Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case–Control Study Using National Health Insurance Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Selection
2.2. Exposure to PPIs
2.3. Outcome (Colorectal Cancer)
2.4. Covariates
2.5. Statistical Analyses
3. Results
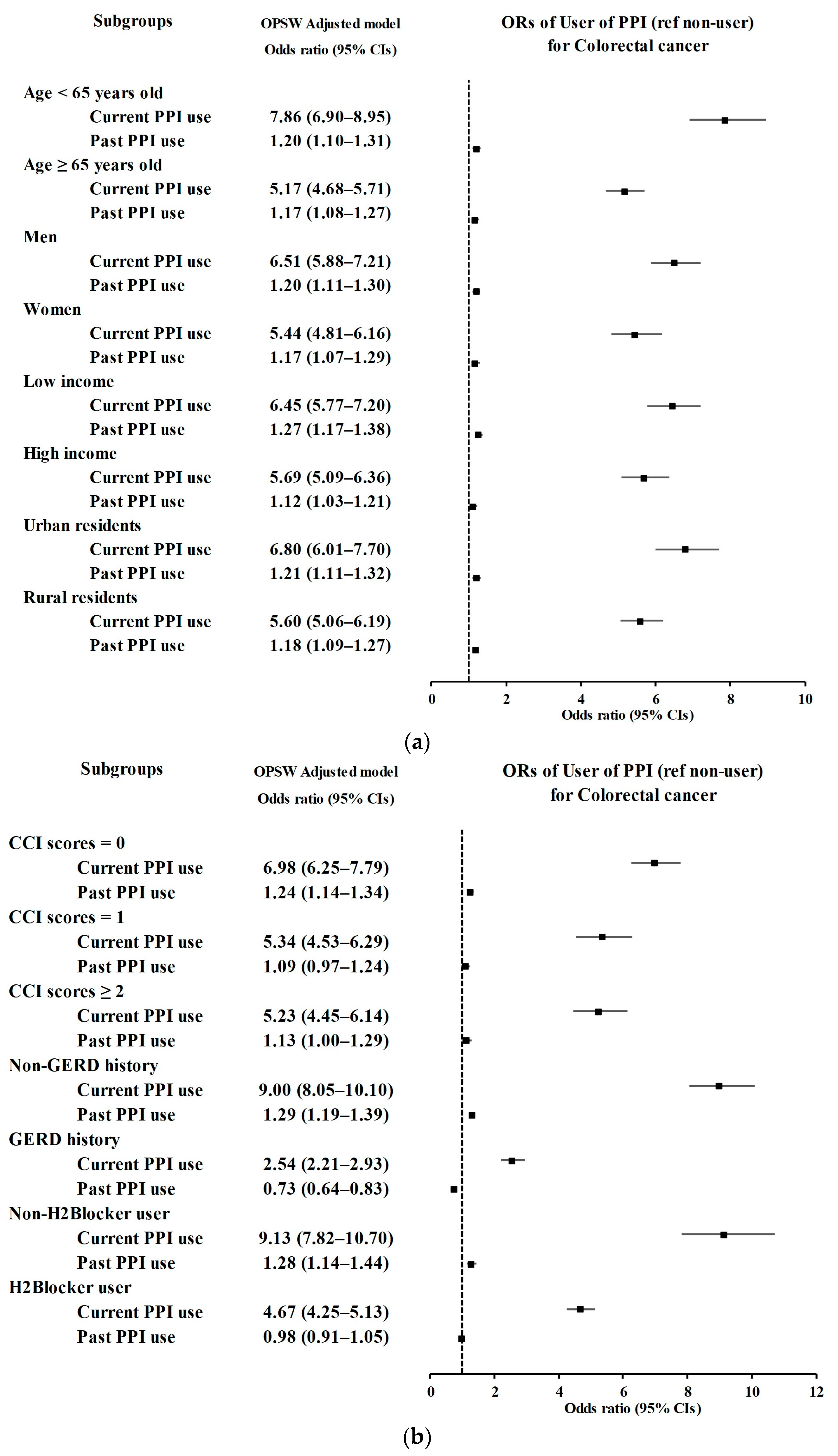
3.1. Relationship between the History of PPI Use and CRC Incidence
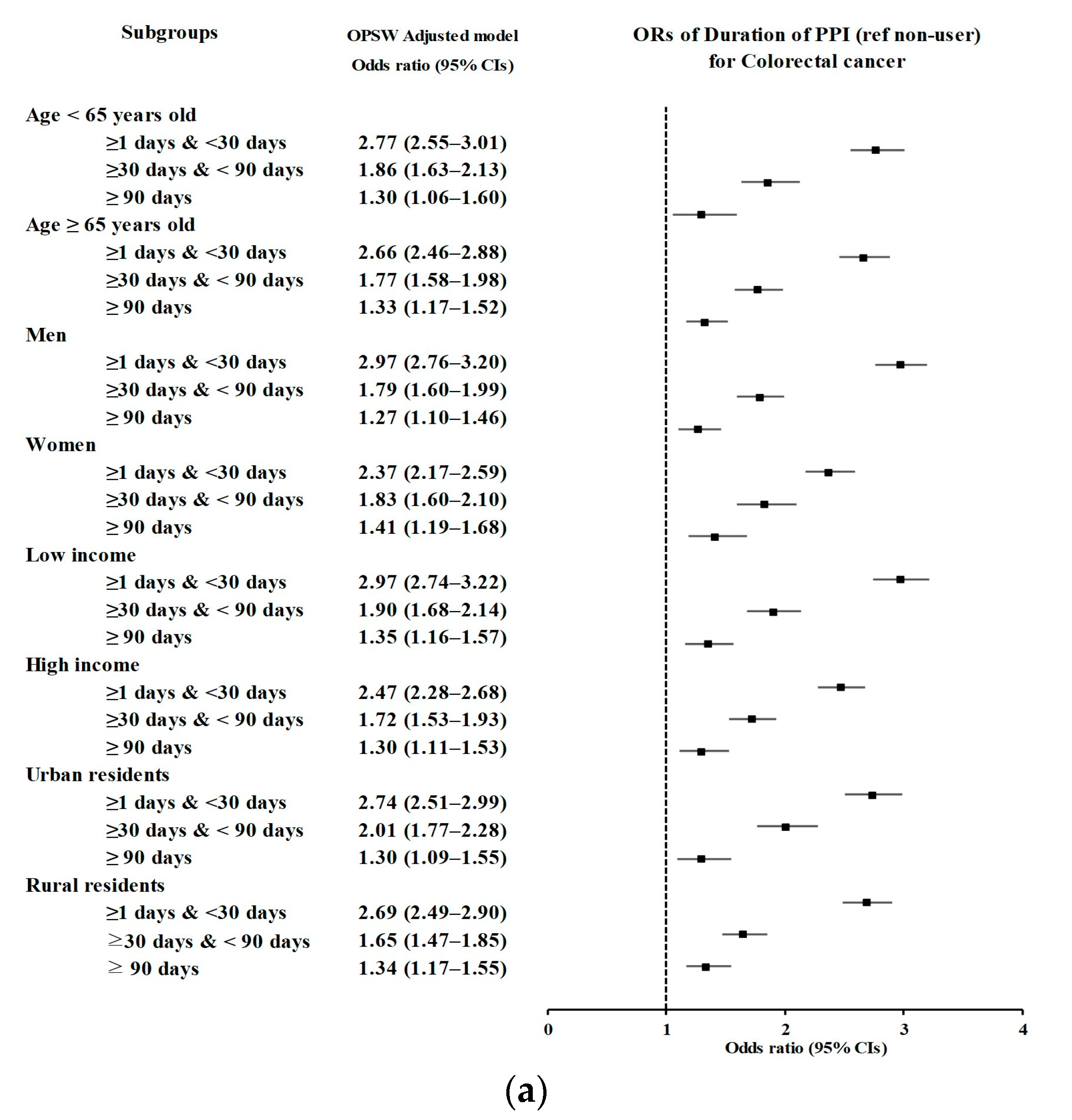
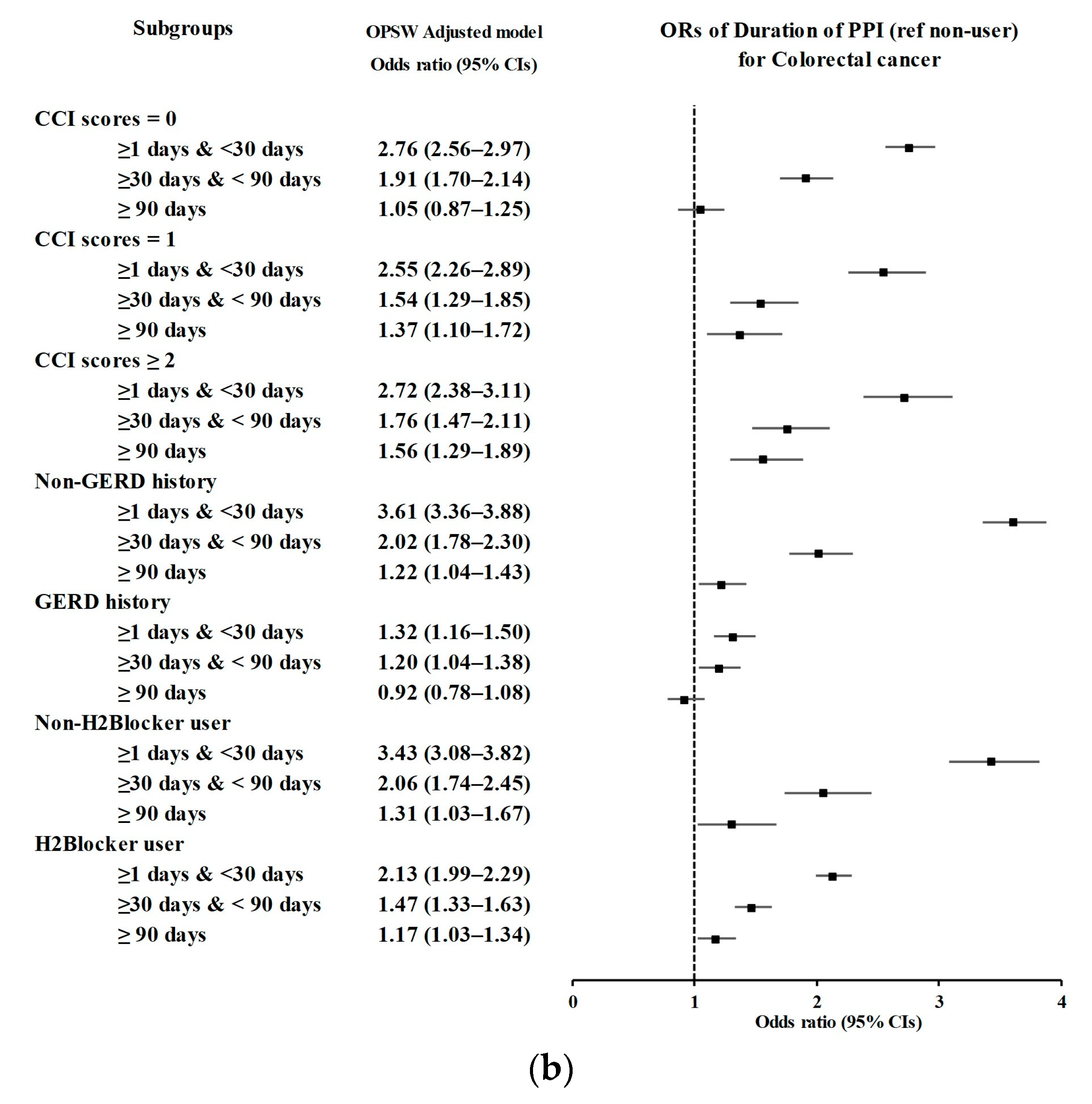
3.2. Relationship between the Duration of PPI Use and Likelihood of CRC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Khil, H.; Kim, S.M.; Hong, S.; Gil, H.M.; Cheon, E.; Lee, D.H.; Kim, Y.A.; Keum, N. Time trends of colorectal cancer incidence and associated lifestyle factors in South Korea. Sci. Rep. 2021, 11, 2413. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.Y.; Chiu, H.M.; Jung, K.W.; Jun, J.K.; Sekiguchi, M.; Matsuda, T.; Kyaw, M.H. Increasing Trend in Young-Onset Colorectal Cancer in Asia: More Cancers in Men and More Rectal Cancers. Am. J. Gastroenterol. 2019, 114, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.; Lau, J.Y.; Goh, K.L.; Leung, W.K. Increasing incidence of colorectal cancer in Asia: Implications for screening. Lancet Oncol. 2005, 6, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jung, K.W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.C.; Ji, B.-T.; Dai, Q.; Gridley, G.; McLaughlin, J.K.; Gao, Y.-T.; Fraumeni, J.F., Jr.; Chow, W.-H. Dietary factors and risk of colon cancer in Shanghai, China. Cancer Epidemiol. Biomark. Prev. 2003, 12, 201–208. [Google Scholar]
- Yiu, H.Y.; Whittemore, A.S.; Shibata, A. Increasing colorectal cancer incidence rates in Japan. Int. J. Cancer 2004, 109, 777–781. [Google Scholar] [CrossRef]
- Haenszel, W.; Berg, J.W.; Segi, M.; Kurihara, M.; Locke, F.B. Large-bowel cancer in Hawaiian Japanese. J. Natl. Cancer Inst. 1973, 51, 1765–1779. [Google Scholar] [CrossRef]
- Koh, S.-J.; Kim, J.S. The reasons for the increased incidence of colorectal cancer in Korea. Korean J. Med. 2010, 79, 97–103. [Google Scholar]
- Namazi, M.R.; Jowkar, F. A succinct review of the general and immunological pharmacologic effects of proton pump inhibitors. J. Clin. Pharm. Ther. 2008, 33, 215–217. [Google Scholar] [CrossRef]
- Oh, J.-A.; Lee, G.-M.; Chung, S.-Y.; Cho, Y.-S.; Lee, H.-J. Utilization trends of Proton Pump Inhibitors in South Korea: Analysis using 2016-2020 Healthcare Bigdata Hub by Health Insurance Review and Assessment Service. Yakhak Hoeji 2021, 65, 276–283. [Google Scholar] [CrossRef]
- Celebi, A.; Aydin, D.; Kocaman, O.; Konduk, B.T.; Senturk, O.; Hulagu, S. Comparison of the effects of esomeprazole 40 mg, rabeprazole 20 mg, lansoprazole 30 mg, and pantoprazole 40 mg on intragastric pH in extensive metabolizer patients with gastroesophageal reflux disease. Turk. J. Gastroenterol. 2016, 27, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.; Azoulay, L. Trends in acid suppressant drug prescriptions in primary care in the UK: A population-based cross-sectional study. BMJ Open 2020, 10, e041529. [Google Scholar] [CrossRef] [PubMed]
- Havu, N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion 1986, 35 (Suppl. S1), 42–55. [Google Scholar] [CrossRef] [PubMed]
- McGwin, G. The Association between Ranitidine Use and Gastrointestinal Cancers. Cancers 2020, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Chubak, J.; Boudreau, D.M.; Rulyak, S.J.; Mandelson, M.T. Colorectal cancer risk in relation to use of acid suppressive medications. Pharmacoepidemiol. Drug Saf. 2009, 18, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Babic, A.; Zhang, X.; Morales-Oyarvide, V.; Yuan, C.; Khalaf, N.; Khalili, H.; Lochhead, P.; Chan, A.T.; Ogino, S.; Wolpin, B.M.; et al. Acid-suppressive medications and risk of colorectal cancer: Results from three large prospective cohort studies. Br. J. Cancer 2020, 123, 844–851. [Google Scholar] [CrossRef]
