An Innovative Non-Linear Prediction Model for Clinical Benefit in Women with Newly Diagnosed Breast Cancer Using Baseline FDG-PET/CT and Clinical Data
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
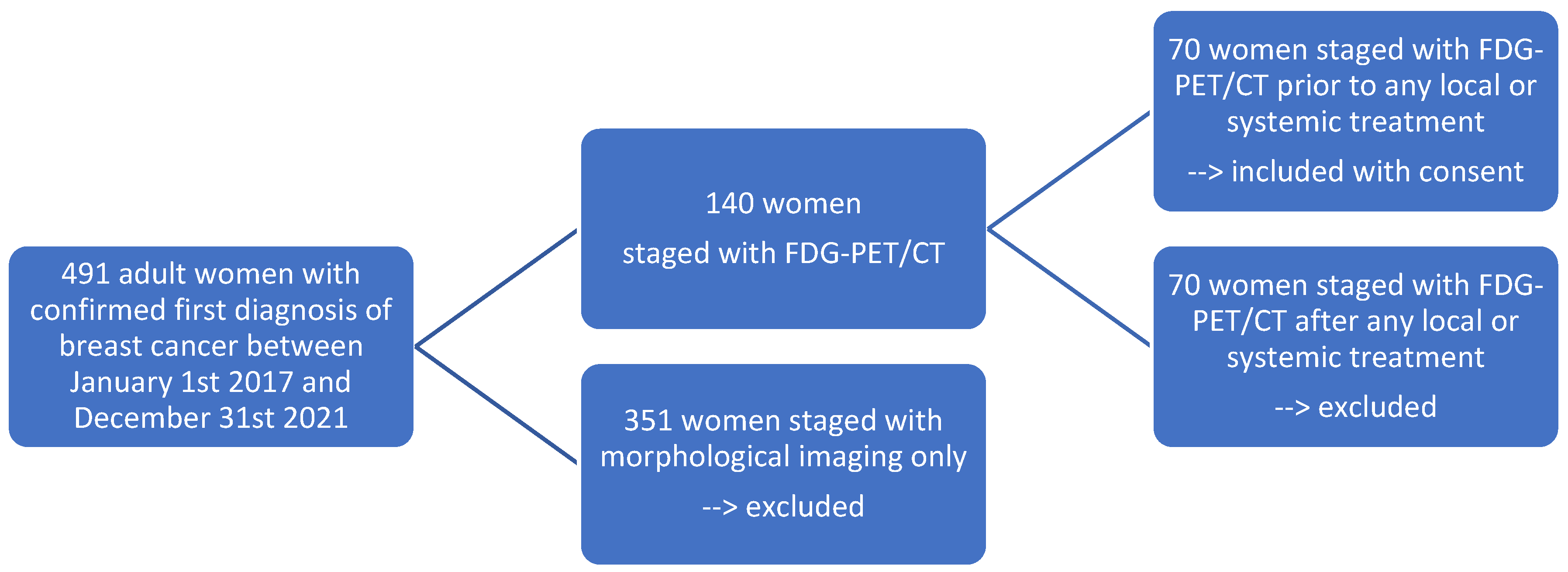
2.1. Patient Selection
2.2. Data Collection
| Data Category | Specifics |
| Patient Demographics | Age, Gender |
| Anthropometric Data | Height, Weight, and Body Mass Index (BMI) |
| Primary Tumor Characteristics | Anatomical site and histology |
| Receptor Status | Estrogen receptor (ER) expression, Progesterone receptor (PR) expression, and Human Epidermal Growth Factor Receptor-2 (Her-2) expression/overexpression |
| Tumor Proliferation Index | ki-67 expression |
| Molecular Subtype | Luminal A, Luminal B, HER-2 enriched, or triple negative |
| Clinical Staging | TNM (8th edition American Joint Committee on Cancer AJCC) |
| Endpoint | Definition |
| Overall survival (OS) | The time from the date of diagnosis to death or the last follow-up |
| Progression-free survival (PFS) | The time from the date of diagnosis to disease progression |
| Clinical benefit (CB) | No death and no disease progression from the date of diagnosis to the last follow-up |
2.3. FDG-PET/CT Acquisition
2.4. Primary Tumor (PT) Segmentation on FDG-PET/CT
- Morphological features: volume, morphology (solid, inflammatory), and margin (sharp, irregular, spiculated)
- Metabolic features: SUVmax, SUVmean, metabolic tumor volume (MTV), and total lesion glycolysis (TLG)
2.5. Statistical Analysis
3. Results
3.1. Patient Selection
3.2. Descriptive Statistics
3.3. Prediction Model Development
3.4. Performance of the Generated Prediction Model
3.4.1. For the Entire Cohort (N = 70)
3.4.2. According to the Molecular Subgroups
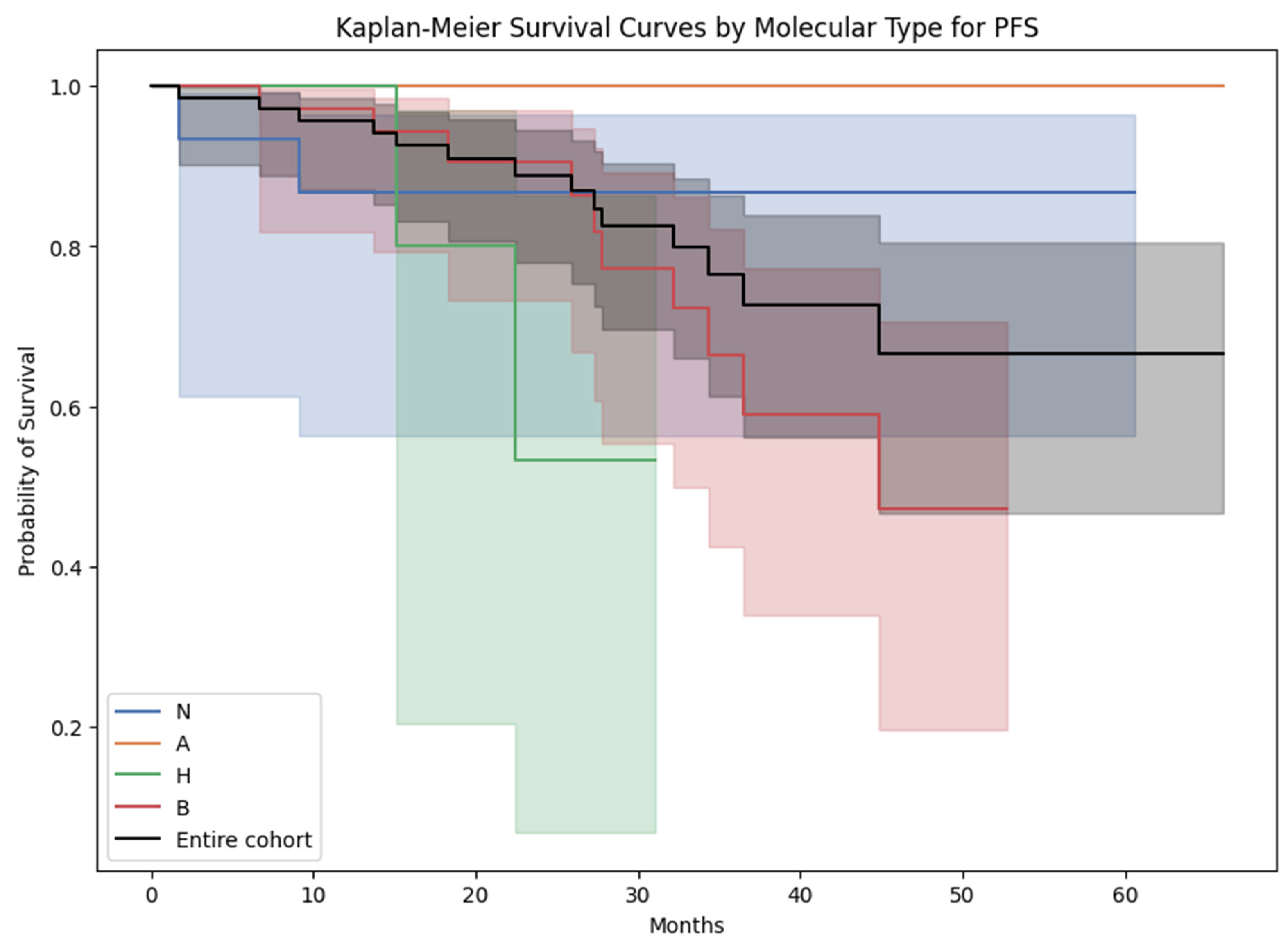
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FDG-PET/CT | [18F]-fluorodeoxyglucose positron emission tomography/computed tomography |
| PT | Primary Tumor |
| SUVmax | Maximum Standardized Uptake Value |
| SUVmean | Mean Standardized Uptake Value |
| MTV | Metabolic Tumor Volume |
| TLG | Total Lesion Glycolysis |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| CB | Clinical Benefit |
| BMI | Body Mass Index |
| MRI | Magnetic Resonance Imaging |
| NSCLC | Non-Small-Cell Lung Cancer |
| ER | Estrogen Receptor |
| PR | Progesterone Receptor |
| Her-2 | Human Epidermal Growth Factor Receptor-2 |
