Granzyme B Expression in the Tumor Microenvironment as a Prognostic Biomarker for Patients with Triple-Negative Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
2.2. Pathological Assessment
2.3. Immunohistochemistry
2.4. Statistics
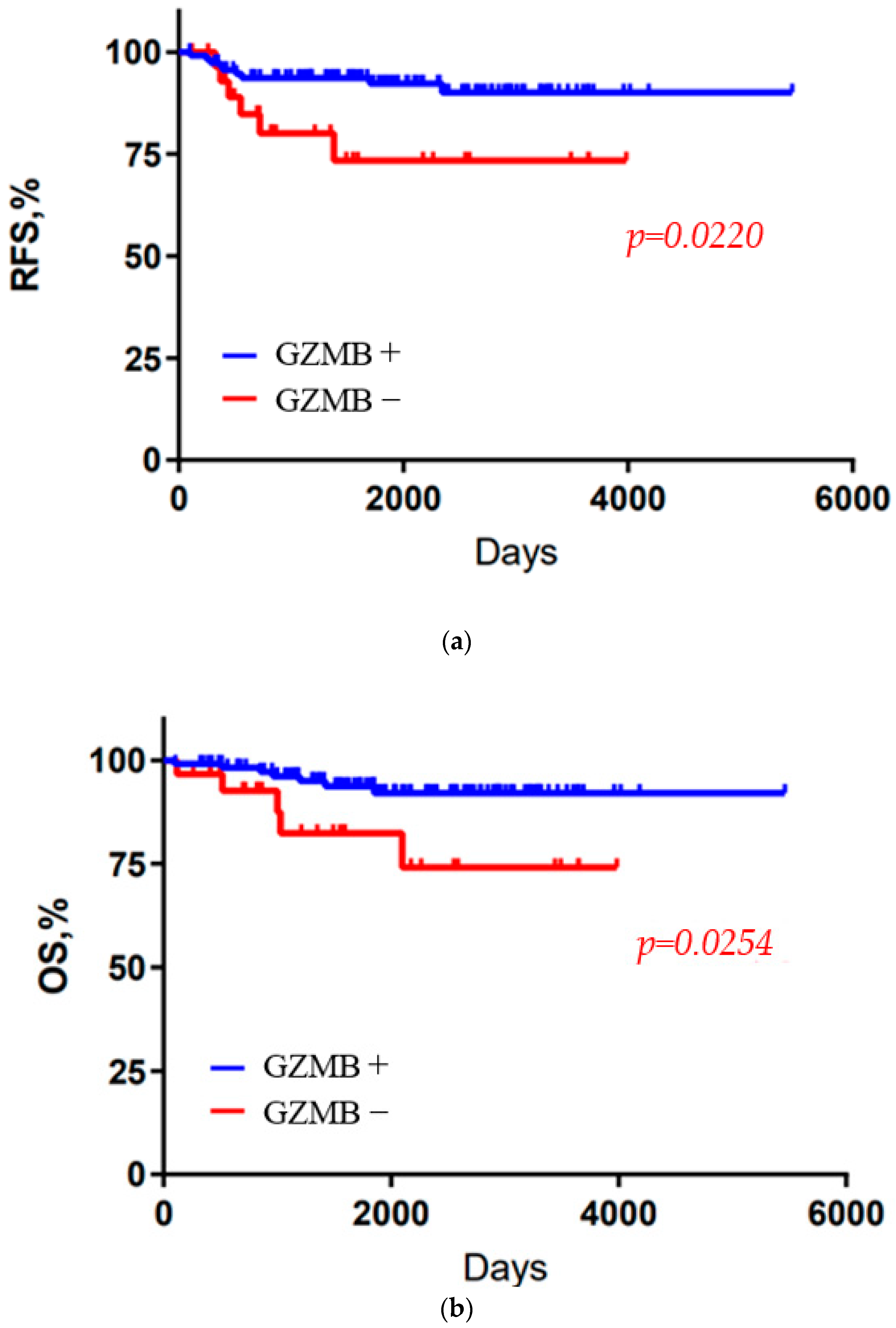
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-Negative Breast Cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2020, 56, 15. [Google Scholar] [CrossRef]
- Loi, S.; Sirtaine, N.; Piette, F.; Salgado, R.; Viale, G.; Van Eenoo, F.; Rouas, G.; Francis, P.; Crown, J.; Hitre, E.; et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 2013, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Kubo, M.; Yamaguchi, R.; Nishimura, R.; Osako, T.; Arima, N.; Okumura, Y.; Okido, M.; Yamada, M.; Kai, M.; et al. The combination of PD-L1 expression and decreased tumor infiltrating lymphocytes is associated with a poor prognosis in triple-negative breast cancer. Oncotarget 2017, 8, 15584–15592. [Google Scholar] [CrossRef]
- Kontani, K.; Sawai, S.; Hanaoka, J.; Tezuka, N.; Inoue, S.S.; Fujino, K. Involvement of granzyme B and perforin in suppressing nodal metastasis of cancer cells in breast and lung cancers. Eur. J. Surg. Oncol. 2001, 27, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hong, Z.; Lei, G.; Guo, A.L.; Wang, F.S.; Jiao, Y.M.; Fu, J. Decreased granzyme-B expression in CD11c+CD8+ T cells associated with disease progression in patients with HBV-related hepatocellular carcinoma. Front. Immunol. 2023, 14, 1107483. [Google Scholar] [CrossRef] [PubMed]
- Noti, L.; Galván, J.A.; Dawson, H.; Lugli, A.; Kirsch, R.; Assarzadegan, N.; Messenger, D.; Krebs, P.; Berger, M.D.; Zlobec, I. A combined spatial score of granzyme B and CD68 surpasses CD8 as an independent prognostic factor in TNM stage II colorectal cancer. BMC Cancer 2022, 22, 987. [Google Scholar] [CrossRef]
- Chung, J.H.; Ha, J.S.; Choi, J.; Kwon, S.M.; Yun, S.M.; Kim, T.; Jeon, D.; Yoon, S.H.; Kim, Y.S. Granzyme B for predicting the durable clinical benefit of anti-PD-1/PD-L1 immunotherapy in patients with non-small cell lung cancer. Transl. Cancer Res. 2022, 11, 316–326. [Google Scholar] [CrossRef]
- Hodge, G.; Barnawi, J.; Jurisevic, C.; Moffat, D.; Holmes, M.; Reynolds, P.N.; Jersmann, H.; Hodge, S. Lung cancer is associated with decreased expression of perforin, granzyme B and interferon (IFN)-γ by infiltrating lung tissue T cells, natural killer (NK) T-like and NK cells. Clin. Exp. Immunol. 2014, 178, 79–85. [Google Scholar] [CrossRef]
