The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms
Abstract
Simple Summary
Abstract
1. Introduction
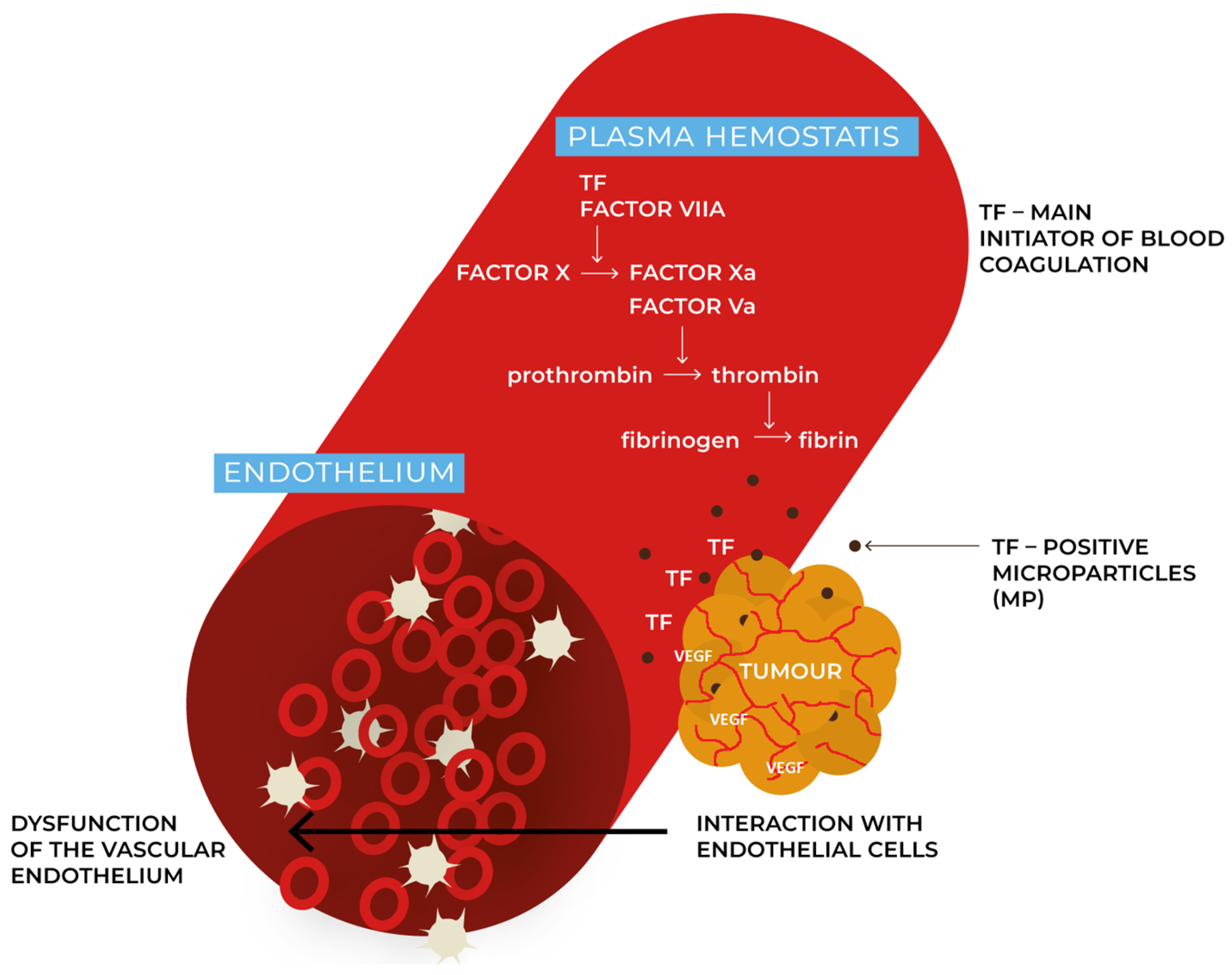
2. Methods
3. The Occurrence of VTE in Neuroendocrine Neoplasms
4. Atypical Locations of VTE in Neuroendocrine Neoplasms
5. VTE in Hormonally Active Neuroendocrine Neoplasms
5.1. Carcinoid Syndrome
5.2. Glucagonoma
5.3. Endogenous Cushing’s Syndrome
6. Antithrombotic Prophylaxis in Neuroendocrine Neoplasms
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Ramcharitar, R.K.; Man, L.; Khaja, M.S.; Barnett, M.E.; Sharma, A. A Review of the Past, Present and Future of Cancer-Associated Thrombosis Management. Heart Int. 2022, 16, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Lugassy, G.; Falanga, A.; Kakkar, A.K.; Rickles Frederick, R. Thrombosis and Cancer; CRC Press: Boca Raton, FL, USA, 2004; ISBN 9781841842875. [Google Scholar]
- Wojtukiewicz, M.Z.; Sierko, E.; Tomkowski, W.; Zawilska, K.; Undas, A.; Podolak-Dawidziak, M.; Wysocki, P.; Krzakowski, M.; Warzocha, K.; Windyga, J. Guidelines for the Prevention and Treatment of Venous Thromboembolism in Patients with Cancers Treated Conservatively. Hematologia 2016, 7, 128–160. [Google Scholar]
- Bagot, C.N.; Arya, R. Virchow and His Triad: A Question of Attribution. Br. J. Haematol. 2008, 143, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.S.; Khorana, A.A.; McCrae, K.R. Mechanisms and Biomarkers of Cancer-Associated Thrombosis. Transl. Res. 2020, 225, 33–53. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Mackman, N.; Bertina, R.M.; Kaider, A.; Marosi, C.; Key, N.S.; Barcel, D.A.; Scheithauer, W.; Kornek, G.; et al. Microparticle-Associated Tissue Factor Activity, Venous Thromboembolism and Mortality in Pancreatic, Gastric, Colorectal and Brain Cancer Patients. J. Thromb. Haemost. 2012, 10, 1363–1370. [Google Scholar] [CrossRef]
- Thaler, J.; Ay, C.; Mackman, N.; Metz-Schimmerl, S.; Stift, J.; Kaider, A.; Müllauer, L.; Gnant, M.; Scheithauer, W.; Pabinger, I. Microparticle-Associated Tissue Factor Activity in Patients with Pancreatic Cancer: Correlation with Clinicopathological Features. Eur. J. Clin. Investig. 2013, 43, 277–285. [Google Scholar] [CrossRef]
- Geddings, J.E.; Mackman, N. Tumor-Derived Tissue Factor-Positive Microparticles and Venous Thrombosis in Cancer Patients. Blood 2013, 122, 1873–1880. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Foltyn, W.; Malczewska, A. Update of the diagnostic and therapeutic guidelines for gastro-enteropancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 387–423. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Baudin, E.; Caplin, M.; Garcia-Carbonero, R.; Fazio, N.; Ferolla, P.; Filosso, P.L.; Frilling, A.; de Herder, W.W.; Hörsch, D.; Knigge, U.; et al. Lung and Thymic Carcinoids: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2021, 32, 439–451. [Google Scholar] [CrossRef]