| AJCC | American Joint Committee on Cancer |
| DMI | Discovery Molecular Insights |
| GE | General Electrics |
| OSEM | Ordered Subset Expectation Maximization |
| TOF | Time-of-Flight |
| DLP | Dose Length Product |
| AW | Advanced Workstation |
| SD | Standard Deviation |
| ANOVA | Analysis of Variance |
| GAM | Generalized Additive Model |
| ROC | Receiver Operating Characteristic |
| AUC | Area Under the Curve |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Autier, P.; Boniol, M.; LaVecchia, C.; Vatten, L.; Gavin, A.; Héry, C.; Heanue, M. Disparities in breast cancer mortality trends between 30 European countries: Retrospective trend analysis of WHO mortality database. BMJ 2010, 341, c3620. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Legood, R.; Dos-Santos-Silva, I.; Gaiha, S.M.; Sadique, Z. Global treatment costs of breast cancer by stage: A systematic review. PLoS ONE 2018, 13, e0207993. [Google Scholar] [CrossRef]
- Duggan, C.; Trapani, D.; Ilbawi, A.M.; Fidarova, E.; Laversanne, M.; Curigliano, G.; Bray, F.; O Anderson, B. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: A population-based analysis. Lancet Oncol. 2021, 22, 1632–1642. [Google Scholar] [CrossRef]
- Paydary, K.; Seraj, S.M.; Zadeh, M.Z.; Emamzadehfard, S.; Shamchi, S.P.; Gholami, S.; Werner, T.J.; Alavi, A. The Evolving Role of FDG-PET/CT in the Diagnosis, Staging, and Treatment of Breast Cancer. Mol. Imaging Biol. 2018, 21, 1–10. [Google Scholar] [CrossRef]
- Brandão, M.; Morais, S.; Lopes-Conceição, L.; Fontes, F.; Araújo, N.; Dias, T.; Pereira, D.; Borges, M.; Pereira, S.; Lunet, N. Healthcare use and costs in early breast cancer: A patient-level data analysis according to stage and breast cancer subtype. ESMO Open 2020, 5, e000984. [Google Scholar] [CrossRef]
- Werner, S.; Sekler, J.; Gückel, B.; la Fougère, C.; Nikolaou, K.; Pfannenberg, C.; Preibsch, H.; Engler, T.; Olthof, S.-C. Influence of [18F]FDG-PET/CT on Clinical Management Decisions in Breast Cancer Patients—A PET/CT Registry Study. Diagnostics 2023, 13, 2420. [Google Scholar] [CrossRef]
- Hadebe, B.; Harry, L.; Ebrahim, T.; Pillay, V.; Vorster, M. The Role of PET/CT in Breast Cancer. Diagnostics 2023, 13, 597. [Google Scholar] [CrossRef]
- Groheux, D. FDG-PET/CT for Primary Staging and Detection of Recurrence of Breast Cancer. Semin. Nucl. Med. 2022, 52, 508–519. [Google Scholar] [CrossRef]
