Simple Summary
Endometrial cancer is the second most common tumor of the female reproductive organs in the world. Taking into account the immunological mechanisms of defense against cancer, an important role is attributed to cytokines. In this case-control study, we aimed to identify the possible associations between selected single nucleotide polymorphisms (SNPs), localized in the CCL2, CCL5, CXCL8, and CXCR2 genes, and the onset and progression of endometrial cancer. We found that CCL5 and CXCR2 polymorphisms were associated with increased cancer risk, while the relationships remained significant after adjustments for age, diabetes, hypertension, or endometrial thickening. The selected haplotypes for CCL5 and CCL2 SNPs also correlated with an increased risk of cancer. We concluded that the four polymorphisms studied were significantly associated with an increased risk of endometrial cancer. The obtained results may be useful for identifying the signaling pathways involved in observed genetic changes, which is important for the tumorigenesis of endometrial cancer.
Abstract
Significant relationships with endometrial cancer were demonstrated, both for CCL2, CCL5, and CXCL8 chemokines and for the chemokine receptor CXCR2. The reported case-control study of genetic associations was designed to establish the role of selected single nucleotide polymorphisms (SNPs) of the CCL2, CCL5, CXCL8, and CXCR2 genes in the onset and progression of endometrial cancer. This study was conducted on 282 women, including 132 (46.8%) patients with endometrial cancer and 150 (53.2%) non-cancerous controls. The genotypes for CCL2 rs4586, CCL5 rs2107538 and rs2280789, CXCL8 rs2227532 and −738 T>A, and CXCR2 rs1126580 were determined, using PCR-RFLP assays. The AA homozygotes in CCL5 rs2107538 were associated with more than a quadruple risk of endometrial cancer (p ≤ 0.050). The GA heterozygotes in the CXCR2 SNP were associated with approximately threefold higher cancer risk (p ≤ 0.001). That association also remained significant after certain adjustments, carried out for age, diabetes mellitus, arterial hypertension, or endometrial thickness above 5 mm (p ≤ 0.050). The A-A haplotypes for the CCL5 polymorphisms and T-A-A haplotypes for the CCL2 and CCL5 SNPs were associated with about a twofold risk of endometrial cancer (p ≤ 0.050). In conclusion, CCL2 rs4586, CCL5 rs2107538 and rs2280789, and CXCR2 rs1126580 demonstrated significant associations with an increased risk of endometrial cancer.
1. Introduction
Endometrial cancer is a common tumor of the female reproductive system, ranking second in the world after cervical cancer [1,2]. It develops in the endometrium, lining the uterine cavity and smoothly flowing into the mucosa of the cervical canal. Two types of endometrial cancer are known, differing in molecular background, aggressiveness, and the age of onset [3,4,5]. The well-promising type I is more prevalent, occurring in the perimenopausal age, and developing from endometrial hyperplasia, after stimulation with estrogens [3,4]. In turn, type II, associated with a worse prognosis, is generally less frequent and targets women in the sixth and seventh decade of their lives, while not being associated with hormonal stimulation [3]. Most cases of endometrial cancer occur in highly developed countries, where it is the fourth most prevalent malignant tumor in women, after breast, lung, and skin cancer, and the most common genital cancer [6,7,8,9]. From a general perspective, the incidence of endometrial cancer is slowly but steadily increasing [4,10]. Most cases are diagnosed early, when the chances of recovery are still high and the five-year relative survival at diagnosis is estimated at over 80–90% [11]. However, the advanced forms of endometrial cancer are characterized by a worse prognosis and require more aggressive treatment, bringing a cure to approximately 30–50% of affected patients [3,5,11].
Many studies have recently aimed at improving the clinical management of endometrial cancer, personalizing patient therapy, adding novel molecular analyses to define cancer risk classes, and developing therapies, based on carcinogenic molecules [12,13]. Taking into account mutations and somatic copy number variations, genome and exome sequencing, and the microsatellite instability (MSI) test, endometrial cancer can be divided into the following four groups, each one being associated with different prognoses in terms of specific progression-free survival (PFS) and the risk of recurrence: polymerase epsilon (POLE) ultramutated, MSI hypermutated, copy-number (CN) low, and CN high [13,14]. In addition, a new model, called ProMisE (Proactive Molecular Risk Classifier for Endometrial Cancer), based on the Institute of Medicine (IOM) guidelines, has been introduced to overcome the methodology limitations in the Cancer Genome Atlas (TCGA) study, including the costs, the complexity, and the lack of immediate clinical application [13].