- Abrahami, D.; McDonald, E.G.; Schnitzer, M.E.; Barkun, A.N.; Suissa, S.; Azoulay, L. Proton pump inhibitors and risk of colorectal cancer. Gut 2022, 71, 111–118. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Kim, L.S.; Yang, Y.-X. The Risks and Benefits of Long-term Use of Proton Pump Inhibitors: Expert Review and Best Practice Advice from the American Gastroenterological Association. Gastroenterology 2017, 152, 706–715. [Google Scholar] [CrossRef]
- Thorburn, C.M.; Friedman, G.D.; Dickinson, C.J.; Vogelman, J.H.; Orentreich, N.; Parsonnet, J. Gastrin and colorectal cancer: A prospective study. Gastroenterology 1998, 115, 275–280. [Google Scholar] [CrossRef]
- Malecka-Panas, E.; Fligiel, S.E.; Jaszewski, R.; Majumdar, A.P. Differential responsiveness of proximal and distal colonic mucosa to gastrin. Peptides 1997, 18, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.A.; Smith, A.M. Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001, 61, 625–631. [Google Scholar] [PubMed]
- Smith, J.P.; Stock, E.A.; Wotring, M.G.; McLaughlin, P.J.; Zagon, I.S. Characterization of the CCK-B/gastrin-like receptor in human colon cancer. Am. J. Physiol. 1996, 271, R797–R805. [Google Scholar] [CrossRef] [PubMed]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Serban, D.E. Gastrointestinal cancers: Influence of gut microbiota, probiotics and prebiotics. Cancer Lett. 2014, 345, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Mori, S.; Kishi, S.; Fujiwara-Tani, R.; Ohmori, H.; Nishiguchi, Y.; Hojo, Y.; Kawahara, I.; Nakashima, C.; Fujii, K.; et al. Effect of Proton Pump Inhibitors on Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 3877. [Google Scholar] [CrossRef]
- Robertson, D.J.; Larsson, H.; Friis, S.; Pedersen, L.; Baron, J.A.; Sørensen, H.T. Proton pump inhibitor use and risk of colorectal cancer: A population-based, case–control study. Gastroenterology 2007, 133, 755–760. [Google Scholar] [CrossRef]
- Yang, Y.X.; Hennessy, S.; Propert, K.; Hwang, W.T.; Sedarat, A.; Lewis, J.D. Chronic proton pump inhibitor therapy and the risk of colorectal cancer. Gastroenterology 2007, 133, 748–754. [Google Scholar] [CrossRef]
- Van Soest, E.M.; van Rossum, L.G.; Dieleman, J.P.; van Oijen, M.G.; Siersema, P.D.; Sturkenboom, M.C.; Kuipers, E.J. Proton pump inhibitors and the risk of colorectal cancer. Am. J. Gastroenterol. 2008, 103, 966–973. [Google Scholar] [CrossRef]
- Hwang, I.C.; Chang, J.; Park, S.M. Emerging hazard effects of proton pump inhibitor on the risk of colorectal cancer in low-risk populations: A Korean nationwide prospective cohort study. PLoS ONE 2017, 12, e0189114. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, J.G.; van Herk-Sukel, M.P.; Lemmens, V.E.; Kuipers, E.J.; Herings, R.M. Proton pump inhibitors are not associated with an increased risk of colorectal cancer. GastroHep 2020, 2, 165–170. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.S.; Park, S.H.; Shin, S.A.; Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int. J. Epidemiol. 2017, 46, e15. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.Y.; Wang, J.H.; Yi, C.H.; Liu, T.T.; Hung, J.S.; Wong, M.W.; Bair, M.J.; Vaezi, M.F.; Orr, W.C.; Chen, C.L. Association between use of proton pump inhibitors and colorectal cancer: A nationwide population-based study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101397. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.W.; Liao, K.F.; Lai, H.C.; Lin, C.L.; Sung, F.C. Use of proton pump inhibitors correlates with increased risk of colorectal cancer in Taiwan. Asia Pac. J. Clin. Oncol. 2013, 9, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.S.; Park, S.M.; Eom, C.S.; Kim, S.; Myung, S.K. Use of Proton Pump Inhibitor and Risk of Colorectal Cancer: A Meta-analysis of Observational Studies. Korean J. Fam. Med. 2012, 33, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Wu, M.; Jia, S.; Yang, L. Proton pump inhibitors and the risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Int. J. Color. Dis. 2020, 35, 2157–2169. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, R.; Zhang, P.; Chen, Z.; Hua, Y.; Huang, X.; Li, X. Association of proton pump inhibitors with gastric and colorectal cancer risk: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1129948. [Google Scholar] [CrossRef]
- Lee, J.K.; Merchant, S.A.; Schneider, J.L.; Jensen, C.D.; Fireman, B.H.; Quesenberry, C.P.; Corley, D.A. Proton pump inhibitor use and risk of gastric, colorectal, liver, and pancreatic cancers in a community-based population. Off. J. Am. Coll. Gastroenterol. ACG 2020, 115, 706–715. [Google Scholar] [CrossRef]
- Wang, Y.H.; Chen, C.B.; Tassaneeyakul, W.; Saito, Y.; Aihara, M.; Choon, S.E.; Lee, H.Y.; Chang, M.M.; Roa, F.D.; Wu, C.W.; et al. The Medication Risk of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis in Asians: The Major Drug Causality and Comparison with the US FDA Label. Clin. Pharmacol. Ther. 2019, 105, 112–120. [Google Scholar] [CrossRef]
- Ala, S.; Saruveish, M.; Elkin, C.D.; Imran, J.; Tarboush, B.A.; Muthusamy, V. Adverse Effects of Proton Pump Inhibitors use Among South Asian Population Systematic Review. Int. J. Med. Res. Health Sci. 2023, 12, 1–8. [Google Scholar]
- Tai, S.Y.; Chien, C.Y.; Wu, D.C.; Lin, K.D.; Ho, B.L.; Chang, Y.H.; Chang, Y.P. Risk of dementia from proton pump inhibitor use in Asian population: A nationwide cohort study in Taiwan. PLoS ONE 2017, 12, e0171006. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E.; Kennerley, P. Proton pump inhibitors—Differences emerge in hepatic metabolism. Dig. Liver Dis. 2002, 34, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Klotz, U. Proton pump inhibitors: An update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.H.; Tseng, C.C.; Bernstein, L. Hiatal hernia, reflux symptoms, body size, and risk of esophageal and gastric adenocarcinoma. Cancer 2003, 98, 940–948. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, S.; Sofia, M.; Agosta, M.; Litrico, G.; Sarva, I.; La Greca, G.; Latteri, S. The impact of bariatric surgery on colorectal cancer risk. Surg. Obes. Relat. Dis. 2023, 19, 144–157. [Google Scholar] [CrossRef]
- Tobi, M.; Cats, A.; Maliakkal, B.J.; Kinzie, J.L.; Maliakkal, R.; Dullaart, R.P.; Luk, G.D. Zollinger-Ellison syndrome, acromegaly, and colorectal neoplasia. J. Clin. Gastroenterol. 1997, 24, 21–24. [Google Scholar] [CrossRef]
- Bielefeld, P.; Meyer, P.; Caillot, D.; Dalac, S.; Camus, P.; Tavernier, C.; Couillaut, G.; Chalopin, J.M.; Besancenot, J.F. Systemic scleroderma and cancers: 21 cases and review of the literature. Rev. Med. Interne 1996, 17, 810–813. [Google Scholar] [CrossRef]
- Tustumi, F.; Arienzo, V.P.; Sunye, I.R.; Lucas, P.F.S.; Colonno, B.B.; Quintas, J.G.; Lisboa, E.N.; Szor, D.J. Esophageal Dysbiosis in Achalasia and Cancer Development: A Critical Review. Genes 2023, 14, 1521. [Google Scholar] [CrossRef]
