Photodynamic Therapy with an Association of Methylene Blue and Toluidine Blue Promoted a Synergic Effect against Oral Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
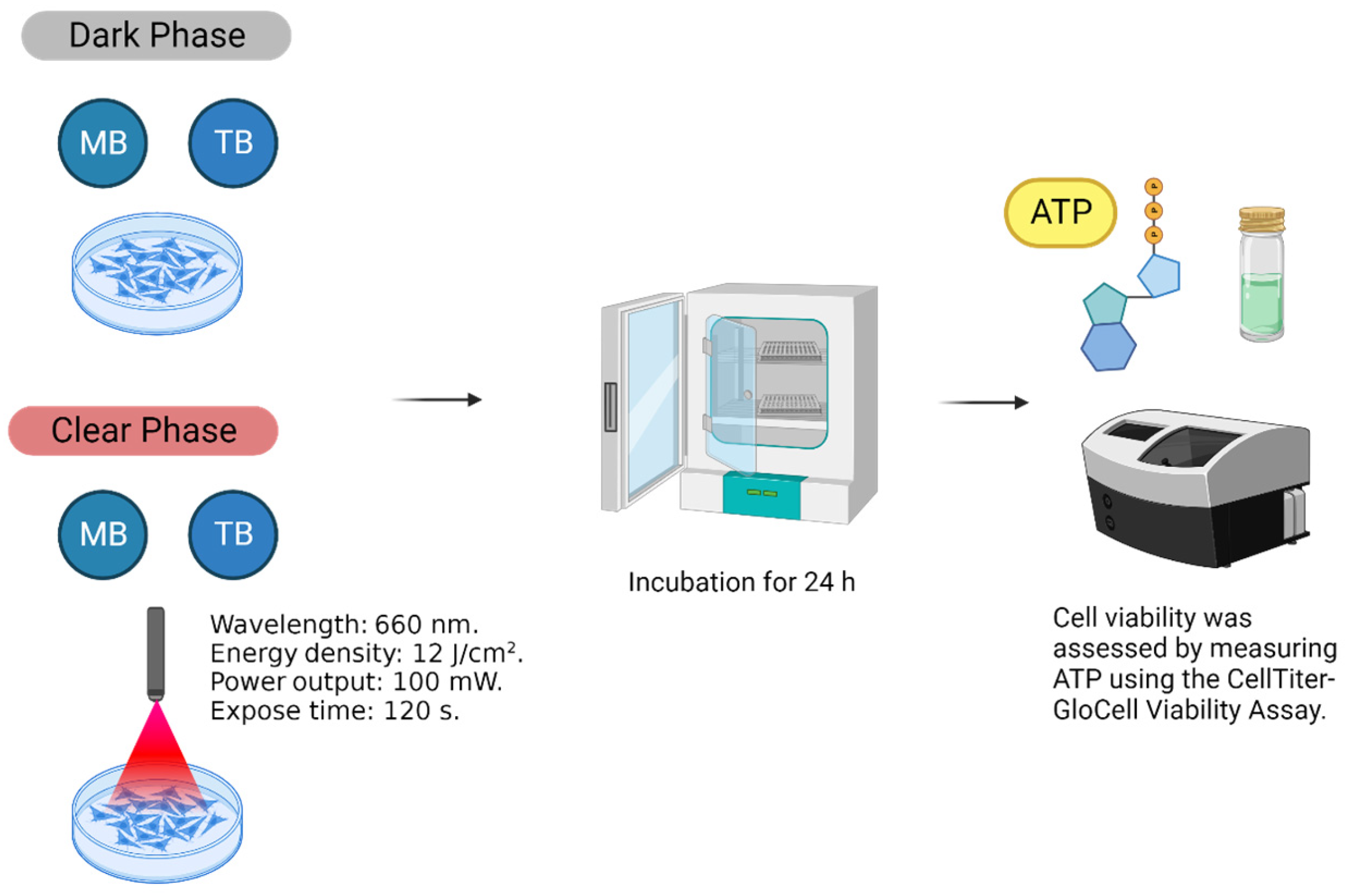
2. Materials and Methods
2.1. Drugs
2.2. Cell Culture
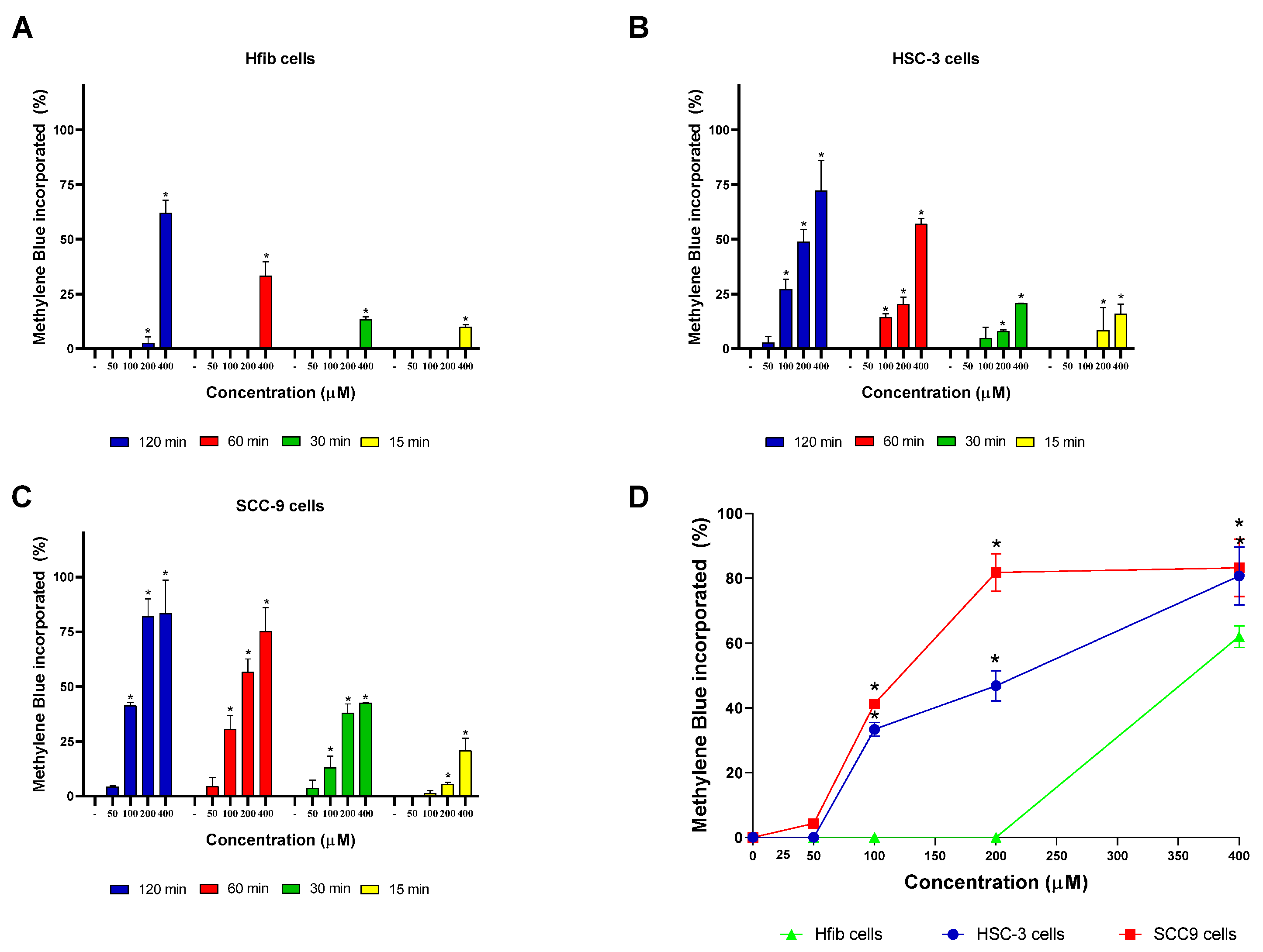
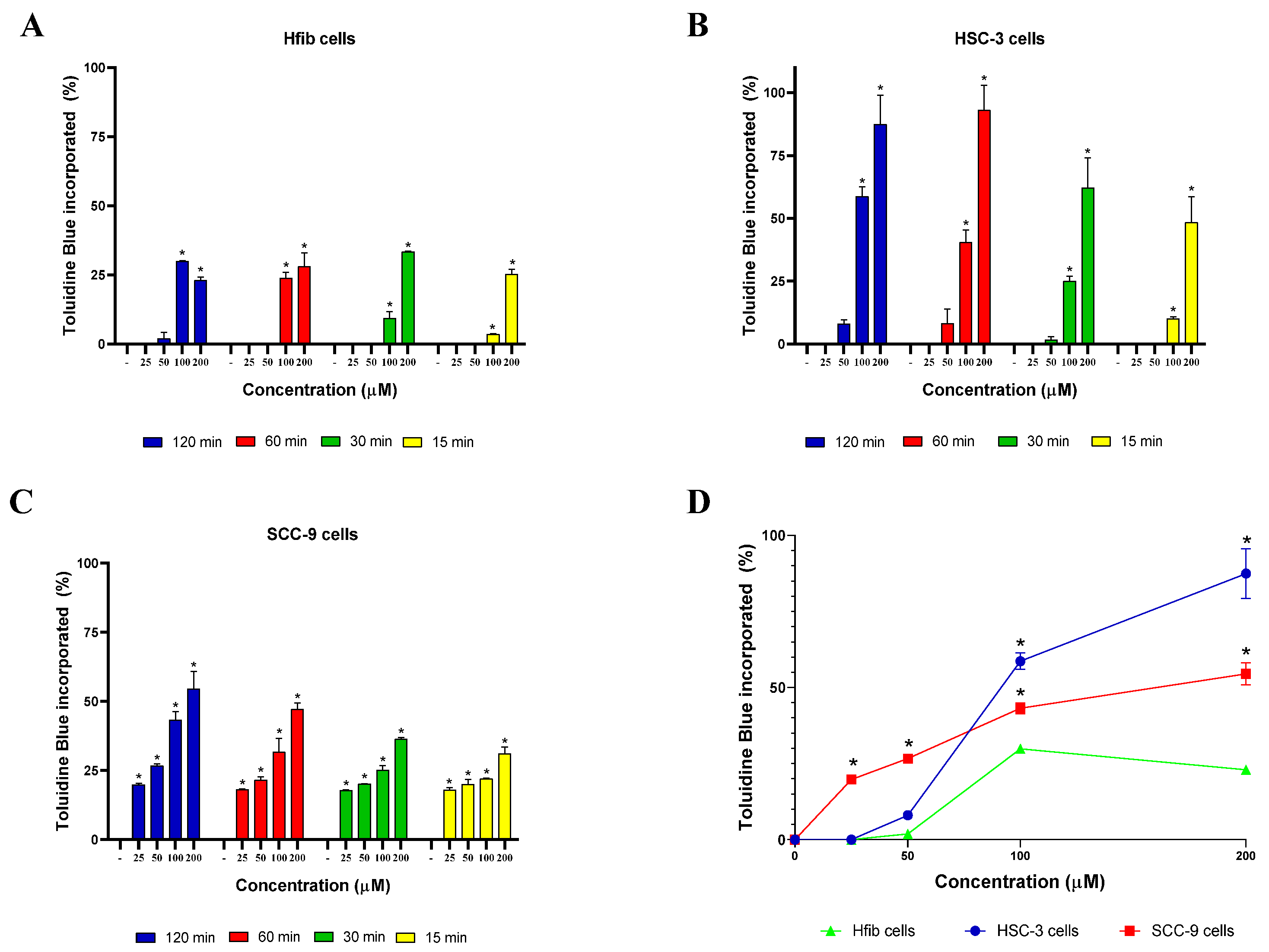
2.3. MB and TB Incorporation Test
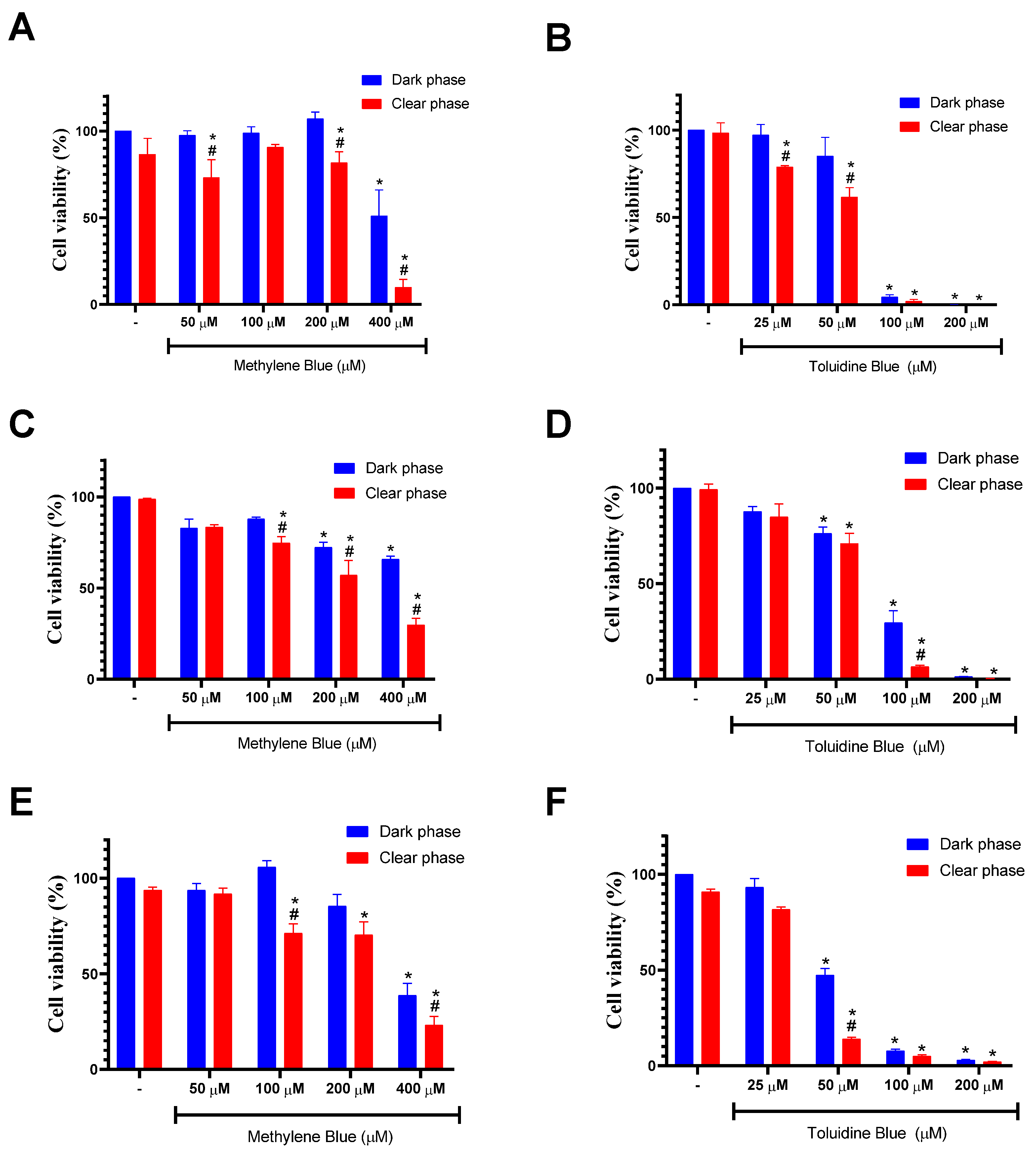
2.4. Cell Viability
2.5. Drug Combination Assay
2.6. Statistical Analyses
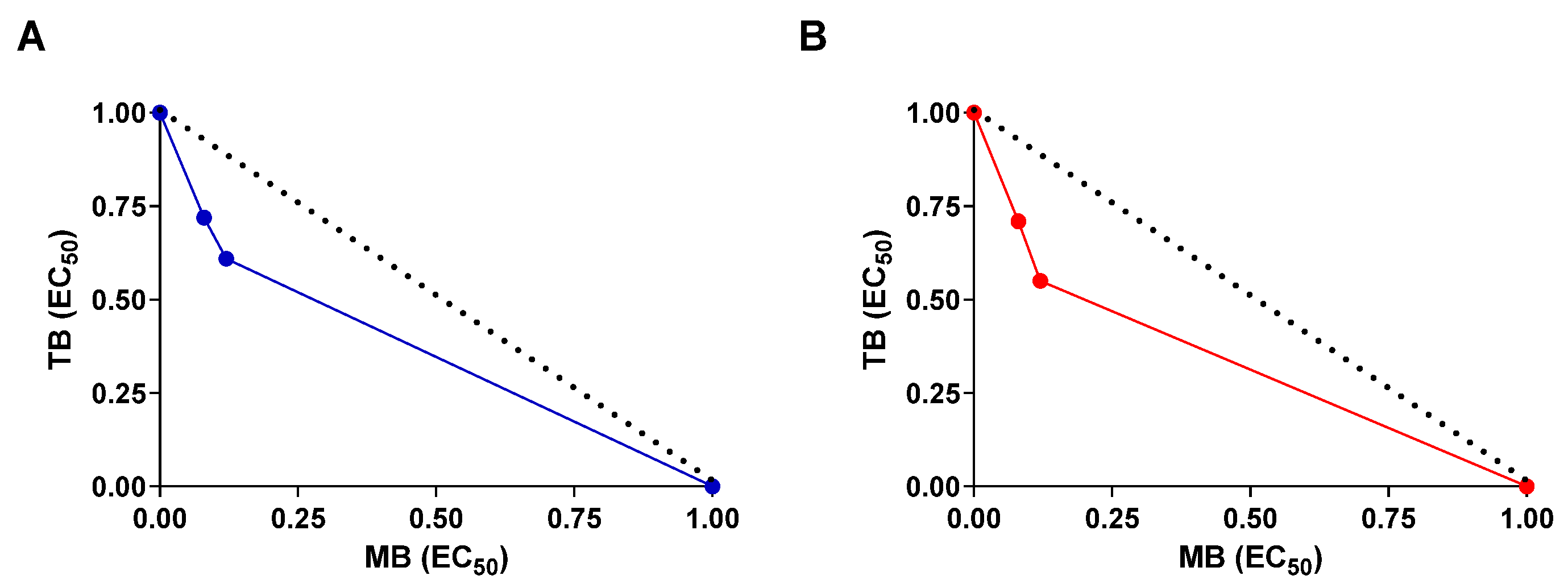
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, Present, and Future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Imbesi Bellantoni, M.; Picciolo, G.; Pirrotta, I.; Irrera, N.; Vaccaro, M.; Vaccaro, F.; Squadrito, F.; Pallio, G. Oral Cavity Squamous Cell Carcinoma: An Update of the Pharmacological Treatment. Biomedicines 2023, 11, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.K.; Carvalho, S.H.; Granville-Garcia, A.F.; Sarmento, D.J.; Agripino, G.G.; Abreu, M.H.; Melo, M.C.; Caldas, A.D., Jr.; Godoy, G.P. Survival and Prognostic Factors in Patients with Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e387–e392. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Nelke, K.H.; Pawlak, W.; Gerber, H.; Leszczyszyn, J. Head and Neck Cancer Patients’ Quality of Life. Adv. Clin. Exp. Med. 2014, 23, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.F.; Terra, L.F.; Wailemann, R.A.; Oliveira, T.C.; Gomes, V.M.; Mineiro, M.F.; Meotti, F.C.; Bruni-Cardoso, A.; Baptista, M.S.; Labriola, L. Methylene Blue Photodynamic