- Larimer, B.M.; Wehrenberg-Klee, E.; Dubois, F.; Mehta, A.; Kalomeris, T.; Flaherty, K.; Boland, G.; Mahmood, U. Granzyme B PET Imaging as a Predictive Biomarker of Immunotherapy Response. Cancer Res. 2017, 77, 2318–2327. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Glick, J.H.; Gelber, R.D.; Coates, A.S.; Thurlimann, B.; Senn, H.J.; Panel Members. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann. Oncol. 2005, 16, 1569–1583. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Gelber, R.D.; Coates, A.S.; Thurlimann, B.; Senn, H.J.; Panel Members. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann. Oncol. 2007, 18, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Ingle, J.N.; Gelber, R.D.; Coates, A.S.; Thurlimann, B.; Senn, H.J.; Panel Members. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann. Oncol. 2009, 20, 1319–1329. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel Members. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Eynden, G.V.; Baehner, F.L.; Llorca, F.P.; et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an international TILs working group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Swisher, S.K.; Wu, Y.; Castaneda, C.A.; Lyons, G.R.; Yang, F.; Tapia, C.; Wang, X.; Casavilca, S.A.A.; Bassett, R.; Castillo, M.; et al. Interobserver agreement between pathologists assessing tumor-infiltrating lymphocytes (TILs) in breast cancer using methodology proposed by the international TILs working group. Ann. Surg. Oncol. 2016, 23, 2242–2248. [Google Scholar] [CrossRef]
- Kurata, K.; Kubo, M.; Kai, M.; Mori, H.; Kawaji, H.; Kaneshiro, K.; Yamada, M.; Nishimura, R.; Osako, T.; Arima, N.; et al. Microsatellite instability in Japanese female patients with triple-negative breast cancer. Breast Cancer 2020, 27, 490–498. [Google Scholar] [CrossRef]
- Mori, H.; Kubo, M.; Kai, M.; Yamada, M.; Kurata, K.; Kawaji, H.; Kazuhisa Kaneshiro, K.; Osako, T.; Nishimura, R.; Arima, N.; et al. T-bet+ lymphocytes infltration as an independent better prognostic indicator for triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 176, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.M.; Pinnaduwage, D.; Tchatchou, S.; Bull, S.B.; Andrulis, I.L. Validation of Intratumoral T-bet+ Lymphoid Cells as Predictors of Disease-Free Survival in Breast Cancer. Cancer Immunol. Res. 2016, 4, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Larimer, B.M.; Bloch, E.; Nesti, S.; Austin, E.E.; Wehrenberg-Klee, E.; Boland, G.; Mahmood, U. The Effectiveness of Checkpoint Inhibitor Combinations and Administration Timing Can Be Measured by Granzyme B PET Imaging. Clin. Cancer Res. 2019, 25, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Bardine, C.; Lourenço, A.L.; Wang, Y.-h.; Huang, Y.; Cleary, S.J.; Wilson, D.M.; Oh, D.Y.; Fong, L.; Looney, M.R.; et al. In Vivo Measurement of Granzyme Proteolysis from Activated Immune Cells with PET. ACS Cent. Sci. 2021, 7, 1638–1649. [Google Scholar] [CrossRef]
