and Feasibility of Aerobic Training on Cardiopulmonary Function and Quality of Life in Postsurgical Nonsmall Cell Lung Cancer Patients: A Pilot Study. Cancer 2008, 113, 3430–3439. [Google Scholar] [CrossRef]
- Johnsen, A.T.; Petersen, M.A.; Pedersen, L.; Groenvold, M. Symptoms and Problems in a Nationally Representative Sample of Advanced Cancer Patients. Palliat. Med. 2009, 23, 491–501. [Google Scholar] [CrossRef]
- Baracos, V.E.; Reiman, T.; Mourtzakis, M.; Gioulbasanis, I.; Antoun, S. Body Composition in Patients with Non-Small Cell Lung Cancer: A Contemporary View of Cancer Cachexia with the Use of Computed Tomography Image Analysis. Am. J. Clin. Nutr. 2010, 91, 1133S–1137S. [Google Scholar] [CrossRef]
- Kenny, P.M.; King, M.T.; Viney, R.C.; Boyer, M.J.; Pollicino, C.A.; McLean, J.M.; Fulham, M.J.; McCaughan, B.C. Quality of Life and Survival in the 2 Years after Surgery for Non Small-Cell Lung Cancer. J. Clin. Oncol. 2008, 26, 233–241. [Google Scholar] [CrossRef]
- Schulte, T.; Schniewind, B.; Dohrmann, P.; Küchler, T.; Kurdow, R. The Extent of Lung Parenchyma Resection Significantly Impacts Long-Term Quality of Life in Patients with Non-Small Cell Lung Cancer. Chest 2009, 135, 322–329. [Google Scholar] [CrossRef]
- Brown, L.M.; Gosdin, M.M.; Cooke, D.T.; Apesoa-Varano, E.C.; Kratz, A.L. Health-Related Quality of Life After Lobectomy for Lung Cancer: Conceptual Framework and Measurement. Ann. Thorac. Surg. 2020, 110, 1840–1846. [Google Scholar] [CrossRef] [PubMed]
- Mustian, K.M.; Alfano, C.M.; Heckler, C.; Kleckner, A.S.; Kleckner, I.R.; Leach, C.R.; Mohr, D.; Palesh, O.G.; Peppone, L.J.; Piper, B.F.; et al. Comparison of Pharmaceutical, Psychological, and Exercise Treatments for Cancer-Related Fatigue: A Meta-Analysis. JAMA Oncol. 2017, 3, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Ferioli, M.; Zauli, G.; Martelli, A.M.; Vitale, M.; McCubrey, J.A.; Ultimo, S.; Capitani, S.; Neri, L.M. Impact of Physical Exercise in Cancer Survivors during and after Antineoplastic Treatments. Oncotarget 2018, 9, 14005–14034. [Google Scholar] [CrossRef] [PubMed]
- Adamsen, L.; Midtgaard, J.; Andersen, C.; Quist, M.; Moeller, T.; Roerth, M. Transforming the Nature of Fatigue through Exercise: Qualitative Findings from a Multidimensional Exercise Programme in Cancer Patients Undergoing Chemotherapy. Eur. J. Cancer Care (Engl.) 2004, 13, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oh, M.; Yoon, Y.J.; Lee, C.W.; Jones, L.W.; Kim, S.I.; Kim, N.K.; Jeon, J.Y. Characteristics of Attitude and Recommendation of Oncologists toward Exercise in South Korea: A Cross Sectional Survey Study. BMC Cancer 2015, 15, 249. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Liu, X.; Yin, Y.Y.; Ma, R.C.; Yang, Z.; Cao, H.P.; Xie, J. Effects of Home-Based Exercise Training for Patients With Lung Cancer. Oncol. Nurs. Forum 2019, 46, E119–E134. [Google Scholar] [CrossRef]
- Adamsen, L.; Stage, M.; Laursen, J.; Rørth, M.; Quist, M. Exercise and Relaxation Intervention for Patients with Advanced Lung Cancer: A Qualitative Feasibility Study. Scand. J. Med. Sci. Sports 2012, 22, 804–815. [Google Scholar] [CrossRef]