- Cigrovski Berković, M.; Čačev, T.; Catela Ivković, T.; Marout, J.; Ulamec, M.; Zjačić-Rotkvić, V.; Kapitanović, S. High VEGF Serum Values Are Associated with Locoregional Spread of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs). Mol. Cell. Endocrinol. 2016, 425, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jia, Z.; Li, Q.; Wang, L.; Rashid, A.; Zhu, Z.; Evans, D.B.; Vauthey, J.N.; Xie, K.; Yao, J.C. Elevated Expression of Vascular Endothelial Growth Factor Correlates with Increased Angiogenesis and Decreased Progression-Free Survival among Patients with Low-Grade Neuroendocrine Tumors. Cancer 2007, 109, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Berardi, R.; Torniai, M.; Partelli, S.; Rubini, C.; Pagliaretta, S.; Savini, A.; Polenta, V.; Santoni, M.; Giampieri, R.; Onorati, S.; et al. Impact of Vascular Endothelial Growth Factor (VEGF) and Vascular Endothelial Growth Factor Receptor (VEGFR) Single Nucleotide Polymorphisms on Outcome in Gastroenteropancreatic Neuroendocrine Neoplasms. PLoS ONE 2018, 13, e0197035. [Google Scholar] [CrossRef]
- Hurtado-Cordovi, J.; Lipka, S.; Avezbakiyev, B.; Multz, A.S. Budd-Chiari Syndrome Induced by Stage IV Rectal Carcinoid. Am. J. Med. Sci. 2013, 345, 246–247. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.; Nishihama, K.; Inoue, C.; Okano, Y.; Eguchi, K.; Tanaka, S.; Maki, K.; D’Alessandro, V.F.; Takeshita, A.; Yasuma, T.; et al. Adrenocorticotropic Hormone-Secreting Pancreatic Neuroendocrine Carcinoma with Multiple Organ Infections and Widespread Thrombosis: A Case Report. World J. Clin. Cases 2022, 10, 5723–5731. [Google Scholar] [CrossRef]
- Delli Colli, M.; Alamri, B.N.; Palma, L.; Rivera, J. A Glucagonoma Presenting as Cerebral Vein Thrombosis and Diabetes. Case Rep. Endocrinol. 2022, 2022, 8–10. [Google Scholar] [CrossRef]
- Tian, Y.; Qi, X.; Aljbri, A.; Xu, K.; Zhong, H. Case Report: Pancreatic Neuroendocrine Tumor With Liver Metastasis and Portal Vein Thrombosis. Front. Oncol. 2022, 11, 1–7. [Google Scholar] [CrossRef]
- Massironi, S.; Cavalcoli, F.; Artoni, A.; Sciola, V.; Zilli, A.; Ciafardini, C.; Elisa Rossi, R. Thrombotic Risk in Gastroenteropancreatic Neuroendocrine Tumor Patients: A Single-Center Experience. Ann. Gastroenterol. 2021, 34, 588–593. [Google Scholar] [CrossRef]
- Lee, M.E.; Ortega-Sustache, Y.M.; Agarwal, S.K.; Tepede, A.; Welch, J.; Mandl, A.; Bansal, R.; Tirosh, A.; Piaggi, P.; Cochran, C.; et al. Patients with MEN1 Are at an Increased Risk for Venous Thromboembolism. J. Clin. Endocrinol. Metab. 2021, 106, E460–E468. [Google Scholar] [CrossRef]
- Amoui, M.; Ahmadi, R.; Qutbi, M.; Asli, I.N. Somatostatin-receptor avidity of pancreatic neuroendocrine tumor thrombus in porto-caval venous systems on 99mTc-Octreotide and posttherapeutic 177Lu-DOTA-TATE scans. World J. Nucl. Med. 2021, 20, 324–326. [Google Scholar] [CrossRef]