- Force, J.; Leal, J.H.S.; McArthur, H.L. Checkpoint Blockade Strategies in the Treatment of Breast Cancer: Where We Are and Where We Are Heading. Curr. Treat. Options Oncol. 2019, 20, 35. [Google Scholar] [CrossRef] [PubMed]
- Napier, T.S.; Hunter, C.L.; Song, P.N.; Larimer, B.M.; Sorace, A.G. Preclinical PET Imaging of Granzyme B Shows Promotion of Immunological Response Following Combination Paclitaxel and Immune Checkpoint Inhibition in Triple Negative Breast Cancer. Pharmaceutics 2022, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Edechi, C.A.; Ikeogu, N.; Uzonna, J.E.; Myal, Y. Regulation of Immunity in Breast Cancer. Cancers 2019, 11, 1080. [Google Scholar] [CrossRef]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Dudley, J.C.; Lin, M.-T.; Le, D.T.; Eshleman, J.R. Microsatellite instability as a biomarker for PD-1 blockade. Clin. Cancer Res. 2016, 22, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, S.H.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]




| Number of Patients | ||
|---|---|---|
| n = 230 | ||
| Age at diagnosis (y), mean (range) | 60 | (30–89) |
| Tumor size | ||
| T1 | 131 | 57.0% |
| T2 | 90 | 39.1% |
| T3 | 8 | 3.5% |
| T4 | 1 | 4.3% |
| Nodal status | ||
| N0 | 152 | 66.1% |
| N1 | 61 | 26.5% |
| N2 | 10 | 4.3% |
| N3 | 7 | 3.0% |
| Pathological stage | ||
| Ⅰ | 152 | 66.1% |
| Ⅱ | 76 | 33.0% |
| Ⅲ | 2 | 8.7% |
| Nuclear grade | ||
| 1/2 | 65 | 28.3% |
| 3 | 159 | 69.1% |
| Unknown | 6 | 2.6% |
| Ki67 | ||
| ≤20 | 27 | 11.7% |
| >20 | 178 | 77.4% |
| Unknown | 25 | 10.9% |
| TIL | ||
| High | 117 | 50.9% |
| Low | 112 | 48.7% |
| CD8 + T cell | ||
| High | 117 | 50.9% |
| Low | 113 | 49.1% |
| PD-L1 (CPS10) | ||
| + | 126 | 54.8% |
| − | 104 | 45.2% |
| Granzyme B-High | Granzyme B-Low | ||||
|---|---|---|---|---|---|
| (n = 181) | (n = 49) | p-Value | |||
| Tumor size | |||||
| T1 | 99 | 54.7% | 32 | 65.3% | 0.5579 |
| T2 | 74 | 40.9% | 16 | 32.7% | |
| T3 | 7 | 3.9% | 1 | 2.0% | |
| T4 | 1 | 0.6% | 0 | 0.0% | |
| Nodal status | |||||
| N0 | 117 | 64.6% | 35 | 71.4% | 0.651 |
| N1 | 50 | 27.6% | 11 | 22.4% | |
| N2 | 9 | 5.0% | 1 | 2.0% | |
| N3 | 5 | 2.8% | 2 | 4.1% | |
| Pathological stage | |||||
| Ⅰ | 117 | 64.6% | 35 | 71.4% | 0.3956 |
| Ⅱ | 63 | 34.8% | 13 | 26.5% | |
| Ⅲ | 1 | 0.6% | 1 | 2.0% | |
| Nuclear grade | |||||
| 1/2 | 48 | 26.5% | 17 | 34.7% | 0.2242 |
| 3 | 129 | 71.3% | 30 | 61.2% | |