Therapy Induces Selective and Massive Cell Death in Human Breast Cancer Cells. BMC Cancer 2017, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, D.; Basilion, J.P. Photodynamic Therapy: Targeting Cancer Biomarkers for the Treatment of Cancers. Cancers 2021, 13, 2992. [Google Scholar] [CrossRef]
- Nafee, N.; Youssef, A.; El-Gowelli, H.; Asem, H.; Kandil, S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int. J. Pharm. 2013, 454, 249–258. [Google Scholar] [CrossRef]
- Li, G.; Sun, L.; Ji, L.; Chao, H. Ruthenium(II) Complexes with dppz: From Molecular Photoswitch to Biological Applications. Dalton Trans. 2016, 45, 13261–13276. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Pantiushenko, I.V.; Rudakovskaya, P.G.; Starovoytova, A.V.; Mikhaylovskaya, A.A.; Abakumov, M.A.; Kaplan, M.A.; Tsygankov, A.A.; Majouga, A.G.; Grin, M.A.; Mironov, A.F. Development of Bacteriochlorophyll a-Based Near-Infrared Photosensitizers Conjugated to Gold Nanoparticles for Photodynamic Therapy of Cancer. Biochemistry 2015, 80, 752–762. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, L. Photodynamic Combinational Therapy in Cancer Treatment. J. BUON 2018, 23, 561–567. [Google Scholar]
- Varga, B.; Csonka, Á.; Csonka, A.; Molnár, J.; Amaral, L.; Spengler, G. Possible Biological and Clinical Applications of Phenothiazines. Anticancer Res. 2017, 37, 5983–5993. [Google Scholar] [CrossRef]
- Andreadis, D.; Pavlou, A.-M.; Sotiriou, E.; Vrani, F.; Ioannides, D.; Kolokotronis, A. Utility of Photodynamic Therapy for the Management of Oral Potentially Malignant Disorders and Oral Cancer. Transl. Res. Oral Oncol. 2016, 1, 2057178X16669161. [Google Scholar] [CrossRef]