- Buffart, L.M.; Kalter, J.; Sweegers, M.G.; Courneya, K.S.; Newton, R.U.; Aaronson, N.K.; Jacobsen, P.B.; May, A.M.; Galvão, D.A.; Chinapaw, M.J.; et al. Effects and Moderators of Exercise on Quality of Life and Physical Function in Patients with Cancer: An Individual Patient Data Meta-Analysis of 34 RCTs. Cancer Treat. Rev. 2017, 52, 91–104. [Google Scholar] [CrossRef]
- Ma, R.C.; Yin, Y.Y.; Liu, X.; Wang, Y.Q.; Xie, J. Effect of Exercise Interventions on Quality of Life in Patients With Lung Cancer: A Systematic Review of Randomized Controlled Trials. Oncol. Nurs. Forum 2020, 47, E58–E72. [Google Scholar] [CrossRef]


| Author, Year and Country | N; IG; CG | Diagnosis | Treatment | Intervention. Physical Activity Program | Main Variables Analyzed. QoL Assessment Tool | Co Intervention | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W Frec. | Int. | Time | Type | Sup. | Duration | Adh. | ||||||
| Arbane et al., 2011. United Kingdom [35] | 43; 22; 21 | Stage I–V NSCLC | Post-surgery | 5 days: twice daily | Mod | 5 days | Strength and mobility training | Sup. | ND | ND | Muscle strength, QoL and exercise tolerance. EORTC QLQ-C30 | No |
| ND | ND | 12 weeks | Strength training and walking | Unsup. | ND | |||||||
| Bade et al., 2021. United States [36] | 39; 20; 19 | Stage III or IV NSCLC | Immunotherapy, chemotherapy, targeted therapy, post-treatment | 7 days | Low | 12 weeks | Walking | Unsup. | ND | ND | Physical activity, QoL, dyspnoea, depression and biomarkers. EORTC QLQ-C30 | No |
| Cavalheri et al., 2017. Australia [37] | 17; 9; 8 | Stages I–IIIA NSCLC | Postoperative chemotherapy or after lobectomy | 3 days | Mod, high | 8 weeks | Aerobic and resistance training | Sup. | 60 min | 44% | Exercise capacity, physical activity, peripheral muscle force, QoL, fatigue, anxiety, depression and lung function. FACT-L; EORTC QLQ-C30 | No |
| Edvardsen et al., 2015. Norway [38] | 61; 30; 31 | Stage I–IV NSCLC | Post-surgery | 3 days | High | 20 weeks | Interval, resistance and inspiratory muscle training | Sup. | 60 min | 88% | Peak oxygen uptake, pulmonary function, muscle strength, total muscle mass, daily physical functioning and QoL. EORTC QLQ-C30 | No |
| Egegaard et al., 2019. Denmark [39] | 12; 7; 5 | Stage IIIa–IV NSCLC | Concomitant chemoradiotherapy | 5 days | Mod, high | 7 weeks | Aerobic interval training | Sup. | 20 min | 88% | VO2 peak, functional capacity, pulmonary function, anxiety, depression and QoL. FACT-L | No |
| Ferreira et al., 2021. Canada [40] | 26; 18; 8 | Lung cancer stages I, II or IIIa | Pre-surgery | 1 day | Mod | 4 weeks | Aerobic and resistance training | Sup. | 60 min | 82% | Functional capacity, QoL, anxiety, depression, energy expenditure, nutritional status, body composition, physical function, anaerobic threshold, V02 peak. FACT-L | Nutritio-nal supplement |
| 6 days | Mod | Aerobic, resistance and stretching training | Unsup. | 35 min | ||||||||
| Henke et al., 2013. Germany [41] | 29; 18; 11 | Stage IIIA/IIIB/ IV NSCLC or SCLC | Palliative platinum-based chemotherapy | 6 days | Mod | 12–14 weeks | Endurance and strength training and breathing techniques | Sup. | ND | >75% | Barthel Index, QoL, endurance capacity, dyspnoea perception and muscle strength. EORTC QLQ-C30 | No |