- Brahmbhatt, P.; Ross, J.; Saleem, A.; McKinney, J.; Patel, P.; Khan, S.; Reddy, C.M.; Young, M. Recurrent Adenocarcinoma of Colon Presenting as Duodenal Metastasis with Partial Gastric Outlet Obstruction: A Case Report with Review of Literature. World J. Oncol. 2013, 4, 102–106. [Google Scholar] [CrossRef]
- Kim, S.Y.; Min, C.; Oh, D.J.; Choi, H.G. Bidirectional Association Between GERD and Asthma: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J. Allergy Clin. Immunol. Pract. 2020, 8, 1005–1013.e9. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Thomas, L.E.; Li, F. Addressing Extreme Propensity Scores via the Overlap Weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap Weighting: A Propensity Score Method That Mimics Attributes of a Randomized Clinical Trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Yen, M.F.; Wang, W.M.; Wong, J.M.; Chen, T.H. A case-cohort study for the disease natural history of adenoma-carcinoma and de novo carcinoma and surveillance of colon and rectum after polypectomy: Implication for efficacy of colonoscopy. Br. J. Cancer 2003, 88, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, T.; Ikegami, M.; Fujisaki, J.; Matsui, T.; Aizawa, S.; Ishikawa, E. Early colorectal carcinoma with special reference to its development de novo. Cancer 1989, 64, 1138–1146. [Google Scholar] [CrossRef]
- Kudo, S.; Tamura, S.; Hirota, S.; Sano, Y.; Yamano, H.; Serizawa, M.; Fukuoka, T.; Mitsuoka, H.; Nakajima, T.; Kusaka, H. The problem of de novo colorectal carcinoma. Eur. J. Cancer 1995, 31A, 1118–1120. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nakajima, T.; Kato, K.; Kouno, T.; Sakamoto, T.; Matsuda, T.; Kushima, R.; Saito, Y. Small invasive colon cancer with systemic metastasis: A case report. BMC Gastroenterol. 2011, 11, 59. [Google Scholar] [CrossRef]
- Umetani, N.; Muto, T.; Kawamura, Y.J.; Watanabe, T.; Nakajima, T.; Nagawa, H. Superficial depressed early carcinoma that developed into protuberant advanced carcinoma in the transverse colon. J. Gastroenterol. 2001, 36, 48–51. [Google Scholar] [CrossRef]
- Lee, Y.; Urbanska, A.M.; Hayakawa, Y.; Wang, H.; Au, A.S.; Luna, A.M.; Chang, W.; Jin, G.; Bhagat, G.; Abrams, J.A.; et al. Gastrin stimulates a cholecystokinin-2-receptor-expressing cardia progenitor cell and promotes progression of Barrett’s-like esophagus. Oncotarget 2017, 8, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Song, J.W.; Chung, K.C. Observational studies: Cohort and case-control studies. Plast. Reconstr. Surg. 2010, 126, 2234–2242. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Fang, C.Y.; Yu, B.H.; Chang, C.M.; Hsu, T.W.; Hung, C.L.; Hung, S.K.; Chiou, W.Y.; Tsai, J.H. Long-Term Usage of Proton Pump Inhibitors Associated with Prognosis in Patients with Colorectal Cancer. Cancers 2023, 15, 5304. [Google Scholar] [CrossRef] [PubMed]
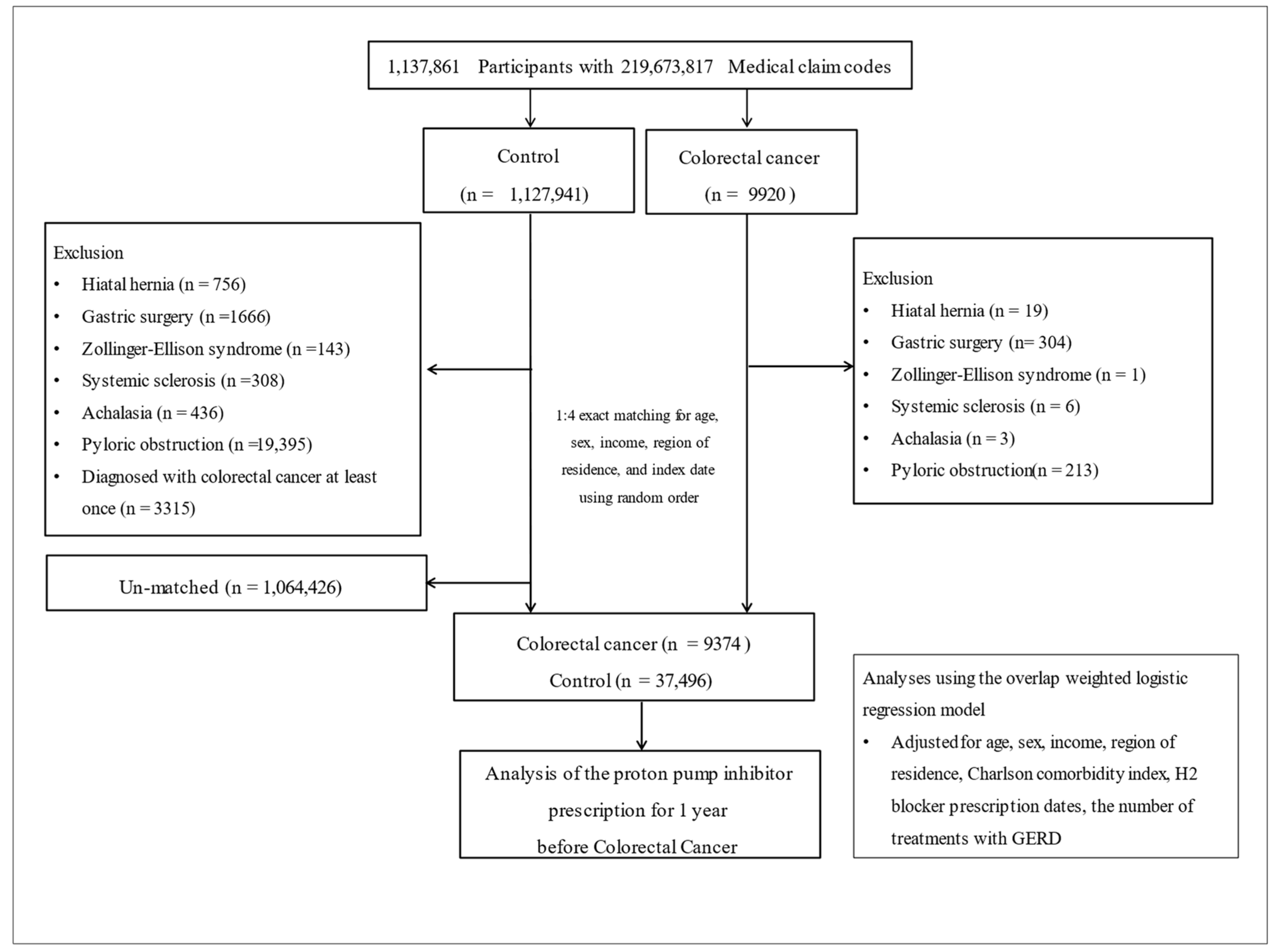
| Characteristics | Before PS Overlap Weighting Adjustment | After PS Overlap Weighting Adjustment | |||||
|---|---|---|---|---|---|---|---|
| Colorectal Cancer | Control | Standardized Difference | Colorectal Cancer | Control | Standardized Difference | ||
| Age (%) | 0.00 | 0.00 | |||||
| 0–4 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 5–9 | N/A | N/A | N/A | N/A | |||
| 10–14 | 3 (0.03) | 12 (0.03) | 2 (0.03) | 2 (0.03) | |||
| 15–19 | 1 (0.01) | 4 (0.01) | 1 (0.01) | 1 (0.01) | |||
| 20–24 | 8 (0.09) | 32 (0.09) | 6 (0.09) | 6 (0.09) | |||
| 25–29 | 26 (0.28) | 104 (0.28) | 21 (0.28) | 21 (0.28) | |||
| 30–34 | 88 (0.94) | 352 (0.94) | 70 (0.94) | 70 (0.94) | |||
| 35–39 | 174 (1.86) | 696 (1.86) | 139 (1.85) | 139 (1.85) | |||
| 40–44 | 346 (3.69) | 1384 (3.69) | 276 (3.68) | 276 (3.68) | |||
| 45–49 | 544 (5.80) | 2176 (5.80) | 434 (5.80) | 434 (5.80) | |||
