Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. CRS/ICANS Grading and Endpoints
2.3. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
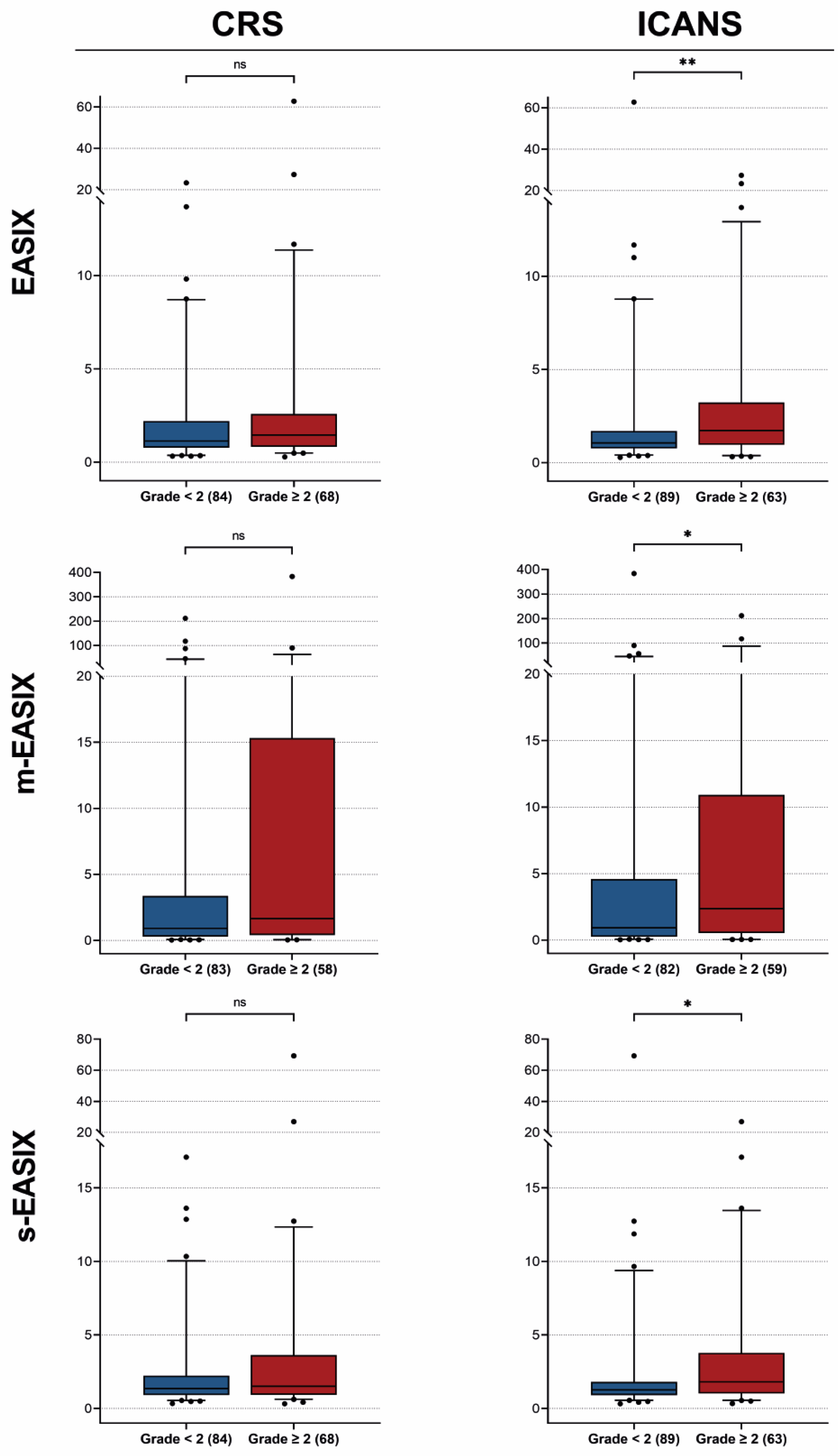
3.2. EASIX/m-EASIX/s-EASIX Distributions
| Author | Year | Patients | Timepoint | Outcome | Used Variable | Variable Form | Statistical Method | Association Descriptives | Performer Descriptives | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|
| Greenbaum et al. [8] | 2021 | r/r LBCL patients treated with axicel (n = 171). | Pre-LD | CRS ≥ grade 2 | EASIX-F | EASIX: >4.6 ferritin: >321 ng/mL | Training model: Fine and Gray regression analyses Validation: bootstrapping 3000 resampled data | EASIX: HR 2.4, p < 0.001 for >UQ | EASIX-F identified 3 risk groups with cumulative incidence of 74% (p < 0.001), 51% (p = 0.04) and 23% (reference) | EASIX combined with ferritin could discriminate three different risk groups for CRS grade 2–4. |
| ICANS grade ≥ 2 | EASIX-FC | EASIX: >2.1 ferritin: >1583 ng/mL CRP: >21 mg/L | EASIX: HR 2.2, p < 0.001 for >median | EASIX-FC identified 3 risk groups with cumulative incidence of 74% (p < 0.001), 51% (p = 0.025) and 29% (reference) | EASIX combined with CRP and ferritin could significantly discriminate three different risk groups for ICANS grade 2–4. | |||||
| Pennisi et al. [16] | 2021 | B-ALL treated with 1928z CAR T cells and r/r LBCL patients treated with axicel and tisacel (n = 118). | Pre-LD D − 1 D + 1 D + 3 | CRS ≥ grade 3 | EASIX (log2) | Continuous | Logistic regression AUC | Pre-LD: OR 1.34, s D − 1: OR 1.51, s D + 1: OR 1.56, s D + 3: OR 1.89, s | Pre-LD: AUC 0.77 D − 1: AUC 0.72 D + 1: AUC 0.72 D + 3: AUC 0.80 | EASIX, m-EASIX and s-EASIX were significantly associated with the occurrence of severe CRS on multiple time points. All three formulas were able to predict severe CRS well. |
| m-EASIX (log2) | Pre-LD: OR 1.32, s D − 1: OR 1.26, s D + 1: OR 1.31, s D +3: 1.56, s | Pre-LD: AUC 0.80 D − 1: AUC 0.73 D + 1: AUC 075 D + 3: AUC 0.73 | ||||||||
| s-EASIX (log2) | Pre-LD: OR 1.49, s D − 1: OR 1.6, s D + 1: OR 1.65, s D + 3: OR 1.92, s | Pre-LD: AUC 0.82 D − 1: AUC 0.75 D + 1: AUC 0.76 D + 3: AUC 0.81 | ||||||||