- Liaqat, A.; Farooq, A.; Gulzar, Q.; Stalcup, S.; Abdalsalam, B. Central Vein Thrombosis in Pulmonary Neuroendocrine Neoplasm: A Novel Presentation. Cureus 2021, 13, e16499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cheng, Y.; Ding, N.; Huo, L. Extensive Tumor Thrombosis of Portal Venous System Demonstrated on 68Ga-DOTATATE and 68Ga-NODAGA-LM3 PET/CT in a Patient With Well-Differentiated Neuroendocrine Tumor. Clin. Nucl. Med. 2020, 45, 902–904. [Google Scholar] [CrossRef]
- Hollander, K.N.; Joshi, B.L.; Joshi, B.L. Bioprosthetic Valve Thrombosis in Carcinoid Heart Disease. Ann. Card. Anaesth. 2019, 22, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Pham, D.; Vierra, A.T.; Azam, S.; Gui, D.; Yoon, J.C. Pulmonary Embolism as the Presenting Symptom and a Confounder in ACTH-Secreting Bronchial Carcinoid. Endocrinol. Diabetes Metab. Case Rep. 2019, 2019, 19-0033. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, R.; Paiella, S.; Cardobi, N.; Landoni, L.; Tinazzi Martini, P.; Ortolani, S.; De Marchi, G.; Gobbo, S.; Giardino, A.; Butturini, G.; et al. Tumor Thrombosis: A Peculiar Finding Associated with Pancreatic Neuroendocrine Neoplasms. A Pictorial Essay. Abdom. Radiol. 2018, 43, 613–619. [Google Scholar] [CrossRef]
- Yu, R. Right Atrial Tumor Thrombus: A New Mechanism for Neuroendocrine Tumor-Induced Heart Complications. Pancreas 2018, 47, e26–e27. [Google Scholar] [CrossRef] [PubMed]
- Moyana, T.N.; Macdonald, D.B.; Martel, G.; Pyatibrat, S.; Lee, G.; Capitano, M. Pancreatic Neuroendocrine Tumors Complicated by Sinistral Portal Hypertension: Insights into Pathogenesis. J. Pancreat. Cancer 2017, 3, 71–77. [Google Scholar] [CrossRef]
- Rodriguez, R.A.; Overton, H.; Morris, K.T. Pancreatic Neuroendocrine Tumor with Splenic Vein Tumor Thrombus: A Case Report. Int. J. Surg. Case Rep. 2014, 5, 1271–1274. [Google Scholar] [CrossRef][Green Version]
- Prakash, L.; Lee, J.E.; Yao, J.; Bhosale, P.; Balachandran, A.; Wang, H.; Fleming, J.B.; Katz, M.H.G. Role and Operative Technique of Portal Venous Tumor Thrombectomy in Patients with Pancreatic Neuroendocrine Tumors. J. Gastrointest. Surg. 2015, 19, 2011–2018. [Google Scholar] [CrossRef]
- Busch, A.; Tschernitz, S.; Thurner, A.; Kellersmann, R.; Lorenz, U. Fatal Paraneoplastic Embolisms in Both Circulations in a Patient with Poorly Differentiated Neuroendocrine Tumour. Case Rep. Vasc. Med. 2013, 2013, 739427. [Google Scholar] [CrossRef]