- Preiser, J.C.; Lejeune, P.; Roman, A.; Carlier, E.; De Backer, D.; Leeman, M.; Kahn, R.J.; Vincent, J.L. Methylene Blue Administration in Septic Shock: A Clinical Trial. Crit. Care Med. 1995, 23, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Clifton, J., 2nd; Leikin, J.B. Methylene Blue. Am. J. Ther. 2003, 10, 289–291. [Google Scholar] [CrossRef] [PubMed]
- Meissner, P.E.; Mandi, G.; Coulibaly, B.; Witte, S.; Tapsoba, T.; Mansmann, U.; Rengelshausen, J.; Schiek, W.; Jahn, A.; Walter-Sack, I.; et al. Methylene Blue for Malaria in Africa: Results from a Dose-finding Study in Combination with Chloroquine. Malar J. 2006, 5, 84. [Google Scholar] [CrossRef]
- Yin, R.; Hamblin, M.R. Antimicrobial Photosensitizers: Drug Discovery Under the Spotlight. Curr. Med. Chem. 2015, 22, 2159–2185. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Petroianu, G.A. Methylene Blue and Alzheimer’s Disease. Biochem. Pharmacol. 2009, 78, 927–932. [Google Scholar] [CrossRef]
- Martins, G.L.S.; Paredes, B.D.; Sampaio, G.L.A.; Nonaka, C.K.V.; da Silva, K.N.; Allahdadi, K.J.; França, L.S.A.; Soares, M.B.P.; Dos Santos, R.R.; Souza, B.S.F. Generation of human iPS cell line CBTCi001-A from dermal fibroblasts obtained from a healthy donor. Stem Cell Res. 2019, 41, 101630. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef] [PubMed]