| ICANS ≥ grade 3 | EASIX (log2) | Continuous | Logistic regressionAUC | Pre-LD: OR 1.11, ns D − 1: OR 1.2, ns D + 1: OR 1.36, s D + 3 OR 1.5, s | D + 1: AUC 0.61 D + 3: AUC 0.68 | EASIX, m-EASIX and s-EASIX on day +1 and +3 were significantly associated with the occurrence of severe ICANS. The predictive power of these three formulas on day +1 and +3 was moderate. | ||||
| m-EASIX (log2) | Pre-LD: OR 1.1, ns D − 1: OR 1.12, ns D + 1: OR 1.2, s D + 3: OR 1.36, s | D + 1: AUC 0.67 D + 3: AUC 0.73 | ||||||||
| s-EASIX (log2) | Pre-LD: OR 1.25, ns D − 1: OR 1.33, ns D + 1: OR 1.46, s D + 3: OR 1.55, s | D + 1: AUC 0.66 D + 3: AUC 0.68 | ||||||||
| Korell et al. [17] | 2022 | Training cohort: r/r LBCL patients treated with axicel (n = 93). Validation cohort: r/r LBCL/MCL/ ALL/FL/CLL patients treated with axi-cel/tisa-cel or HD-CAR-1 (n = 121). | Pre-LD | CRS/ICANS ≥ grade 3 | EASIX (log2) | Continuous Cut-off point 4.67 | Multivariate logistic regression Validation cohort: AUC, Brier scores | Continuous: OR 1.72 p = 0.001 † Cut-off > 4.67: OR 4.32, p = 0.006 † | Continuous: AUC 0.81 | EASIX, s-EASIX and m-EASIX pre-LD were significantly associated with CRS or ICANS grade ≥ 3. All three formulas could predict the occurrence of toxicity and out-performed the reference model in multivariate analysis. |
| m-EASIX (log2) | Continuous | OR 1.22 p = 0.015 † | AUC 0.74 | |||||||
| s-EASIX (log2) | OR 1.63, p = 0.004 † | AUC 0.79 | ||||||||
| Acosta-Medina et al. [18] | 2023 | r/r LBCL patients treated with axicel (n = 84). | Pre-LD D0 | ICANS ≥ grade 3 | EASIX | Continuous | Univariable logistic regressionAUC | Continuous: Pre-LD: OR 1.14, p = 0.047 D0: OR 1.19, p = 0.008 | Continuous: Pre-LD: 0.57 D0: 0.62 | EASIX and m-EASIX were associated with increased risk of ICANS G3–4 at lymphodepletion, but were further optimized when calculated from laboratory values at infusion. Only m-EASIX at infusion was able to categorically predict high-risk patients. |
| m-EASIX | Continuous, Cut-off point 4 | Continuous: Pre-LD: OR 1.007, p = 0.205 D0: OR 1.007, p = 0.086 Cut-off ≥ 4: D0: OR 4.086, p = 0.034 | Continuous: D0: 0.72 |