| 50–54 | 926 (9.88) | 3704 (9.88) | 739 (9.87) | 739 (9.87) | |||
| 55–59 | 1183 (12.62) | 4732 (12.62) | 945 (12.62) | 945 (12.62) | |||
| 60–64 | 1321 (14.09) | 5284 (14.09) | 1054 (14.07) | 1054 (14.07) | |||
| 65–69 | 1396 (14.89) | 5584 (14.89) | 1115 (14.89) | 1115 (14.89) | |||
| 70–74 | 1383 (14.75) | 5532 (14.75) | 1104 (14.75) | 1104 (14.75) | |||
| 75–79 | 987 (10.53) | 3948 (10.53) | 790 (10.54) | 790 (10.54) | |||
| 80–84 | 626 (6.68) | 2504 (6.68) | 502 (6.71) | 502 (6.71) | |||
| 85+ | 361 (3.85) | 1444 (3.85) | 289 (3.86) | 289 (3.86) | |||
| Sex (%) | 0.00 | 0.00 | |||||
| Male | 5596 (59.70) | 22,384 (59.70) | 4469 (59.68) | 4469 (59.68) | |||
| Female | 3778 (40.30) | 15,112 (40.30) | 3019 (40.32) | 3019 (40.32) | |||
| Income (%) | 0.00 | 0.00 | |||||
| 1 (lowest) | 1861 (19.85) | 7444 (19.85) | 1487 (19.85) | 1487 (19.85) | |||
| 2 | 1196 (12.76) | 4784 (12.76) | 954 (12.75) | 954 (12.75) | |||
| 3 | 1480 (15.79) | 5920 (15.79) | 1181 (15.77) | 1181 (15.77) | |||
| 4 | 1954 (20.84) | 7816 (20.84) | 1562 (20.86) | 1562 (20.86) | |||
| 5 (highest) | 2883 (30.76) | 11,532 (30.76) | 2304 (30.77) | 2304 (30.77) | |||
| Region of residence (%) | 0.00 | 0.00 | |||||
| Urban | 4220 (45.02) | 16,880 (45.02) | 3370 (45.01) | 3370 (45.01) | |||
| Rural | 5154 (54.98) | 20,616 (54.98) | 4118 (54.99) | 4118 (54.99) | |||
| CCI score (Mean, SD) | 0.79 (1.17) | 0.79 (1.17) | 0.09 | 0.77 (1.03) | 0.77 (0.57) | 0.00 | |
| Number of treatments with GERD (Mean, SD) | 0.49 (1.74) | 0.49 (1.74) | 0.03 | 0.48 (1.51) | 0.48 (0.95) | 0.00 | |
| H2-blocker prescription dates (Mean, SD) | 30.08 (62.38) | 30.08 (62.38) | 0.05 | 29.58 (54.96) | 29.59 (31.48) | 0.00 | |
| PPI users (n, %) | 0.46 | 0.46 | |||||
| Non-use | 6607 (70.48) | 31,362 (83.64) | 5287 (70.61) | 6221 (83.08) | |||
| Current PPI use | 1666 (17.77) | 1401 (3.74) | 1325 (17.70) | 295 (3.93) | |||
| Past PPI use | 1101 (11.75) | 4733 (12.62) | 875 (11.69) | 972 (12.99) | |||
| Duration of PPI use (n, %) | 0.35 | 0.33 | |||||
| Non-use | 6607 (70.48) | 31,362 (83.64) | 5287 (70.61) | 6221 (83.08) | |||
| ≥1 days and <30 days | 1839 (19.62) | 3249 (8.66) | 1468 (19.60) | 659 (8.81) | |||
| ≥30 days and <90 days | 606 (6.46) | 1649 (4.40) | 481 (6.42) | 343 (4.58) | |||
| ≥90 days | 322 (3.44) | 1236 (3.30) | 252 (3.37) | 265 (3.54) | |||
| Characteristics | Colorectal Cancer | Control | Odds Ratios (95% Confidence Intervals) | |||
|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Adjusted Model with OW † | p | |
| Exposure to PPI | ||||||
| Past | 1101/9374 (11.75) | 4733/37,496 (12.62) | 1.10 (1.03–1.19) | 0.006 * | 1.19 (1.12–1.26) | <0.001 * |
| Current | 1666/9374 (17.77) | 1401/37,496 (3.74) | 5.64 (5.23–6.09) | <0.001 * | 6.06 (5.60–6.56) | <0.001 * |
| Duration of PPI use | ||||||
| <30 days | 1839/9374 (19.62) | 3249/37,496 (8.66) | 2.69 (2.52–2.86) | <0.001 * | 2.71 (2.56–2.87) | <0.001 * |
| 30–90 days | 606/9374 (6.46) | 1649/37,496 (4.4) | 1.74 (1.58–1.92) | <0.001 * | 1.80 (1.66–1.96) | <0.001 * |
| ≥90 days | 322/9374 (3.44) | 1236/37,496 (3.3) | 1.24 (1.09–1.40) | <0.001 * | 1.33 (1.19–1.48) | <0.001 * |
| User of PPI | Colorectal Cancer | Control | Odds Ratios (95% Confidence Intervals) | ||||
|---|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Adjusted Model with OW † | p | ||
| Age < 65 years old (n = 23,105) | |||||||
| Current PPI use | 738/4621 (15.97) | 482/18,484 (2.61) | 7.19 (6.38–8.12) | <0.001 * | 7.86 (6.90–8.95) | <0.001 * | |
| Past PPI use | 488/4621 (10.56) | 2052/18,484 (11.1) | 1.12 (1.01–1.24) | 0.039 * | 1.20 (1.10–1.31) | <0.001 * | |