- Fivelman, Q.L.; Adagu, I.S.; Warhurst, D.C. Modified Fixed-Ratio Isobologram Method for Studying In Vitro Interactions Between Atovaquone and Proguanil or Dihydroartemisinin Against Drug-Resistant Strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 2004, 48, 4097–4102. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Brown, S.B.; Moan, J.; Nesland, J.M.; Wainwright, M.; Griffiths, J.; Dixon, B.; Cruse-Sawyer, J.; Vernon, D. Biodistribution of a Methylene Blue Derivative in Tumor and Normal Tissues of Rats. J. Photochem. Photobiol. B 1993, 20, 63–71. [Google Scholar] [CrossRef]
- Tremblay, J.F.; Dussault, S.; Viau, G.; Gad, F.; Boushira, M.; Bissonnette, R. Photodynamic Therapy with Toluidine Blue in Jurkat Cells: Cytotoxicity, Subcellular Localization and Apoptosis Induction. Photochem. Photobiol. Sci. 2002, 1, 852–856. [Google Scholar] [CrossRef]
- Gandolfo, S.; Pentenero, M.; Broccoletti, R.; Pagano, M.; Carrozzo, M.; Scully, C. Toluidine Blue Uptake in Potentially Malignant Oral Lesions In Vivo: Clinical and Histological Assessment. Oral Oncol. 2006, 42, 89–95. [Google Scholar] [CrossRef]
- Warnakulasuriya, K.A.; Johnson, N.W. Sensitivity and Specificity of OraScan® Toluidine Blue Mouthrinse in the Detection of Oral Cancer and Precancer. J. Oral Pathol. Med. 1996, 25, 97–103. [Google Scholar] [CrossRef]
- Kirszberg, C.; Rumjanek, V.M.; Capella, M.A. Methylene Blue Is More Toxic to Erythroleukemic Cells Than to Normal Peripheral Blood Mononuclear Cells: A Possible Use in Chemotherapy. Cancer Chemother. Pharmacol. 2005, 56, 659–665. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy-Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Newman, E.L. Photosensitizer Targeting in Photodynamic Therapy. II. Conjugates of Haematoporphyrin with Serum Lipoproteins. J. Photochem. Photobiol. B 1994, 26, 147–157. [Google Scholar] [CrossRef]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part Three-Photosensitizer Pharmacokinetics, Biodistribution, Tumor Localization and Modes of Tumor Destruction. Photodiagnosis Photodyn. Ther. 2005, 2, 91–106. [Google Scholar] [CrossRef]
- Cruz, P.M.; Mo, H.; McConathy, W.J.; Sabnis, N.; Lacko, A.G. The Role of Cholesterol Metabolism and Cholesterol Transport in Carcinogenesis: A Review of Scientific Findings, Relevant to Future Cancer Therapeutics. Front. Pharmacol. 2013, 4, 119. [Google Scholar] [CrossRef]
- de Freitas, L.M.; Serafim, R.B.; de Sousa, J.F.; Moreira, T.F.; Dos Santos, C.T.; Baviera, A.M.; Valente, V.; Soares, C.P.; Fontana, C.R. Photodynamic Therapy Combined to Cisplatin Potentiates Cell Death Responses of Cervical Cancer Cells. BMC Cancer 2017, 17, 123. [Google Scholar] [CrossRef]
- Guan, J.; Lai, X.; Wang, X.; Leung, A.W.; Zhang, H.; Xu, C. Photodynamic Action of Methylene Blue in Osteosarcoma Cells In Vitro. Photodiagnosis Photodyn. Ther. 2014, 11, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Bojang, P.; Esquivel, R.; Hernández, P.; Ramos, K.S.; Guzmán-Zamudio, R.; Lucero-Acuña, A. Differential Response of BEAS-2B and H-441 Cells to Methylene Blue Photoactivation. Anticancer Res. 2019, 39, 3739–3744. [Google Scholar] [CrossRef] [PubMed]
- Kofler, B.; Romani, A.; Pritz, C.; Steinbichler, T.B.; Schartinger, V.H.; Riechelmann, H.; Dudas, J. Photodynamic Effect of Methylene Blue and Low Level Laser Radiation in Head and Neck Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2018, 19, 1107. [Google Scholar] [CrossRef] [PubMed]