- Groheux, D.; Hindie, E. Breast cancer: Initial workup and staging with FDG PET/CT. Clin. Transl. Imaging 2021, 9, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Aroztegui, A.P.C.; Vicente, A.M.G.; Ruiz, S.A.; Bolton, R.C.D.; Rincon, J.O.; Garzon, J.R.G.; Torres, M.d.A.; Garcia-Velloso, M.J. 18F-FDG PET/CT in breast cancer: Evidence-based recommendations in initial staging. Tumor Biol. 2017, 39, 1–23. [Google Scholar] [CrossRef]
- Lother, D.; Robert, M.; Elwood, E.; Smith, S.; Tunariu, N.; Johnston, S.R.; Parton, M.; Bhaludin, B.; Millard, T.; Downey, K.; et al. Imaging in metastatic breast cancer, CT, PET/CT, MRI, WB-DWI, CCA: Review and new perspectives. Cancer Imaging 2023, 23, 53. [Google Scholar] [CrossRef] [PubMed]
- De Jong, D.; Desperito, E.; Al Feghali, K.A.; Dercle, L.; Seban, R.-D.; Das, J.P.; Ma, H.; Sajan, A.; Braumuller, B.; Prendergast, C.; et al. Advances in PET/CT Imaging for Breast Cancer. J. Clin. Med. 2023, 12, 4537. [Google Scholar] [CrossRef] [PubMed]
- Koolen, B.B.; Peeters, M.-J.T.F.D.V.; Aukema, T.S.; Vogel, W.V.; Oldenburg, H.S.A.; van der Hage, J.A.; Hoefnagel, C.A.; Stokkel, M.P.M.; Loo, C.E.; Rodenhuis, S.; et al. 18F-FDG PET/CT as a staging procedure in primary stage II and III breast cancer: Comparison with conventional imaging techniques. Breast Cancer Res. Treat. 2011, 131, 117–126. [Google Scholar] [CrossRef]
- Cè, M.; Caloro, E.; Pellegrino, M.E.; Basile, M.; Sorce, A.; Fazzini, D.; Oliva, G.; Cellina, M. Artificial intelligence in breast cancer imaging: Risk stratification, lesion detection and classification, treatment planning and prognosis—A narrative review. Explor. Target. Anti-Tumor Ther. 2022, 3, 795–816. [Google Scholar] [CrossRef]
- Kudura, K.; Ritz, N.; Kutzker, T.; Hoffmann, M.H.K.; Templeton, A.J.; Foerster, R.; Kreissl, M.C.; Antwi, K. Predictive Value of Baseline FDG-PET/CT for the Durable Response to Immune Checkpoint Inhibition in NSCLC Patients Using the Morphological and Metabolic Features of Primary Tumors. Cancers 2022, 14, 6095. [Google Scholar] [CrossRef]
- Kudura, K.; Dimitriou, F.; Basler, L.; Förster, R.; Mihic-Probst, D.; Kutzker, T.; Dummer, R.; Mangana, J.; Burger, I.A.; Kreissl, M.C. Prediction of Early Response to Immune Checkpoint Inhibition Using FDG-PET/CT in Melanoma Patients. Cancers 2021, 13, 3830. [Google Scholar] [CrossRef]