| Total (n = 154) | |
|---|---|
| Age, median (range) | 60 (18–84) |
| Gender, male, n % | 101 (65.6) |
| Diagnosis, n % | |
| DLBCL | 79 (51.3) |
| tFL | 50 (32.5) |
| HGBCL DH/TH | 14 (9.1) |
| HGBCL NOS | 6 (3.9) |
| PMBCL | 5 (3.2) |
| ECOG, n % | |
| 0–1 | 138 (89.6) |
| 2–4 | 11 (7.1) |
| Missing, n % | 5 (3.3) |
| Disease stage a, n % | |
| Stage I–II | 34 (22.1) |
| Stage III–IV | 120 (77.9) |
| Bulky disease a, n % | 51 (33.1) |
| Missing, n % | 3 (2.0) |
| Nr. of extranodal sites a, n % | |
| 0 | 52 (33.8) |
| 1 | 55 (35.7) |
| ≥2 | 45 (29.2) |
| Missing, n % | 2 (1.3) |
| LDH at screening, median (IQR) | 269 (215–446) |
| Missing, n % | 15 (9.7) |
| LDH at lymphodepletion, median (IQR) | 238 (195–329) |
| Missing, n% | 2 (1.3) |
| IPI a, n % | |
| Low | 32 (20.8) |
| Low-intermediate | 45 (29.2) |
| Intermediate-high | 43 (27.9) |
| High | 13 (8.4) |
| Missing, n % | 21 (14) |
| Patients refractory to first-line treatment b, n % | 94 (61.0) |
| Patients refractory to second-line treatment b, n % | 114 (74.0) |
| Missing, n % | 12 (7.8) |
| Previous lines of therapy, median (range) | 2 (2–10) |
| Previous stem cell transplant, n % | 45 (29.2) |
| Allogenic | 3 (1.9) |
| Autologous | 45 (29.2) |
| Bridging therapy, n % | |
| No bridging | 32 (20.8) |
| Radiotherapy | 37 (24.0) |
| Systemic therapy | 34 (22.1) |
| Steroids | 19 (12.3) |
| Combination | 32 (20.8) |
| CRS grade, n % | |
| No CRS | 14 (9.1) |
| 1 | 71 (46.1) |
| 2 | 61 (39.6) |
| 3 | 7 (4.5) |
| 4 | 1 (0.6) |
| ICANS grade, n % | |
| No ICANS | 61 (39.6) |
| 1 | 30 (19.5) |
| 2 | 31 (20.1) |
| 3 | 28 (18.2) |
| 4 | 4 (2.6) |
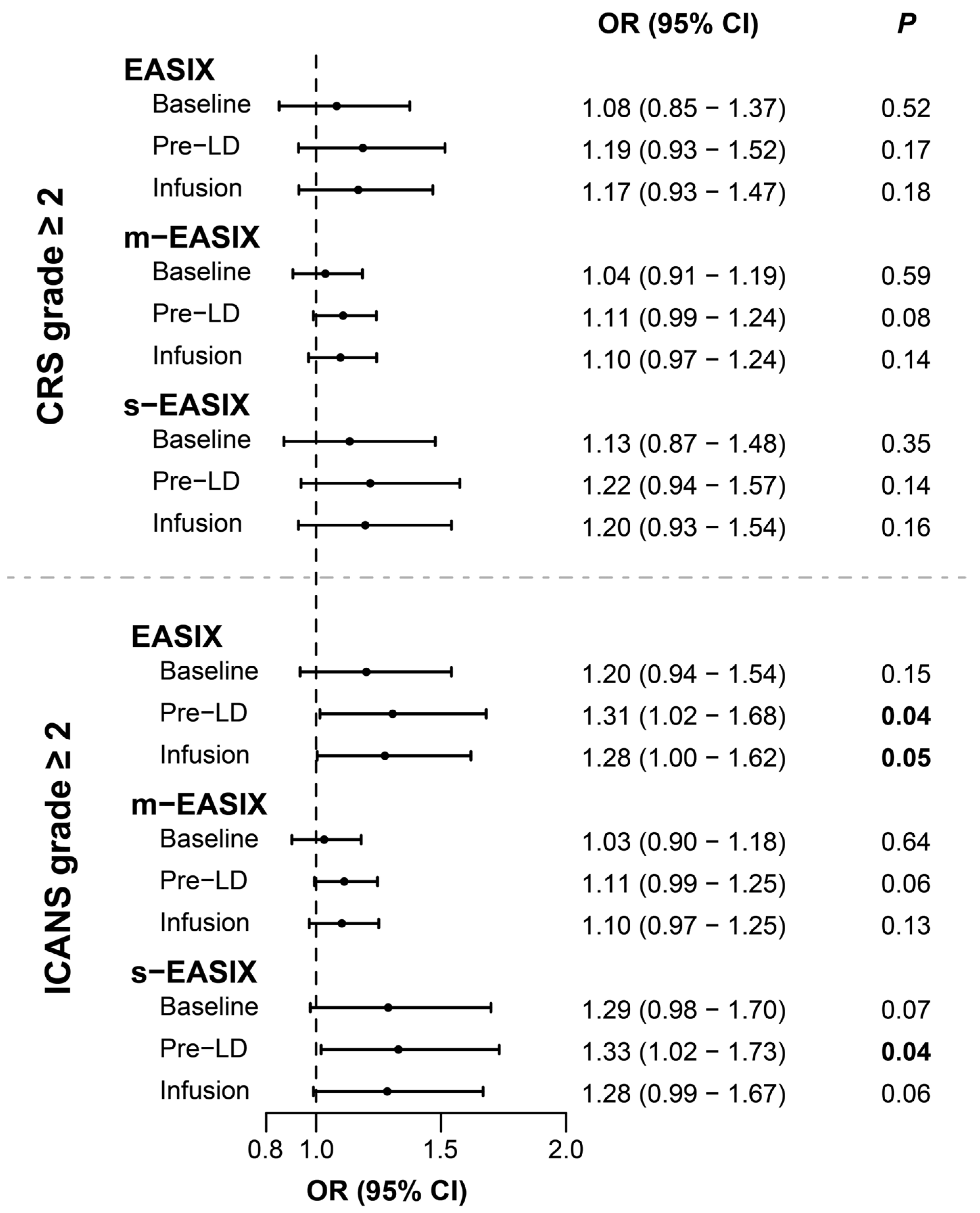
3.3. Univariable Associations with CRS and ICANS Development
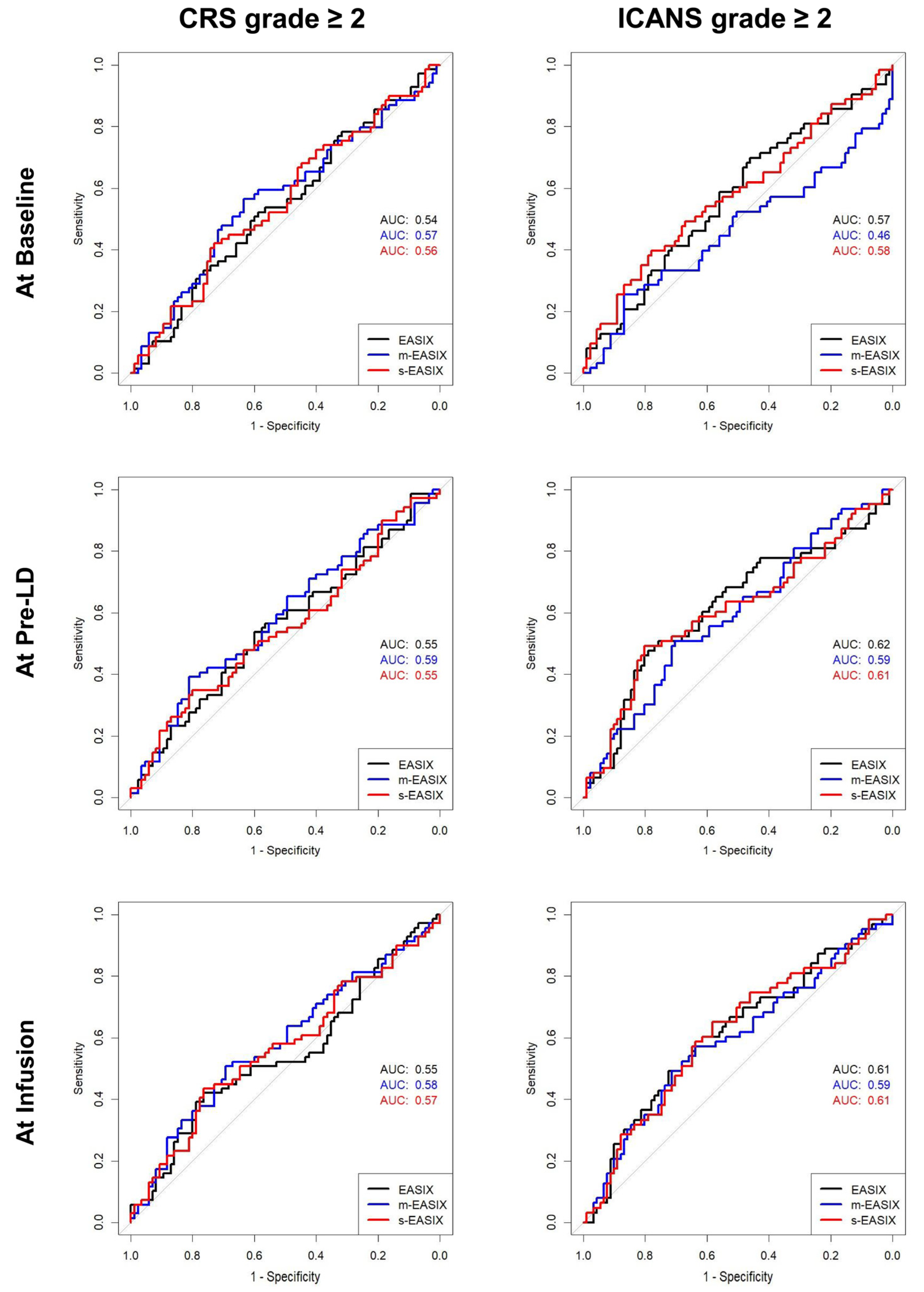
3.4. ROC Curve Analysis
3.5. EASIX Risk-Stratification
3.6. EASIX Cutoff