- Balachandran, A.; Tamm, E.P.; Bhosale, P.R.; Katz, M.H.; Fleming, J.B.; Yao, J.C.; Charnsangavej, C. Venous Tumor Thrombus in Nonfunctional Pancreatic Neuroendocrine Tumors. Am. J. Roentgenol. 2012, 199, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Teh, R.W.; Tsoi, D.T. Acute Disseminated Intravascular Coagulation in Neuroendocrine Carcinoma. Case Rep. Oncol. 2012, 5, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Naswa, N.; Kumar, R.; Bal, C.; Malhotra, A. Vascular thrombosis as a cause of abdominal pain in a patient with neuroendocrine carcinoma of pancreas: Findings on (68)Ga-DOTANOC PET/CT. Indian J. Nucl. Med. 2012, 27, 35–37. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lim, T.C.; Tan, E.H.; Zaheer, S. Use of Ga-68 DOTATATE PET/CT to confirm portal vein tumor thrombosis in a patient with pancreatic neuroendocrine tumor. Clin. Nucl. Med. 2011, 36, 498–499. [Google Scholar] [CrossRef] [PubMed]
- Tsuchikawa, T.; Kondo, S.; Hirano, S.; Tanaka, E.; Kawasaki, R.; Kato, K.; Matsumoto, J.; Shichinohe, T. Distal Pancrea- Tectomy and Portal Vein Resection without Vascular Reconstruction for Endocrine Tumors with Massive Intraportal Growth: Report of a Case. Hepatogastroenterology 2011, 58, 1029–1031. [Google Scholar]
- Barbier, L.; Turrini, O.; Sarran, A.; Delpero, J.R. Pancreatic Endocrine Tumor with Neoplastic Venous Thrombus and Bilobar Liver Metastasis. A Case Report. J. Visc. Surg. 2010, 147, 58–62. [Google Scholar] [CrossRef]
- Kawakami, H.; Kuwatani, M.; Hirano, S.; Kondo, S.; Nakanishi, Y.; Itoh, T.; Asaka, M. Pancreatic Endocrine Tumors with Intraductal Growth into the Main Pancreatic Duct and Tumor Thrombus within the Portal Vein: A Case Report and Review of the Literature. Intern. Med. 2007, 46, 273–277. [Google Scholar] [CrossRef][Green Version]
- Nguyen, B.D. Pancreatic neuroendocrine tumor with portal vein tumor thrombus: PET demonstration. Clin. Nucl. Med. 2005, 30, 628–629. [Google Scholar] [CrossRef] [PubMed]
- Bedirli, A.; Patiroglu, T.E.; Sakrak, O.; Aritas, Y. Portal Vein Resection for a Portal Vein Thrombus Caused by Nonfunctioning Islet Cell Carcinoma: Report of a Case. Surg. Today 2004, 34, 802–804. [Google Scholar] [CrossRef]
- Di Micco, P.; Federico, A.; De Lucia, D.; De Sio, I.; Niglio, A.; Romano, M. Procoagulant Activities in the Plasma of a Patient with Peritoneal Metastasis from Pancreatic Neuroendocrine Tumor: A Case Report. Exp. Oncol. 2002, 24, 213–215. [Google Scholar]
- Watase, M.; Sakon, M.; Monden, M.; Miyoshi, Y.; Tono, T.; Ichikawa, T.; Kubota, N.; Shiozaki, H.; Okuda, H.; Okamura, J.; et al. A Case of Splenic Vein Occlusion Caused by the Intravenous Tumor Thrombus of Nonfunctioning Islet Cell Carcinoma. Surg Today 1992, 22, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Gervaso, L.; Dave, H.; Khorana, A.A. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncology 2021, 3, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.J.; Card, T.R.; West, J.; Crooks, C.; Grainge, M.J. Incidence of Venous Thromboembolism in Patients with Cancer-A Cohort Study Using Linked United Kingdom Databases. Eur. J. Cancer 2013, 49, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.K.; Wun, T.; Harvey, D.; Zhou, H.; White, R.H. Incidence of Venous Thromboembolism and Its Effect on Survival among Patients with Common Cancers. Arch. Intern. Med. 2006, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Durczynski, A.; Kumor, A.; Hogendorf, P.; Szymanski, D.; Grzelak, P.; Strzelczyk, J. Preoperative High Level of D-Dimers Predicts Unresectability of Pancreatic Head Cancer. World J. Gastroenterol. 2014, 20, 13167–13171. [Google Scholar] [CrossRef]
- Chan, S.L.; Chong, C.C.N.; Chan, A.W.H.; Poon, D.M.C.; Chok, K.S.H. Management of Hepatocellular Carcinoma with Portal Vein Tumor Thrombosis: Review and Update at 2016. World J. Gastroenterol. 2016, 22, 7289–7300. [Google Scholar] [CrossRef]
- Ng, C.S.; Wood, C.G.; Silverman, P.M.; Tannir, N.M.; Tamboli, P.; Sandler, C.M. Renal Cell Carcinoma: Diagnosis, Staging, and Surveillance. Am. J. Roentgenol. 2008, 191, 1220–1232. [Google Scholar] [CrossRef]
- Sainz-Esteban, A.; Prasad, V.; Baum, R.P. Pancreatic Neuroendocrine Tumor With Involvement of the Inferior Mesenteric Vein Diagnosed by Ga-68 DOTA-TATE PET/CT. Clinical Nuclear Med. 2010, 35, 40–41. [Google Scholar] [CrossRef]
- Zhou, J.; Tang, Z.Y.; Fan, J.; Wu, Z.Q.; Li, X.M.; Liu, Y.K.; Liu, F.; Sun, H.C.; Ye, S.L. Expression of Platelet-Derived Endothelial Cell Growth Factor and Vascular Endothelial Growth Factor in Hepatocellular Carcinoma and Portal Vein Tumor Thrombus. J. Cancer Res. Clin. Oncol. 2000, 126, 57–61. [Google Scholar] [CrossRef]
- Falconi, M.; Bartsch, D.K.; Eriksson, B.; Klöppel, G.; Lopes, J.M.; O’Connor, J.M.; Salazar, R.; Taal, B.G.; Vullierme, M.P.; O’Toole, D. ENETS Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Neoplasms of the Digestive System: Well-Differentiated Pancreatic Non-Functioning Tumors. Neuroendocrinology 2012, 95, 120–134. [Google Scholar] [CrossRef]
- Bednarczuk, T.; Zemczak, A.; Borowska, M.; Borowska, M.; Chmielik, E.; Ćwikła, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; Hubalewska-Dydejczyk, A.; et al. Neuroendocrine Neoplasms of the Small Intestine and the Appendix—Update of the Diagnostic and Therapeutic Guidelines (Recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 549–567. [Google Scholar] [CrossRef] [PubMed]
- Kos-Kudła, B.; Rosiek, V.; Borowska, M.; Bednarczuk, T.; Bolanowski, M.; Chmielik, E.; Ćwikla, J.B.; Foltyn, W.; Gisterek, I.; Handkiewicz-Junak, D.; et al. Pancreatic Neuroendocrine Neoplasms—Update of the Diagnostic and Therapeutic Guidelines (Recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol. Pol. 2022, 73, 491–548. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, D.; Kuang, T.; Rong, Y.; Lou, W. Glucagonoma Syndrome: Report of One Case. Transl. Gastroenterol. Hepatol. 