- Kudura, K.; Ritz, N.; Templeton, A.J.; Kutzker, T.; Foerster, R.; Antwi, K.; Kreissl, M.C.; Hoffmann, M.H.K. Predictive Value of Total Metabolic Tumor Burden Prior to Treatment in NSCLC Patients Treated with Immune Checkpoint Inhibition. J. Clin. Med. 2023, 12, 3725. [Google Scholar] [CrossRef]
- Antunovic, L.; De Sanctis, R.; Cozzi, L.; Kirienko, M.; Sagona, A.; Torrisi, R.; Tinterri, C.; Santoro, A.; Chiti, A.; Zelic, R.; et al. PET/CT radiomics in breast cancer: Promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur. J. Nucl. Med. 2019, 46, 1468–1477. [Google Scholar] [CrossRef] [PubMed]
- Dos Reis, F.J.C.; Wishart, G.C.; Dicks, E.M.; Greenberg, D.; Rashbass, J.; Schmidt, M.K.; Broek, A.J.v.D.; Ellis, I.O.; Green, A.; Rakha, E.; et al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. Breast Cancer Res. 2017, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Park, S.; Bang, J.-I.; Kim, E.-K.; Lee, H.-Y. Metabolic Radiomics for Pretreatment 18F-FDG PET/CT to Characterize Locally Advanced Breast Cancer: Histopathologic Characteristics, Response to Neoadjuvant Chemotherapy, and Prognosis. Sci. Rep. 2017, 7, 1556. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Yamamoto, Y.; Iwase, H. Clinical imaging for the prediction of neoadjuvant chemotherapy response in breast cancer. Chin. Clin. Oncol. 2020, 9, 31. [Google Scholar] [CrossRef]
- Kaise, H.; Shimizu, F.; Akazawa, K.; Hasegawa, Y.; Horiguchi, J.; Miura, D.; Kohno, N.; Ishikawa, T. Prediction of pathological response to neoadjuvant chemotherapy in breast cancer patients by imaging. J. Surg. Res. 2018, 225, 175–180. [Google Scholar] [CrossRef]
- Lee, A.H.S.; Ellis, I.O. The Nottingham Prognostic Index for Invasive Carcinoma of the Breast. Pathol. Oncol. Res. 2008, 14, 113–115. [Google Scholar] [CrossRef]
- Elwood, J.M.; Tawfiq, E.; TinTin, S.; Marshall, R.J.; Phung, T.M.; Campbell, I.; Harvey, V.; Lawrenson, R. Development and validation of a new predictive model for breast cancer survival in New Zealand and comparison to the Nottingham prognostic index. BMC Cancer 2018, 18, 897. [Google Scholar] [CrossRef]
- Min, N.; Wei, Y.; Zheng, Y.; Li, X. Advancement of prognostic models in breast cancer: A narrative review. Gland. Surg. 2021, 10, 2815–2831. [Google Scholar] [CrossRef]
- Oliveira, C.; Oliveira, F.; Vaz, S.C.; Marques, H.P.; Cardoso, F. Prediction of pathological response after neoadjuvant chemotherapy using baseline FDG PET heterogeneity features in breast cancer. Br. J. Radiol. 2023, 96, 20220655. [Google Scholar] [CrossRef]