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.-A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023, 389, 147–157. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Tummala, S.; Kebriaei, P.; Wierda, W.; Gutierrez, C.; Locke, F.L.; Komanduri, K.V.; Lin, Y.; Jain, N.; Daver, N.; et al. Chimeric Antigen Receptor T-Cell Therapy—Assessment and Management of Toxicities. Nat. Rev. Clin. Oncol. 2018, 15, 47–62. [Google Scholar] [CrossRef]
- Neelapu, S.S. Managing the Toxicities of CAR T-Cell Therapy. Hematol. Oncol. 2019, 37 (Suppl. S1), 48–52. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.L.; Schmitt, M.; Wang, L.; Ramos, C.A.; Jordan, K.; Müller-Tidow, C.; Dreger, P. Side-Effect Management of Chimeric Antigen Receptor (CAR) T-Cell Therapy. Ann. Oncol. 2021, 32, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.C.; Neelapu, S.S.; Giavridis, T.; Sadelain, M. Cytokine Release Syndrome and Associated Neurotoxicity in Cancer Immunotherapy. Nat. Rev. Immunol. 2022, 22, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Topp, M.; Van Meerten, T.; Houot, R.; Minnema, M.C.; Milpied, N.; Lugtenburg, P.J.; Thieblemont, C.; Wermke, M.; Song, K.; Avivi, I.; et al. Earlier Steroid Use with Axicabtagene Ciloleucel (Axi-Cel) in Patients with Relapsed/Refractory Large B Cell Lymphoma. Blood 2019, 134, 243. [Google Scholar] [CrossRef]
- Oluwole, O.O.; Bouabdallah, K.; Muñoz, J.; De Guibert, S.; Vose, J.M.; Bartlett, N.L.; Lin, Y.; Deol, A.; McSweeney, P.A.; Goy, A.H.; et al. Prophylactic Corticosteroid Use in Patients Receiving Axicabtagene Ciloleucel for Large B-Cell Lymphoma. Br. J. Haematol. 2021, 194, 690–700. [Google Scholar] [CrossRef]
- Greenbaum, U.; Strati, P.; Saliba, R.M.; Torres, J.; Rondon, G.; Nieto, Y.; Hosing, C.; Srour, S.A.; Westin, J.; Fayad, L.E.; et al. CRP and Ferritin in Addition to the EASIX Score Predict CAR-T-Related Toxicity. Blood Adv. 2021, 5, 2799–2806. [Google Scholar] [CrossRef]
- Gust, J.; Hay, K.A.; Hanafi, L.A.; Li, D.; Myerson, D.; Gonzalez-Cuyar, L.F.; Yeung, C.; Liles, W.C.; Wurfel, M.; Lopez, J.A.; et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017, 7, 1404–1419. [Google Scholar] [CrossRef]
- Tedesco, V.E.; Mohan, C. Biomarkers for Predicting Cytokine Release Syndrome Following CD19-Targeted CAR T Cell Therapy. J. Immunol. 2021, 206, 1561–1568. [Google Scholar] [CrossRef]