- Le, M.N.; Wuertz, B.R.; Biel, M.A.; Thompson, R.L.; Ondrey, F.G. Effects of Methylene Blue Photodynamic Therapy on Oral Carcinoma and Leukoplakia Cells. Laryngoscope Investig. Otolaryngol. 2022, 7, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Nowak, E.; Kammerer, S.; Küpper, J.H. ATP-Based Cell Viability Assay Is Superior to Trypan Blue Exclusion and XTT Assay in Measuring Cytotoxicity of Anticancer Drugs Taxol and Imatinib, and Proteasome Inhibitor MG-132 on Human Hepatoma Cell Line HepG2. Clin. Hemorheol. Microcirc. 2018, 69, 327–336. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Stockert, J.C.; Sanz-Rodríguez, F.; Zamarrón, A.; Juarranz, A. Differential Photodynamic Response of Cultured Cells to Methylene Blue and Toluidine Blue: Role of Dark Redox Processes. Photochem. Photobiol. Sci. 2009, 8, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, H.; Deng, H.; Zhang, Y.; Yang, X.; Zhang, W. Facile Preparation of Toluidine Blue-Loaded DNA Nanogels for Anticancer Photodynamic Therapy. Front. Bioeng. Biotechnol. 2023, 11, 1180448. [Google Scholar] [CrossRef]
- Mills, S. How Effective Is Toluidine Blue for Screening and Diagnosis of Oral Cancer and Premalignant Lesions? Evid. Based Dent. 2022, 23, 34–35. [Google Scholar] [CrossRef]