- Phung, M.T.; Tin, S.T.; Elwood, J.M. Prognostic models for breast cancer: A systematic review. BMC Cancer 2019, 19, 230. [Google Scholar] [CrossRef]
- Rodrigues-Ferreira, S.; Nahmias, C. Predictive biomarkers for personalized medicine in breast cancer. Cancer Lett. 2022, 545, 215828. [Google Scholar] [CrossRef] [PubMed]
- Seban, R.-D.; Arnaud, E.; Loirat, D.; Cabel, L.; Cottu, P.; Djerroudi, L.; Hescot, S.; Loap, P.; Bonneau, C.; Bidard, F.-C.; et al. [18F]FDG PET/CT for predicting triple-negative breast cancer outcomes after neoadjuvant chemotherapy with or without pembrolizumab. Eur. J. Nucl. Med. 2023, 50, 4024–4035. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Chao, J.; Liu, P.; Zhang, B.; Zhang, N.; Luo, Z.; Han, J. Prognostic models for breast cancer: Based on logistics regression and Hybrid Bayesian Network. BMC Med. Inform. Decis. Mak. 2023, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Sollini, M.; Cozzi, L.; Ninatti, G.; Antunovic, L.; Cavinato, L.; Chiti, A.; Kirienko, M. PET/CT radiomics in breast cancer: Mind the step. Methods 2020, 188, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Modi, N.D.; Sorich, M.J.; Rowland, A.; Logan, J.M.; McKinnon, R.A.; Kichenadasse, G.; Wiese, M.D.; Hopkins, A.M. A literature review of treatment-specific clinical prediction models in patients with breast cancer. Crit. Rev. Oncol. Hematol. 2020, 148, 102908. [Google Scholar] [CrossRef] [PubMed]
- Kent, D.M.; Steyerberg, E.; van Klaveren, D. Personalized evidence based medicine: Predictive approaches to heterogeneous treatment effects. BMJ 2018, 363, k4245. [Google Scholar] [CrossRef]
- Park, H.; Petkova, E.; Tarpey, T.; Ogden, R.T. Functional additive models for optimizing individualized treatment rules. Biometrics 2021, 79, 113–126. [Google Scholar] [CrossRef]
- Park, H.; Petkova, E.; Tarpey, T.; Ogden, R.T. A sparse additive model for treatment effect-modifier selection. Biostatistics 2022, 23, 412–429. [Google Scholar] [CrossRef]
- Shin, S.; Fu, J.; Shin, W.-K.; Huang, D.; Min, S.; Kang, D. Association of food groups and dietary pattern with breast cancer risk: A systematic review and meta-analysis. Clin. Nutr. 2023, 42, 282–297. [Google Scholar] [CrossRef]
- De Mooij, C.M.; Ploumen, R.A.W.; Nelemans, P.J.; Mottaghy, F.M.; Smidt, M.L.; van Nijnatten, T.J.A. The influence of receptor expression and clinical subtypes on baseline [18F]FDG uptake in breast cancer: Systematic review and meta-analysis. EJNMMI Res. 2023, 13, 5. [Google Scholar] [CrossRef]