| Huang et al., 2017. China [42] | 60; 30; 30 | Stage I–III NSCLC | Pre-surgery | 7 days | High | 1 week | Aerobic and inspiratory muscle training | Sup. | 20–40 min | 90% | Postoperative pulmonary complications, length of hospital stay, QoL, functional capacity and peak expiratory flow. EORTC QLQ-C30 | No |
| Hwang et al., 2012. Taiwan [43] | 24; 13; 11 | Stage IIIA–IV NSCLC | Targeted therapy | 3 days | High, very high | 8 weeks | Aerobic interval training | Sup. | 30–40 min | 71% | VO2peak, muscle strength, endurance and oxygenation during exercise, insulin resistance, inflammatory response and QoL. EORTC QLQ-C30 | No |
| Lai et al., 2017a. China [44] | 101; 51; 50 | Stage I–IV NSCLC | Pre-surgery | 7 days | High | 1 week | Aerobic training. Thoracic expansion and breathing exercises. | Sup. | 15–30 min | ND | Postoperative pulmonary complications, QoL, functional capacity, changes in blood gas. EORTC QLQ-C30 | No |
| Lai et al., 2017b. China [45] | 60; 30; 30 | Stage I–IV NSCLC | Pre-surgery | 7 days | High | 1 week | Aerobic and inspiratory muscle training | Sup. | 30 min | ND | Postoperative pulmonary complications, QoL, functional capacity and peak expiratory flow. EORTC QLQ-C30 | No |
| Messaggi-Sartor et al., 2019. Spain [46] | 23; 10; 13 | Stage I or II NSCLC | Post-surgery | 3 days | Mod | 8 weeks | Aerobic and inspiratory and expiratory muscle training | Sup. | 60 min | 80% | Exercise capacity, respiratory muscle strength, QoL, levels of serum insulin growth factor I (IGF-I) and IGF binding protein 3 (IGFBP-3). EORTC QLQ-C30 | No |
| Quist et al., 2020. Denmark [47] | 133; 66; 67 | Stage III or IV NSCLC or ED-SCLC | Chemotherapy | 2 days | High, very high | 12 weeks | Strength, aerobic, stretching and relaxation exercises | Sup. | 90 min | 44% | V02 peak, muscle strength, functional capacity, pulmonary function, QoL, anxiety and depression. FACT-L | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barrera-Garcimartín, A.; Sánchez-Polán, M.; López-Martín, A.; Echarri-González, M.J.; Marquina, M.; Barakat, R.; Cordente-Martínez, C.; Refoyo, I. Effects of Physical Activity Interventions on Self-Perceived Health Status among Lung Cancer Patients: Systematic Review and Meta-Analysis. Cancers 2023, 15, 5610. https://doi.org/10.3390/cancers15235610
Barrera-Garcimartín A, Sánchez-Polán M, López-Martín A, Echarri-González MJ, Marquina M, Barakat R, Cordente-Martínez C, Refoyo I. Effects of Physical Activity Interventions on Self-Perceived Health Status among Lung Cancer Patients: Systematic Review and Meta-Analysis. Cancers. 2023; 15(23):5610. https://doi.org/10.3390/cancers15235610
Chicago/Turabian StyleBarrera-Garcimartín, Alejandro, Miguel Sánchez-Polán, Ana López-Martín, María José Echarri-González, Moisés Marquina, Rubén Barakat, Carlos Cordente-Martínez, and Ignacio Refoyo. 2023. "Effects of Physical Activity Interventions on Self-Perceived Health Status among Lung Cancer Patients: Systematic Review and Meta-Analysis" Cancers 15, no. 23: 5610. https://doi.org/10.3390/cancers15235610
APA StyleBarrera-Garcimartín, A., Sánchez-Polán, M., López-Martín, A., Echarri-González, M. J., Marquina, M., Barakat, R., Cordente-Martínez, C., & Refoyo, I. (2023). Effects of Physical Activity Interventions on Self-Perceived Health Status among Lung Cancer Patients: Systematic Review and Meta-Analysis. Cancers, 15(23), 5610. https://doi.org/10.3390/cancers15235610