| Age ≥ 65 years old (n = 23,765) | |||||||
| Current PPI use | 928/4753 (19.52) | 919/19,012 (4.83) | 4.85 (4.39–5.35) | <0.001 * | 5.17 (4.68–5.71) | <0.001 * | |
| Past PPI use | 613/4753 (12.9) | 2681/19,012 (14.1) | 1.10 (1.00–1.21) | 0.058 | 1.17 (1.08–1.27) | <0.001 * | |
| Males (n = 27,980) | |||||||
| Current PPI use | 1040/5596 (18.58) | 820/22,384 (3.66) | 6.09 (5.52–6.71) | <0.001 * | 6.51 (5.88–7.21) | <0.001 * | |
| Past PPI use | 645/5596 (11.53) | 2783/22,384 (12.43) | 1.11 (1.01–1.22) | 0.023 * | 1.20 (1.11–1.30) | <0.001 * | |
| Females (n = 18,890) | |||||||
| Current PPI use | 626/3778 (16.57) | 581/15,112 (3.84) | 5.03 (4.46–5.67) | <0.001 * | 5.44 (4.81–6.16) | <0.001 * | |
| Past PPI use | 456/3778 (12.07) | 1950/15,112 (12.9) | 1.09 (0.98–1.22) | 0.12 | 1.17 (1.07–1.29) | <0.001 * | |
| Low income (n = 22,685) | |||||||
| Current PPI use | 868/4537 (19.13) | 711/18148 (3.92) | 5.93 (5.33–6.59) | <0.001 * | 6.45 (5.77–7.20) | <0.001 * | |
| Past PPI use | 554/4537 (12.21) | 2310/18148 (12.73) | 1.16 (1.05–1.29) | 0.003 * | 1.27 (1.17–1.38) | <0.001 * | |
| High income (n = 24,185) | |||||||
| Current PPI use | 798/4837 (16.5) | 690/19,348 (3.57) | 5.38 (4.82–5.99) | <0.001 * | 5.69 (5.09–6.36) | <0.001 * | |
| Past PPI use | 547/4837 (11.31) | 2423/19,348 (12.52) | 1.05 (0.95–1.16) | 0.342 | 1.12 (1.03–1.21) | 0.008 * | |
| Urban (n = 21,100) | |||||||
| Current PPI use | 721/4220 (17.09) | 543/16,880 (3.22) | 6.29 (5.59–7.08) | <0.001 * | 6.80 (6.01–7.70) | <0.001 * | |
| Past PPI use | 476/4220 (11.28) | 2016/16,880 (11.94) | 1.12 (1.00–1.25) | 0.041 * | 1.21 (1.11–1.32) | <0.001 * | |
| Rural (n = 25,770) | |||||||
| Current PPI use | 945/5154 (18.34) | 858/20,616 (4.16) | 5.24 (4.74–5.78) | <0.001 * | 5.60 (5.06–6.19) | <0.001 * | |
| Past PPI use | 625/5154 (12.13) | 2717/20,616 (13.18) | 1.09 (1.00–1.20) | 0.062 | 1.18 (1.09–1.27) | <0.001 * | |
| CCI scores = 0 (n = 28,945) | |||||||
| Current PPI use | 823/5175 (15.9) | 705/23,770 (2.97) | 6.28 (5.64–6.98) | <0.001 * | 6.98 (6.25–7.79) | <0.001 * | |
| Past PPI use | 559/5175 (10.8) | 2667/23,770 (11.22) | 1.13 (1.02–1.24) | 0.016 * | 1.24 (1.14–1.34) | <0.001 * | |
| CCI scores = 1 (n = 9926) | |||||||
| Current PPI use | 456/2453 (18.59) | 341/7473 (4.56) | 4.79 (4.12–5.56) | <0.001 * | 5.34 (4.53–6.29) | <0.001 * | |
| Past PPI use | 300/2453 (12.23) | 1058/7473 (14.16) | 1.01 (0.88–1.17) | 0.835 | 1.09 (0.97–1.24) | 0.161 | |
| CCI scores ≥ 2 (n = 7999) | |||||||
| Current PPI use | 387/1746 (22.16) | 355/6253 (5.68) | 4.77 (4.07–5.59) | <0.001 * | 5.23 (4.45–6.14) | <0.001 * | |
| Past PPI use | 242/1746 (13.86) | 1008/6253 (16.12) | 1.05 (0.90–1.23) | 0.528 | 1.13 (1.00–1.29) | 0.058 | |
| Without GERD (n = 40,708) | |||||||
| Current PPI use | 1071/7918 (13.53) | 557/32,790 (1.7) | 9.24 (8.31–10.3) | <0.001 * | 9.00 (8.05–10.1) | <0.001 * | |
| Past PPI use | 594/7918 (7.5) | 2186/32,790 (6.67) | 1.31 (1.19–1.44) | <0.001 * | 1.29 (1.19–1.39) | <0.001 * | |
| With GERD (n = 6162) | |||||||
| Current PPI use | 595/1456 (40.87) | 844/4706 (17.93) | 2.62 (2.24–3.07) | <0.001 * | 2.54 (2.21–2.93) | <0.001 * | |
| Past PPI use | 507/1456 (34.82) | 2547/4706 (54.12) | 0.74 (0.64–0.86) | <0.001 * | 0.73 (0.64–0.83) | <0.001 * | |
| Without H2-blocker use (n = 19191) | |||||||
| Current PPI use | 314/2281 (13.77) | 314/16,910 (1.86) | 8.57 (7.27–10.1) | <0.001 * | 9.13 (7.82–10.7) | <0.001 * | |
| Past PPI use | 171/2281 (7.5) | 1209/16,910 (7.15) | 1.21 (1.02–1.43) | 0.025 * | 1.28 (1.14–1.44) | <0.001 * | |
| With H2-blocker use (n = 27,679) | |||||||