- Groheux, D.; Cochet, A.; Humbert, O.; Alberini, J.-L.; Hindié, E.; Mankoff, D. 18F-FDG PET/CT for Staging and Restaging of Breast Cancer. J. Nucl. Med. 2016, 57, 17S–26S. [Google Scholar] [CrossRef]
- Groheux, D.; Mankoff, D.; Espié, M.; Hindié, E. 18F-FDG PET/CT in the early prediction of pathological response in aggressive subtypes of breast cancer: Review of the literature and recommendations for use in clinical trials. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 983–993. [Google Scholar] [CrossRef]
- Morris, P.G.; Ulaner, G.A.; Eaton, A.; Fazio, M.; Jhaveri, K.; Patil, S.; Evangelista, L.; Park, J.Y.; Serna-Tamayo, C.; Howard, J.; et al. Standardized uptake value by positron emission tomography/computed tomography as a prognostic variable in metastatic breast cancer. Cancer 2012, 118, 5454–5462. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Lee, S.-W.; Jeong, S.Y.; Song, B.-I.; Chae, Y.S.; Ahn, B.-C.; Lee, J. Whole-Body Metabolic Tumor Volume, as Determined by 18F-FDG PET/CT, as a Prognostic Factor of Outcome for Patients with Breast Cancer Who Have Distant Metastasis. Am. J. Roentgenol. 2015, 205, 878–885. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Shigematsu, H.; Tsutani, Y.; Emi, A.; Masumoto, N.; Ozaki, S.; Kadoya, T.; Okada, M. Role of FDG-PET/CT in evaluating surgical outcomes of operable breast cancer—Usefulness for malignant grade of triple-negative breast cancer. Breast 2013, 22, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Aogi, K.; Kadoya, T.; Sugawara, Y.; Kiyoto, S.; Shigematsu, H.; Masumoto, N.; Okada, M. Utility of 18F FDG-PET/CT for predicting prognosis of luminal-type breast cancer. Breast Cancer Res. Treat. 2015, 150, 209–217. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Wang, B.; Hu, X.; Gong, C.; Zhao, Y.; Xie, Y.; Zhang, Y.; Song, S.; Yang, Z.; et al. Prediction of Pretreatment 18F-FDG-PET/CT Parameters on the Outcome of First-Line Therapy in Patients with Metastatic Breast Cancer. Int. J. Gen. Med. 2021, 14, 1797–1809. [Google Scholar] [CrossRef]
- Alam Khan, S.; Hernandez-Villafuerte, K.; Hernandez, D.; Schlander, M. Estimation of the stage-wise costs of breast cancer in Germany using a modeling approach. Front. Public Health 2023, 10, 946544. [Google Scholar] [CrossRef]