- Kang, S.; Tanaka, T.; Inoue, H.; Ono, C.; Hashimoto, S.; Kioi, Y.; Matsumoto, H.; Matsuura, H.; Matsubara, T.; Shimizu, K.; et al. IL-6 Trans-Signaling Induces Plasminogen Activator Inhibitor-1 from Vascular Endothelial Cells in Cytokine Release Syndrome. Proc. Natl. Acad. Sci. USA 2020, 117, 22351–22356. [Google Scholar] [CrossRef] [PubMed]
- Teachey, D.T.; Lacey, S.F.; Shaw, P.A.; Melenhorst, J.J.; Maude, S.L.; Frey, N.; Pequignot, E.; Gonzalez, V.E.; Chen, F.; Finklestein, J.; et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-Cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016, 6, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Norelli, M.; Camisa, B.; Barbiera, G.; Falcone, L.; Purevdorj, A.; Genua, M.; Sanvito, F.; Ponzoni, M.; Doglioni, C.; Cristofori, P.; et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat. Med. 2018, 24, 739–748. [Google Scholar] [CrossRef]
- Mackall, C.L.; Miklos, D.B. CNS Endothelial Cell Activation Emerges as a Driver of CAR T Cell-Associated Neurotoxicity. Cancer Discov. 2017, 7, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Obstfeld, A.E.; Frey, N.V.; Mansfield, K.; Lacey, S.F.; June, C.H.; Porter, D.L.; Melenhorst, J.J.; Wasik, M.A. Cytokine Release Syndrome Associated with Chimeric-Antigen Receptor T-Cell Therapy: Clinicopathological Insights. Blood 2017, 130, 2569–2572. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, M.; Sanchez-Escamilla, M.; Flynn, J.R.; Shouval, R.; Tomas, A.A.; Silverberg, M.L.; Batlevi, C.; Brentjens, R.J.; Dahi, P.B.; Devlin, S.M.; et al. Modified EASIX Predicts Severe Cytokine Release Syndrome and Neurotoxicity after Chimeric Antigen Receptor T Cells. Blood Adv. 2021, 5, 3397–3406. [Google Scholar] [CrossRef] [PubMed]
- Korell, F.; Penack, O.; Mattie, M.; Schreck, N.; Benner, A.; Krzykalla, J.; Wang, Z.; Schmitt, M.; Bullinger, L.; Müller-Tidow, C.; et al. EASIX and Severe Endothelial Complications After CD19-Directed CAR-T Cell Therapy—A Cohort Study. Front. Immunol. 2022, 13, 877477. [Google Scholar] [CrossRef]