| Unknown | 4 | 2.2% | 2 | 4.1% | |
| Ki67 | |||||
| ≤20 | 21 | 11.6% | 6 | 12.2% | 0.6496 |
| >20 | 145 | 80.1% | 33 | 67.3% | |
| Unknown | 15 | 8.3% | 10 | 20.4% | |
| TIL | |||||
| High | 81 | 44.8% | 36 | 73.5% | 0.0006 |
| Low | 99 | 54.7% | 13 | 26.5% | |
| CD8 + T cell | |||||
| High | 102 | 56.4% | 15 | 30.6% | 0.002 |
| Low | 79 | 43.6% | 34 | 69.4% | |
| PD-L1(CPS10) | |||||
| + | 104 | 57.5% | 22 | 44.9% | 0.01171 |
| − | 77 | 42.5% | 27 | 55.1% |
| OS | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| GZMB (high) | 0.28 (0.09–0.89) | 0.04 | 0.25 (0.07–0.88) | 0.03 |
| CD8 (positive) | 1.76 (0.47–6.49) | 0.38 | ||
| TIL (high) | 0.47 (0.15–1.46) | 0.19 | ||
| Tumor size (>2 cm) | 3.26 (0.98–10.84) | 0.04 | 3.70 (1.02–13.36) | 0.05 |
| Lymph node (positive) | 4.06 (1.22–13.51) | 0.02 | 3.19 (0.92–11.02) | 0.06 |
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| GZMB (high) | 0.27 (0.09–0.72) | 0.009 | 0.21 (0.07–0.69) | 0.009 |
| CD8 (positive) | 1.88 (0.60–5.83) | 0.25 | ||
| TIL (high) | 0.47 (0.15–1.46) | 0.19 | ||
| Tumor size (>2 cm) | 2.87 (1.04–7.90) | 0.04 | 3.08 (1.06–8.92) | 0.04 |
| Lymph node (positive) | 3.32 (1.20–9.17) | 0.02 | 2.53 (0.86–7.42) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mizoguchi, K.; Kawaji, H.; Kai, M.; Morisaki, T.; Hayashi, S.; Takao, Y.; Yamada, M.; Shimazaki, A.; Osako, T.; Arima, N.; et al. Granzyme B Expression in the Tumor Microenvironment as a Prognostic Biomarker for Patients with Triple-Negative Breast Cancer. Cancers 2023, 15, 4456. https://doi.org/10.3390/cancers15184456
Mizoguchi K, Kawaji H, Kai M, Morisaki T, Hayashi S, Takao Y, Yamada M, Shimazaki A, Osako T, Arima N, et al. Granzyme B Expression in the Tumor Microenvironment as a Prognostic Biomarker for Patients with Triple-Negative Breast Cancer. Cancers. 2023; 15(18):4456. https://doi.org/10.3390/cancers15184456
Chicago/Turabian StyleMizoguchi, Kimihisa, Hitomi Kawaji, Masaya Kai, Takafumi Morisaki, Saori Hayashi, Yuka Takao, Mai Yamada, Akiko Shimazaki, Tomofumi Osako, Nobuyuki Arima, and et al. 2023. "Granzyme B Expression in the Tumor Microenvironment as a Prognostic Biomarker for Patients with Triple-Negative Breast Cancer" Cancers 15, no. 18: 4456. https://doi.org/10.3390/cancers15184456
APA StyleMizoguchi, K., Kawaji, H., Kai, M., Morisaki, T., Hayashi, S., Takao, Y., Yamada, M., Shimazaki, A., Osako, T., Arima, N., Okido, M., Oda, Y., Nakamura, M., & Kubo, M. (2023). Granzyme B Expression in the Tumor Microenvironment as a Prognostic Biomarker for Patients with Triple-Negative Breast Cancer. Cancers, 15(18), 4456. https://doi.org/10.3390/cancers15184456