| N | All | CB | No CB | p-Value | |||
|---|---|---|---|---|---|---|---|
| 70 | 55 | 15 | |||||
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 63.3 | 15.4 | 63.5 | 15.3 | 62.8 | 16.3 | 0.88 |
| BMI (kg/m2) | 26.5 | 5.7 | 26.2 | 5.7 | 27.7 | 5.7 | 0.36 |
| Blood glucose (mmol/L) | 5.7 | 0.9 | 5.7 | 0.9 | 6.0 | 1.1 | 0.33 |
| Injected activity (MBq) | 304.8 | 102.1 | 300.1 | 98.2 | 323.1 | 118.1 | 0.46 |
| Total DLP (mGy·cm) | 833.6 | 388.5 | 826.6 | 390.4 | 859.4 | 393.9 | 0.77 |
| PT Volume | 12.8 | 30.4 | 7.5 | 11.1 | 32.1 | 59.7 | <0.01 |
| PT SUVmax | 8.1 | 7.2 | 9.0 | 7.8 | 4.7 | 2.4 | 0.04 |
| PT SUVmean | 4.9 | 4.4 | 5.5 | 4.7 | 2.8 | 1.5 | 0.03 |
| PT MTV | 12.7 | 30.4 | 7.5 | 10.9 | 32.0 | 60.0 | <0.01 |
| PT TLG | 47.4 | 80.2 | 44.3 | 77.3 | 58.9 | 92.1 | 0.54 |
| Ki-67 expression (%) | 35.1 | 24.5 | 35.5 | 23.9 | 34.0 | 27.4 | 0.84 |
| Observation time (months) | 34.4 | 12.7 | 33.7 | 12.7 | 36.9 | 12.8 | 0.39 |
| OS (months) | 31.7 | 14.2 | 32.5 | 13.4 | 28.8 | 17.2 | 0.37 |
| PFS (months) | 30.2 | 14.1 | 32.5 | 13.4 | 21.4 | 13.4 | <0.01 |
| Clinical Data | All | CB | No CB | p-Value |
|---|---|---|---|---|
| Anatomical site | 1.00 | |||
| 1 = right | 33 (47.1%) | 26 (42.3%) | 7 (46.7%) | |
| 2 = left | 37 (52.9%) | 29 (52.7%) | 8 (53.3%) | |
| Quadrant | 0.86 | |||
| 1 = central position | 7 (10.0%) | 5 (9.1%) | 2 (13.3%) | |
| 2 = upper inner quadrant | 11 (15.7%) | 9 (16.4%) | 2 (13.3%) | |
| 3 = lower inner quadrant | 7 (10.0%) | 6 (10.9%) | 1 (6.8%) | |
| 4 = upper outer quadrant | 28 (40.0%) | 23 (41.8%) | 5 (33.3%) | |
| 5 = lower outer quadrant | 16 (22.9%) | 11 (20.0%) | 5 (33.3%) | |
| 9 = not further described | 1 (1.4%) | 1 (1.8%) | 0 | |
| Histology PT | 0.25 | |||
| 1 = invasive ductal adenocarcinoma | 62 (88.6%) | 50 (91.0%) | 12 (80.0%) | |
| 2 = invasive lobular adenocarcinoma | 5 (7.1%) | 3 (5.4%) | 2 (13.3%) | |
| 3 = invasive papillary adenocarcinoma | 1 (1.4%) | 0 (0.0%) | 1 (6.7%) | |
| 4 = mucinous carcinoma | 1 (1.4%) | 1 (1.8%) | 0 (0.0%) | |
| 5 = apocrine carcinoma | 1 (1.4%) | 1 (1.8%) | 0 (0.0%) | |
| Molecular subtype PT | 0.33 | |||
| A = Luminal A | 13 (18.6%) | 12 (21.8%) | 1 (6.7%) | |
| B = Luminal B | 36 (51.4%) | 26 (47.3%) | 10 (66.7%) | |
| H = Her-2 enriched | 6 (8.6%) | 4 (7.3%) | 2 (13.3%) | |
| N = triple negative | 15 (21.4%) | 13 (23.6%) | 2 (13.3%) | |
| T | 0.17 | |||
| 1 | 21 (30.0%) | 19 (34.5%) | 2 (13.3%) | |