- Acosta-Medina, A.A.; Johnson, I.M.K.; Bansal, R.; Hathcock, M.; Kenderian, S.J.; Durani, U.; Khurana, A.; Wang, Y.; Paludo, J.; Villasboas, J.C.; et al. Pre-Lymphodepletion & Infusion Endothelial Activation and Stress Index as Predictors of Clinical Outcomes in CAR-T Therapy for B-Cell Lymphoma. Blood Cancer J. 2023, 13, 7. [Google Scholar] [CrossRef]
- Spanjaart, A.M.; Pennings, E.R.A.; Mutsaers, P.G.N.J.; van Dorp, S.; Jak, M.; van Doesum, J.A.; de Boer, J.W.; Niezink, A.G.H.; Kos, M.; Vermaat, J.S.P.; et al. The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands. Cancers 2023, 15, 4334. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Jiang, S.; Penack, O.; Terzer, T.; Schult, D.; Majer-Lauterbach, J.; Radujkovic, A.; Blau, I.W.; Bullinger, L.; Müller-Tidow, C.; Dreger, P.; et al. Predicting Sinusoidal Obstruction Syndrome after Allogeneic Stem Cell Transplantation with the EASIX Biomarker Panel. Haematologica 2021, 106, 446. [Google Scholar] [CrossRef]
- Luft, T.; Benner, A.; Terzer, T.; Jodele, S.; Dandoy, C.E.; Storb, R.; Kordelas, L.; Beelen, D.; Gooley, T.; Sandmaier, B.M.; et al. EASIX and Mortality after Allogeneic Stem Cell Transplantation. Bone Marrow Transplant. 2020, 55, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Luft, T.; Benner, A.; Jodele, S.; Dandoy, C.E.; Storb, R.; Gooley, T.; Sandmaier, B.M.; Becker, N.; Radujkovic, A.; Dreger, P.; et al. EASIX in Patients with Acute Graft-versus-Host Disease: A Retrospective Cohort Analysis. Lancet Haematol. 2017, 4, e414–e423. [Google Scholar] [CrossRef] [PubMed]
- Song, G.Y.; Jung, S.H.; Kim, K.; Kim, S.J.; Yoon, S.E.; Lee, H.S.; Kim, M.; Ahn, S.Y.; Ahn, J.S.; Yang, D.H.; et al. Endothelial Activation and Stress Index (EASIX) Is a Reliable Predictor for Overall Survival in Patients with Multiple Myeloma. BMC Cancer 2020, 20, 803. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Fein, J.A.; Shouval, A.; Danylesko, I.; Shem-Tov, N.; Zlotnik, M.; Yerushalmi, R.; Shimoni, A.; Nagler, A. External Validation and Comparison of Multiple Prognostic Scores in Allogeneic Hematopoietic Stem Cell Transplantation. Blood Adv. 2019, 3, 1881–1890. [Google Scholar] [CrossRef]