2016, 2016, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Langlois, F.; Ting Lim, D.S.; McCartney, S.; Fleseriu, M. Hypercoagulability and Risk of Venous Thromboembolic Events in Endogenous Cushing’s Syndrome: A Systematic Meta-Analysis. Front. Endocrinol. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Nakano, K.; Sugiyama, K.; Satoh, H.; Shiromori, S.; Sugitate, K.; Arifuku, H.; Yoshida, N.; Watanabe, H.; Tokita, S.; Wakayama, T.; et al. Risk Factors for Disseminated Intravascular Coagulation in Patients with Lung Cancer. Thorac. Cancer 2018, 9, 931–938. [Google Scholar] [CrossRef]

| No. | Author, Year | Primary Tumor Localization | Pathology | TNM Stage | Functionality Status | Number of Patients with VTE (% VTE) | Type of Thromboembolic Complications | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Yoshihara et al. (2022) | Pancreatic | NEC (Ki-67 40%) | N/A | F: Ectopic Cushing’s syndrome (ectopic ACTH syndrome) | 1/1 (CR) | VTE (deep vein thrombosis, pulmonary embolism) DIC | [16] |
| 2 | Delli Colli et al. (2022) | Pancreatic | G1 (Ki-67 2%) | I | F: Glucagonoma | 1/1 (CR) | VTE (cerebral sinus venous thrombosis) | [17] |
| 3 | Tian et al. (2022) | Pancreatic | N/A | IV | N/A | 1/1 (CR) | VTE (portal vein thrombosis) | [18] |
| 4 | Massironi et al. (2021) | Pancreatic: 9/12 (75%) Small intestinal: 2/12 (16.7%) Unknown: 1/12 (8.3%) | G1: 3/12 (25%) G2: 6/12 (50%) G3: 2/12 (16.7%) N/A: 1/12 | I: 2/12 (16.7%) II: 3/12 (25%) III: 2/12 (16.7%) IV: 5/12 (41.7%) | NF: 10/12 (83.33%), F: 2/12 (16.67%) | 12/160 (7.5%) | VTE (deep venous thrombosis: 9/12 (75%), pulmonary embolism: 3/12 (25%)) | [19] |
| 5 | Lee et al. (2021) | Pancreatic: 29/36 (80%) | N/A | 7/36 metastatic (19.4%) | NF: 22/36 (75.9%) F: Insulinoma 7/36 (24%) Gastrinoma and/or Zollinger–Ellison 16/36 (44.4%) Glucagonoma (None) | 36/286 MEN-1 pts (12.9%) | VTE (deep venous thrombosis, pulmonary embolism) | [20] |
| 6 | Amoui et al. (2021) | Pancreatic | NEC | IV | N/A | 1/1 (CR) | VTE (portal vein tumor thrombus) | [21] |
| 7 | Liaqat et al. (2021) | Pulmonary | NEC | N/A | N/A | 1/1 (CR) | VTE (right internal jugular vein (IJV) thrombosis and right subclavian vein thrombosis) | [22] |
| 8 | Zhu et al. (2020) | Pancreatic | N/A | N/A | N/A | 1 (CR) | VTE (portal vein tumor thrombus) | [23] |
| 9 | Hollander et al. (2019) | Small intestinal | N/A | Metastatic disease | F: Carcinoid syndrome | 1/1 (CR) | Bioprosthetic valve thrombosis | [24] |
| 10 | Yang et al. (2019) | Bronchial | Typical carcinoid | IV | F: Ectopic Cushing’s syndrome (ectopic ACTH syndrome) | 1/1 (CR) | VTE (pulmonary embolism) | [25] |
| 11 | De Robertis et al. (2018) | Pancreatic | G2: 4/6 (66.7%) G3: 2/6 (33.3%) | N/A | N/A | 6/6 (CR) | VTE (tumor thrombus) | [26] |
| 12 | Yu (2018) | Duodenal | G2 (Ki-67 5–10%) | Metastatic | N/A | 1/1 (CR) | VTE (right atrial tumor thrombus) | [27] |