| 2 | 34 (48.6%) | 27 (49.1%) | 7 (46.7%) | |
| 3 | 2 (2.9%) | 1 (1.8%) | 1 (6.7%) | |
| 4 | 13 (18.6%) | 8 (14.6%) | 5 (33.3%) | |
| N | 0.54 | |||
| 0 | 19 (27.1%) | 17 (30.8%) | 2 (13.3%) | |
| 1 | 34 (48.6%) | 26 (47.3%) | 8 (53.3%) | |
| 2 | 6 (8.6%) | 4 (7.3%) | 2 (13.3%) | |
| 3 | 11 (15.7%) | 8 (14.6%) | 3 (20.1%) | |
| M | <0.01 | |||
| 0 | 58 (82.3%) | 51 (92.7%) | 7 (46.7%) | |
| 1 | 12 (17.1%) | 4 (7.3%) | 8 (53.3%) | |
| Hybrid Imaging | All | CB | No CB | p-Value |
| Margin PT | 0.78 | |||
| 1 = sharp | 10 (14.3%) | 7 (12.7%) | 3 (20.0%) | |
| 2 = irregular | 55 (78.6%) | 44 (80.0%) | 11 (73.3%) | |
| 3 = spiculated | 5 (7.1%) | 4 (7.3%) | 1 (6.7%) | |
| Morphology PT | 0.42 | |||
| 1 = solid | 66 (94.3%) | 53 (96.4%) | 13 (86.7%) | |
| 2 = inflammatory | 4 (5.7%) | 2 (3.6%) | 2 (13.3%) | |
| Death | <0.01 | |||
| 0 = no | 65 (92.8%) | 55 (100.0%) | 10 (66.7%) | |
| 1 = yes | 5 (7.2%) | 0 (0.0%) | 5 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudura, K.; Ritz, N.; Templeton, A.J.; Kutzker, T.; Hoffmann, M.H.K.; Antwi, K.; Zwahlen, D.R.; Kreissl, M.C.; Foerster, R. An Innovative Non-Linear Prediction Model for Clinical Benefit in Women with Newly Diagnosed Breast Cancer Using Baseline FDG-PET/CT and Clinical Data. Cancers 2023, 15, 5476. https://doi.org/10.3390/cancers15225476
Kudura K, Ritz N, Templeton AJ, Kutzker T, Hoffmann MHK, Antwi K, Zwahlen DR, Kreissl MC, Foerster R. An Innovative Non-Linear Prediction Model for Clinical Benefit in Women with Newly Diagnosed Breast Cancer Using Baseline FDG-PET/CT and Clinical Data. Cancers. 2023; 15(22):5476. https://doi.org/10.3390/cancers15225476
Chicago/Turabian StyleKudura, Ken, Nando Ritz, Arnoud J. Templeton, Tim Kutzker, Martin H. K. Hoffmann, Kwadwo Antwi, Daniel R. Zwahlen, Michael C. Kreissl, and Robert Foerster. 2023. "An Innovative Non-Linear Prediction Model for Clinical Benefit in Women with Newly Diagnosed Breast Cancer Using Baseline FDG-PET/CT and Clinical Data" Cancers 15, no. 22: 5476. https://doi.org/10.3390/cancers15225476
APA StyleKudura, K., Ritz, N., Templeton, A. J., Kutzker, T., Hoffmann, M. H. K., Antwi, K., Zwahlen, D. R., Kreissl, M. C., & Foerster, R. (2023). An Innovative Non-Linear Prediction Model for Clinical Benefit in Women with Newly Diagnosed Breast Cancer Using Baseline FDG-PET/CT and Clinical Data. Cancers, 15(22), 5476. https://doi.org/10.3390/cancers15225476