- Barbosa, A.F.S.; Santos, I.P.; Santos, G.M.P.; Bastos, T.M.; Rocha, V.P.C.; Meira, C.S.; Soares, M.B.P.; Pitta, I.R.; Pinheiro, A.L.B. Anti-Trypanosoma cruzi Effect of the Photodynamic Antiparasitic Chemotherapy Using Phenothiazine Derivatives as Photosensitizers. Lasers Med. Sci. 2020, 35, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bulhões Portapilla, G.; Pereira, L.M.; Bronzon da Costa, C.M.; Voltarelli Providello, M.; Sampaio Oliveira, P.A.; Goulart, A.; Ferreira Anchieta, N.; Wainwright, M.; Leite Braga, G.Ú.; de Albuquerque, S. Phenothiazinium Dyes Are Active against Trypanosoma cruzi In Vitro. Biomed Res. Int. 2019, 2019, 8301569. [Google Scholar] [CrossRef] [PubMed]
- Zancan, N.L.B.; Tebaldi, N.D. Terapia fotodinâmica no controle de Xanthomonas campestris pv. campestris in vitro e no tratamento de sementes de canola naturalmente contaminadas. Summa Phytopathol. 2020, 46, 327–332. [Google Scholar] [CrossRef]
- Hosseinzadeh, R.; Khorsandi, K.; Jahanshiri, M. Combination Photodynamic Therapy of Human Breast Cancer Using Salicylic Acid and Methylene Blue. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 184, 198–203. [Google Scholar] [CrossRef]
- Yousefi Sadeghloo, A.; Khorsandi, K.; Kianmehr, Z. Synergistic Effect of Photodynamic Treatment and Doxorubicin on Triple Negative Breast Cancer Cells. Photochem. Photobiol. Sci. 2020, 19, 1580–1589. [Google Scholar] [CrossRef]
| Sample | IC50 ± S.D (μM) HSC-3 | IC50 ± S.D. (μM) SCC-9 | IC50 ± S.D. (μM) Hfib | |||
|---|---|---|---|---|---|---|
| Dark Phase | Clear Phase | Dark Phase | Clear Phase | Dark Phase | Clear Phase | |
| MB | >400 | 234.5 ± 9.6 | 362.6 ± 15.6 | 307.4 ± 28.8 | >400 | 294.4 ± 12.1 |
| TB | 73.2 ± 2.6 | 58.7 ± 2.3 | 41.4 ± 4.0 | 33.8. ± 1.4 | 66.6 ± 5.9 | 53.7 ± 3.6 |