- Hay, K.A.; Hanafi, L.A.; Li, D.; Gust, J.; Liles, W.C.; Wurfel, M.M.; López, J.A.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome after CD19 Chimeric Antigen Receptor-Modified T-Cell Therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Dean, E.A.; Mhaskar, R.S.; Lu, H.; Mousa, M.S.; Krivenko, G.S.; Lazaryan, A.; Bachmeier, C.A.; Chavez, J.C.; Nishihori, T.; Davila, M.L.; et al. High Metabolic Tumor Volume Is Associated with Decreased Efficacy of Axicabtagene Ciloleucel in Large B-Cell Lymphoma. Blood Adv. 2020, 4, 3268–3276. [Google Scholar] [CrossRef]
- Hong, R.; Tan Su Yin, E.; Wang, L.; Zhao, X.; Zhou, L.; Wang, G.; Zhang, M.; Zhao, H.; Wei, G.; Wang, Y.; et al. Tumor Burden Measured by 18F-FDG PET/CT in Predicting Efficacy and Adverse Effects of Chimeric Antigen Receptor T-Cell Therapy in Non-Hodgkin Lymphoma. Front. Oncol. 2021, 11, 713577. [Google Scholar] [CrossRef]
- Cohen, D.; Luttwak, E.; Beyar-Katz, O.; Hazut Krauthammer, S.; Bar-On, Y.; Amit, O.; Gold, R.; Perry, C.; Avivi, I.; Ram, R.; et al. [18F]FDG PET-CT in Patients with DLBCL Treated with CAR-T Cell Therapy: A Practical Approach of Reporting Pre- and Post-Treatment Studies. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 953–962. [Google Scholar] [CrossRef]
- Derlin, T.; Schultze-Florey, C.; Werner, R.A.; Möhn, N.; Skripuletz, T.; David, S.; Beutel, G.; Eder, M.; Ross, T.L.; Bengel, F.M.; et al. 18F-FDG PET/CT of off-Target Lymphoid Organs in CD19-Targeting Chimeric Antigen Receptor T-Cell Therapy for Relapsed or Refractory Diffuse Large B-Cell Lymphoma. Ann. Nucl. Med. 2021, 35, 132–138. [Google Scholar] [CrossRef]
| CRS ≥ Grade 2 | ICANS ≥ Grade 2 | CRS ≥ Grade 3 | ICANS ≥ Grade 3 | CRS/ICANS ≥ Grade 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Published ‡ | Our Cohort | Published ‡ | Our Cohort | Published | Our Cohort | Published | Our Cohort | Published + | Our Cohort | |
| EASIX † | NR | 0.17 | NR | 0.04 | s | 0.81 | ns/0.05 | 0.45 | 0 | 0.71 |
| m-EASIX † | NR | 0.08 | NR | 0.06 | s | 0.75 | ns/0.21 | 0.59 | 0.02 | 0.99 |
| s-EASIX † | NR | 0.14 | NR | 0.04 | s | 0.77 | ns | 0.58 | 0 | 0.87 |