| Current PPI use | 1352/7093 (19.06) | 1087/20,586 (5.28) | 4.13 (3.79–4.50) | <0.001 * | 4.67 (4.25–5.13) | <0.001 * | |
| Past PPI use | 930/7093 (13.11) | 3524/20,586 (17.12) | 0.88 (0.81–0.95) | 0.001 * | 0.98 (0.91–1.05) | 0.613 | |
| Duration of PPI | Colorectal Cancer | Control | Odds Ratios (95% Confidence Intervals) | ||||
|---|---|---|---|---|---|---|---|
| (Exposure/Total, %) | (Exposure/Total, %) | Crude | p | Adjusted Model with OW † | p | ||
| Age < 65 years old (n = 23,105) | |||||||
| <30 days | 872/4621 (18.87) | 1499/18,484 (8.11) | 2.73 (2.49–2.99) | <0.001 * | 2.77 (2.55–3.01) | <0.001 * | |
| 30–90 days | 261/4621 (5.65) | 687/18,484 (3.72) | 1.78 (1.54–2.07) | <0.001 * | 1.86 (1.63–2.13) | <0.001 * | |
| ≥90 days | 93/4621 (2.01) | 348/18,484 (1.88) | 1.26 (1.00–1.58) | 0.054 | 1.30 (1.06–1.60) | 0.012 * | |
| Age ≥ 65 years old (n = 23,765) | |||||||
| <30 days | 967/4753 (20.35) | 1750/19,012 (9.2) | 2.65 (2.43–2.89) | <0.001 * | 2.66 (2.46–2.88) | <0.001 * | |
| 30–90 days | 345/4753 (7.26) | 962/19,012 (5.06) | 1.72 (1.51–1.96) | <0.001 * | 1.77 (1.58–1.98) | <0.001 * | |
| ≥90 days | 229/4753 (4.82) | 888/19,012 (4.67) | 1.24 (1.06–1.44) | 0.005 * | 1.33 (1.17–1.52) | <0.001 * | |
| Males (n = 27,980) | |||||||
| <30 days | 1128/5596 (20.16) | 1836/22,384 (8.2) | 2.95 (2.72–3.20) | <0.001 * | 2.97 (2.76–3.20) | <0.001 * | |
| 30–90 days | 371/5596 (6.63) | 1025/22,384 (4.58) | 1.74 (1.54–1.97) | <0.001 * | 1.79 (1.60–1.99) | <0.001 * | |
| ≥90 days | 186/5596 (3.32) | 742/22,384 (3.31) | 1.20 (1.02–1.42) | 0.027 * | 1.27 (1.10–1.46) | 0.001 * | |
| Females (n = 18,890) | |||||||
| <30 days | 711/3778 (18.82) | 1413/15,112 (9.35) | 2.35 (2.13–2.59) | <0.001 * | 2.37 (2.17–2.59) | <0.001 * | |
| 30–90 days | 235/3778 (6.22) | 624/15,112 (4.13) | 1.76 (1.50–2.05) | <0.001 * | 1.83 (1.60–2.10) | <0.001 * | |
| ≥90 days | 136/3778 (3.6) | 494/15,112 (3.27) | 1.28 (1.06–1.56) | 0.011 * | 1.41 (1.19–1.68) | <0.001 * | |
| Low income (n = 22,685) | |||||||
| <30 days | 953/4537 (21.01) | 1570/18,148 (8.65) | 2.95 (2.70–3.22) | <0.001 * | 2.97 (2.74–3.22) | <0.001 * | |
| 30–90 days | 298/4537 (6.57) | 790/18,148 (4.35) | 1.83 (1.59–2.10) | <0.001 * | 1.90 (1.68–2.14) | <0.001 * | |
| ≥90 days | 171/4537 (3.77) | 661/18,148 (3.64) | 1.26 (1.06–1.49) | 0.01 * | 1.35 (1.16–1.57) | <0.001 * | |
| High income (n = 24,185) | |||||||
| <30 days | 886/4837 (18.32) | 1679/19,348 (8.68) | 2.45 (2.24–2.68) | <0.001 * | 2.47 (2.28–2.68) | <0.001 * | |
| 30–90 days | 308/4837 (6.37) | 859/19,348 (4.44) | 1.67 (1.46–1.91) | <0.001 * | 1.72 (1.53–1.93) | <0.001 * | |
| ≥90 days | 151/4837 (3.12) | 575/19,348 (2.97) | 1.22 (1.02–1.47) | 0.032 * | 1.30 (1.11–1.53) | 0.001 * | |
| Urban (n = 21,100) | |||||||
| <30 days | 788/4220 (18.67) | 1378/16,880 (8.16) | 2.71 (2.46–2.98) | <0.001 * | 2.74 (2.51–2.99) | <0.001 * | |
| 30–90 days | 289/4220 (6.85) | 708/16,880 (4.19) | 1.93 (1.68–2.23) | <0.001 * | 2.01 (1.77–2.28) | <0.001 * | |
| ≥90 days | 120/4220 (2.84) | 473/16,880 (2.8) | 1.20 (0.98–1.47) | 0.077 | 1.30 (1.09–1.55) | 0.004 * | |
| Rural (n = 25,770) | |||||||
| <30 days | 1051/5154 (20.39) | 1871/20,616 (9.08) | 2.67 (2.46–2.90) | <0.001 * | 2.69 (2.49–2.90) | <0.001 * | |
| 30–90 days | 317/5154 (6.15) | 941/20,616 (4.56) | 1.60 (1.40–1.83) | <0.001 * | 1.65 (1.47–1.85) | <0.001 * | |
| ≥90 days | 202/5154 (3.92) | 763/20,616 (3.7) | 1.26 (1.07–1.48) | 0.005 * | 1.34 (1.17–1.55) | <0.001 * | |
| CCI scores = 0 (n = 28,945) | |||||||
| <30 days | 981/5175 (18.96) | 1930/23,770 (8.12) | 2.73 (2.51–2.97) | <0.001 * | 2.76 (2.56–2.97) | <0.001 * | |
| 30–90 days | 305/5175 (5.89) | 895/23,770 (3.77) | 1.83 (1.60–2.10) | <0.001 * | 1.91 (1.70–2.14) | <0.001 * | |