| Sample | IC50 ± S.D. (μM) Dark Phase | IC50 ± S.D. (μM) Clear Phase | CI a | |||
|---|---|---|---|---|---|---|
| Drug Alone | Combination | Drug Alone | Combination | CI Dark Phase | CI Clear Phase | |
| MB | 368.5 ± 10.8 | 30.5 ± 4.8 | 302.3 ± 25.7 | 23.4 ± 1.4 | 0.69 ± 0.05 | 0.73 ± 0.08 |
| TB | 43.8 ± 2.9 | 26.9 ± 1.4 | 33.7 ± 1.2 | 22.4 ± 2.4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, É.; Bomfim, L.; Junior, S.; Santos, G.; Meira, C.; Soares, M. Photodynamic Therapy with an Association of Methylene Blue and Toluidine Blue Promoted a Synergic Effect against Oral Squamous Cell Carcinoma. Cancers 2023, 15, 5509. https://doi.org/10.3390/cancers15235509
Rocha É, Bomfim L, Junior S, Santos G, Meira C, Soares M. Photodynamic Therapy with an Association of Methylene Blue and Toluidine Blue Promoted a Synergic Effect against Oral Squamous Cell Carcinoma. Cancers. 2023; 15(23):5509. https://doi.org/10.3390/cancers15235509
Chicago/Turabian StyleRocha, Évilin, Larissa Bomfim, Sérgio Junior, Gustavo Santos, Cássio Meira, and Milena Soares. 2023. "Photodynamic Therapy with an Association of Methylene Blue and Toluidine Blue Promoted a Synergic Effect against Oral Squamous Cell Carcinoma" Cancers 15, no. 23: 5509. https://doi.org/10.3390/cancers15235509
APA StyleRocha, É., Bomfim, L., Junior, S., Santos, G., Meira, C., & Soares, M. (2023). Photodynamic Therapy with an Association of Methylene Blue and Toluidine Blue Promoted a Synergic Effect against Oral Squamous Cell Carcinoma. Cancers, 15(23), 5509. https://doi.org/10.3390/cancers15235509