| Parameters | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Risk Group | EASIX | Ferritin | n * | Events, n * | CumInc, % * | HR | 95% CI | p | |
| CRS grade ≥ 2 | High risk | High | Any level | 17 | 8 | 47 | 0.96 | 0.43–2.12 | 0.92 |
| Intermediate risk | Low | High | 41 | 13 | 32 | 0.87 | 0.47–1.60 | 0.64 | |
| Low risk | Low | Low | 21 | 9 | 43 | 1.00 | - | - | |
| Ferritin | EASIX/CRP | ||||||||
| ICANS grade ≥ 2 | High risk | High | Any Level | 14 | 8 | 57 | 1.64 | 0.82–3.26 | 0.16 |
| Intermediate risk | Low | High EASIX | 14 | 12 | 86 | 2.04 | 1.26–3.32 | <0.01 | |
| High CRP | 14 | 5 | 36 | ||||||
| Low risk | Low | Low EASIX and low CRP | 29 | 11 | 38 | 1.00 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Boer, J.W.; Keijzer, K.; Pennings, E.R.A.; van Doesum, J.A.; Spanjaart, A.M.; Jak, M.; Mutsaers, P.G.N.J.; van Dorp, S.; Vermaat, J.S.P.; van der Poel, M.W.M.; et al. Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities. Cancers 2023, 15, 5443. https://doi.org/10.3390/cancers15225443
de Boer JW, Keijzer K, Pennings ERA, van Doesum JA, Spanjaart AM, Jak M, Mutsaers PGNJ, van Dorp S, Vermaat JSP, van der Poel MWM, et al. Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities. Cancers. 2023; 15(22):5443. https://doi.org/10.3390/cancers15225443
Chicago/Turabian Stylede Boer, Janneke W., Kylie Keijzer, Elise R. A. Pennings, Jaap A. van Doesum, Anne M. Spanjaart, Margot Jak, Pim G. N. J. Mutsaers, Suzanne van Dorp, Joost S. P. Vermaat, Marjolein W. M. van der Poel, and et al. 2023. "Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities" Cancers 15, no. 22: 5443. https://doi.org/10.3390/cancers15225443
APA Stylede Boer, J. W., Keijzer, K., Pennings, E. R. A., van Doesum, J. A., Spanjaart, A. M., Jak, M., Mutsaers, P. G. N. J., van Dorp, S., Vermaat, J. S. P., van der Poel, M. W. M., van Dijk, L. V., Kersten, M. J., Niezink, A. G. H., & van Meerten, T., on behalf of the Dutch CAR-T Tumorboard Consortium. (2023). Population-Based External Validation of the EASIX Scores to Predict CAR T-Cell-Related Toxicities. Cancers, 15(22), 5443. https://doi.org/10.3390/cancers15225443