| 13 | Moyana et al. (2017) | Pancreatic | G2: 7/8 (87.5%) G3: 1/8 (12.5%) | II: 1/8 (12.5%) III: 2/8 (25%) IV: 5/8 (62.5%) | NF: 8/8 (100%) | 8/61 (13.11%) | VTE (splenic vein thrombosis and gastric varices in CT imaging) | [28] |
| 14 | Rodriguez et al. (2014) | Pancreatic | G2 | II | NF | 1/1 (CR) | VTE (portal vein tumor thrombus) | [29] |
| 15 | Prakash et al. (2015) | Pancreatic | G2: 4/9 (44.4%) G3: 1/9 (11.1%) N/A: 4/9 | II: 7/9 (77.8%) IV: 2/9 (22.2%) | NF: 9/9 (100%) | 26/245 (11%) 9 pts (3.8%) underwent portal venous tumor thrombectomy | VTE (portal vein tumor thrombus) | [30] |
| 16 | Hurtado-Cordovi et al. (2013) | Rectal | N/A | IV | N/A | 1/1 (CR) | VTE (Budd–Chiari syndrome) | [15] |
| 17 | Busch et al. (2013) | Pulmonary | Small cell NEC | IV | F: SIADH | 1/1 (CR) | VTE (pulmonary embolism) | [31] |
| 18 | Balachandran et al. (2012) | Pancreatic | N/A | N/A | NF: 29/29 (100%) | 29/88 (33%) | VTE (tumor thrombus) | [32] |
| 19 | Teh RW et al. (2012) | Pancreatic (probably) | NEC | N/A | NF | 1/1 (CR) | DIC | [33] |
| 20 | Naswa et al. (2012) | Pancreatic | G2 (Ki-67 4%) | N/A | NF | 1/1 (CR) | VTE (tumor thrombus) | [34] |
| 21 | Lim et al. (2011) | Pancreatic | N/A | IV | N/A | 1/1 (CR) | VTE (portal vein tumor thrombus) | [35] |
| 22 | Tsuchikawa et al. (2011) | Pancreatic | N/A | N/A | N/A | 1/1 (CR) | VTE (portal vein tumor thrombus) | [36] |
| 23 | Barbier et al. (2010) | Pancreatic | G2 (Ki-67 3%) | IV | NF | 1/1 (CR) | VTE (portal vein tumor thrombus) | [37] |
| 24 | Kawakami et al. (2006) | Pancreatic | NEC | N/A | NF | 1/1 (CR) | VTE (portal vein tumor thrombus) | [38] |
| 25 | Nguyen (2005) | Pancreatic | N/A | N/A | N/A | 1/1 (CR) | VTE (portal vein tumor thrombus) | [39] |
| 26 | Bedirli et al. (2004) | Pancreatic | Islet cell carcinoma | N/A | NF | 1/1 (CR) | VTE (portal vein tumor thrombus) | [40] |
| 27 | Di Micco et al. (2002) | Pancreatic | N/A | IV | N/A | 1/1 (CR) | VTE | [41] |
| 28 | Watase et al. (1992) | Pancreatic | Islet cell carcinoma | N/A | NF | 1/1 (CR) | VTE (tumor thrombus) | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Giertuga, M.; Malczewska-Herman, A.; Kos-Kudła, B. The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms. Cancers 2023, 15, 5477. https://doi.org/10.3390/cancers15225477
Wójcik-Giertuga M, Malczewska-Herman A, Kos-Kudła B. The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms. Cancers. 2023; 15(22):5477. https://doi.org/10.3390/cancers15225477
Chicago/Turabian StyleWójcik-Giertuga, Monika, Anna Malczewska-Herman, and Beata Kos-Kudła. 2023. "The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms" Cancers 15, no. 22: 5477. https://doi.org/10.3390/cancers15225477
APA StyleWójcik-Giertuga, M., Malczewska-Herman, A., & Kos-Kudła, B. (2023). The Risk of Venous Thromboembolism in Neuroendocrine Neoplasms. Cancers, 15(22), 5477. https://doi.org/10.3390/cancers15225477