| ≥90 days | 96/5175 (1.86) | 547/23,770 (2.3) | 0.94 (0.76–1.18) | 0.606 | 1.05 (0.87–1.25) | 0.627 | |
| CCI scores = 1 (n = 9926) | |||||||
| <30 days | 500/2453 (20.38) | 719/7473 (9.62) | 2.49 (2.19–2.82) | <0.001 * | 2.55 (2.26–2.89) | <0.001 * | |
| 30–90 days | 158/2453 (6.44) | 384/7473 (5.14) | 1.47 (1.21–1.79) | <0.001 * | 1.54 (1.29–1.85) | <0.001 * | |
| ≥90 days | 98/2453 (4) | 296/7473 (3.96) | 1.19 (0.94–1.50) | 0.156 | 1.37 (1.10–1.72) | 0.006 * | |
| CCI scores ≥ 2 (n = 7999) | |||||||
| <30 days | 358/1746 (20.5) | 600/6253 (9.6) | 2.61 (2.26–3.02) | <0.001 * | 2.72 (2.38–3.11) | <0.001 * | |
| 30–90 days | 143/1746 (8.19) | 370/6253 (5.92) | 1.69 (1.38–2.07) | <0.001 * | 1.76 (1.47–2.11) | <0.001 * | |
| ≥90 days | 128/1746 (7.33) | 393/6253 (6.28) | 1.43 (1.16–1.76) | <0.001 * | 1.56 (1.29–1.89) | <0.001 * | |
| Without GERD (n = 40,708) | |||||||
| <30 days | 1288/7918 (16.27) | 1699/32,790 (5.18) | 3.64 (3.37–3.94) | <0.001 * | 3.61 (3.36–3.88) | <0.001 * | |
| 30–90 days | 257/7918 (3.25) | 591/32,790 (1.8) | 2.09 (1.80–2.43) | <0.001 * | 2.02 (1.78–2.30) | <0.001 * | |
| ≥90 days | 120/7918 (1.52) | 453/32,790 (1.38) | 1.27 (1.04–1.56) | 0.02 * | 1.22 (1.04–1.43) | 0.017 * | |
| With GERD (n = 6162) | |||||||
| <30 days | 551/1456 (37.84) | 1550/4706 (32.94) | 1.32 (1.13–1.54) | <0.001 * | 1.32 (1.16–1.50) | <0.001 * | |
| 30–90 days | 349/1456 (23.97) | 1058/4706 (22.48) | 1.23 (1.04–1.45) | 0.018 * | 1.20 (1.04–1.38) | 0.013 * | |
| ≥90 days | 202/1456 (13.87) | 783/4706 (16.64) | 0.96 (0.79–1.16) | 0.667 | 0.92 (0.78–1.08) | 0.288 | |
| Without H2-blocker use (n = 19,191) | |||||||
| <30 days | 334/2281 (14.64) | 831/16,910 (4.91) | 3.44 (3.01–3.95) | <0.001 * | 3.43 (3.08–3.82) | <0.001 * | |
| 30–90 days | 101/2281 (4.43) | 397/16,910 (2.35) | 2.18 (1.74–2.73) | <0.001 * | 2.06 (1.74–2.45) | <0.001 * | |
| ≥90 days | 50/2281 (2.19) | 295/16,910 (1.74) | 1.45 (1.07–1.97) | 0.016 * | 1.31 (1.03–1.67) | 0.029 * | |
| With H2-blocker use (n = 26,679) | |||||||
| <30 days | 1505/7093 (21.22) | 2418/20,586 (11.75) | 2.07 (1.92–2.22) | <0.001 * | 2.13 (1.99–2.29) | <0.001 * | |
| 30–90 days | 505/7093 (7.12) | 1252/20,586 (6.08) | 1.34 (1.20–1.49) | <0.001 * | 1.47 (1.33–1.63) | <0.001 * | |
| ≥90 days | 272/7093 (3.83) | 941/20,586 (4.57) | 0.96 (0.84–1.10) | 0.562 | 1.17 (1.03–1.34) | 0.018 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Han, K.M.; Kim, J.-H.; Kim, J.H.; Kim, M.-J.; Kim, N.Y.; Choi, H.G.; Kang, H.S. Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case–Control Study Using National Health Insurance Data. Cancers 2023, 15, 5606. https://doi.org/10.3390/cancers15235606
Kwon MJ, Han KM, Kim J-H, Kim JH, Kim M-J, Kim NY, Choi HG, Kang HS. Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case–Control Study Using National Health Insurance Data. Cancers. 2023; 15(23):5606. https://doi.org/10.3390/cancers15235606
Chicago/Turabian StyleKwon, Mi Jung, Kyeong Min Han, Joo-Hee Kim, Ji Hee Kim, Min-Jeong Kim, Nan Young Kim, Hyo Geun Choi, and Ho Suk Kang. 2023. "Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case–Control Study Using National Health Insurance Data" Cancers 15, no. 23: 5606. https://doi.org/10.3390/cancers15235606
APA StyleKwon, M. J., Han, K. M., Kim, J.-H., Kim, J. H., Kim, M.-J., Kim, N. Y., Choi, H. G., & Kang, H. S. (2023). Proton Pump Inhibitors and Likelihood of Colorectal Cancer in the Korean Population: Insights from a Nested Case–Control Study Using National Health Insurance Data. Cancers, 15(23), 5606. https://doi.org/10.3390/cancers15235606