Among the immunological mechanisms of defense against cancer, an important role is assigned to the activity of cytokines, belonging to many groups, including: interleukins, interferons, chemokines, and the tumor necrosis factor (TNF) superfamily [4,15,16]. Cancer cells can secrete both monocyte and macrophage chemotactic factors, e.g., C-C motif chemokine ligand 2 (CCL2), as well as the agents that accelerate the maturation of these cells and activate them, e.g., the colony stimulating factor 1 (CSF1) and CSF2, and also inhibit their chemotaxis [4,6,8,17]. Moreover, the chemokines, secreted by macrophages, may have an opposite effect on tumor growth [17]. They can chemotactically attract many cells (neutrophils, monocytes, and effector T lymphocytes) and increase their inflow into the tumor [16,17]. Some of the chemokines, including the platelet factor 4 (PF4), the C-X-C motif chemokine ligand 9 (CXCL9), and CXCL10, may inhibit tumor vessel formation [17]. In turn, other chemokines, including CCL2, CCL5, CXCL1, CXCL5, and CXCL8, can stimulate angiogenesis [8,15,18,19].
Considering endometrial cancer, a significant relationship was noted between its incidence and pathogenesis on the one hand and CCL2, CCL5, and CXCL8 chemokines, and their selected receptors, including CCR2 (the receptor for CCL2) and CXCR2 (the receptor for CXCL8) on the other [3,5,6,8,9]. Genetic studies confirm the association of the single nucleotide polymorphisms (SNPs) −2518 G>A (rs1024611) of the CCL2 gene and 190 G>A (rs1799864) of the CCR2 gene with the occurrence of endometrial cancer [6,16]. In this reported case-control genetic association study, we aimed to determine the role of selected SNPs of the CCL2 (rs4586 (903 T>C)), as well as CCL5 (rs2107538 (−403 G>A) and rs2280789 (351 A>G)), CXCL8 (IL8, rs2227532 (−845 T>C) and −738 T>A), and CXCR2 (rs1126580 (1440 G>A)) genes, in the onset and progression of endometrial cancer.
2. Materials and Methods
This study involved 282 women, including 132 (46.8%) patients with endometrial cancer and 150 (53.2%) non-cancerous control individuals (see Table 1). The females, classified in the research project, were all hospitalized at the Department of Gynecology and Oncological Gynecology of the Polish Mother’s Memorial Hospital—Research Institute in Lodz, Poland. The women with endometrial cancer were aged between 44 and 88 years, while the controls were aged between 36 and 75 years. Further clinical characteristics of the enrolled women, including diabetes mellitus, arterial hypertension, and endometrial thickness, are presented in Table 1. The endometrial cancers were graded and staged, according to the criteria of the International Federation of Gynecology and Obstetrics (FIGO) [20]. For the non-cancer control group, normal endometrial tissue specimens were analyzed. Both cancerous and non-cancerous endometrial samples were collected by the dilation and curettage (D&C) procedure, performed on medical grounds. All the samples, previously collected for diagnostic purposes, were anonymized in the reported project. This study was approved by the Research Ethics Committee at the Polish Mother’s Memorial Hospital—Research Institute (the Institutional Review Board, approval number 42/2018). Informed consent forms were signed by all the study women, as recommended by the Research Ethics Committee.

Table 1.
Characteristic features of women with endometrial cancer and of non-cancerous controls.
2.1. DNA Extraction
Genomic DNA of the women with endometrial cancer and of the control individuals was extracted from paraffin-embedded sections, using a Syngen DNA Micro Kit (Cat No. SY244020, Syngen Biotech, Wroclaw, Poland). The obtained DNA was diluted in 100 µL of elution buffer and stored at −20 °C until further genetic studies.
2.2. Genotypes within SNPs of Chemokine and Chemokine Receptor Genes
The genotypes from CCL2 903 T>C, CCL5 −403 G>A and 351 A>G, IL8 −845 T>C and −738 T>A, as well as CXCR2 +1440 G>A polymorphisms were assayed by the PCR-RFLP method (see Figure 1). Primer sequences, annealing temperatures, and the amplicon lengths, specific to the performed PCR methods, are presented in Table 2. The external oligonucleotides for all the polymorphisms were obtained from the literature [21,22,23,24]. Additional internal primer sequences for nested PCR assays were designed, using the PerlPrimer v1.1.21 software. PCR products were resolved on 1% agarose gels, stained with ethidium bromide, and then digested overnight at 37 °C, with SatI, RsaI, MboII, AseI, XbaI, or HphI restriction enzymes, to estimate genotypes within the analyzed polymorphic sites (see Table 3). Genotypes were determined, based on the restriction profiles of digested products, obtained on 2% agarose gels (see Figure 2 and Figure S1, Table 2 and Table S1). The selected PCR products were additionally sequenced by the Sanger method at the Genomed Joint-Stock Company (Warsaw, Poland) to corroborate the genotypes previously estimated by the PCR-RFLP method. For CCL2 SNP, DNA fragments were sequenced for ten TT, six CT, and six CC genotypes. In case of CCL5 −403 G>A polymorphism, nine GG and 12 GA genotypes were verified by a sequencing process. Regarding CCL5 351 A>G SNP, PCR products were sequenced for seven AA and eight AG genotypes. In case of IL8 −845 T>C SNP, sequencing was performed for 14 TT genotypes, while, in case of IL8 −738 T>A, sequencing was performed for seven TT and two TA genotypes. The sequenced DNA fragments were analyzed by the Sequence Scanner 1.0 (Applied Biosystems) program.
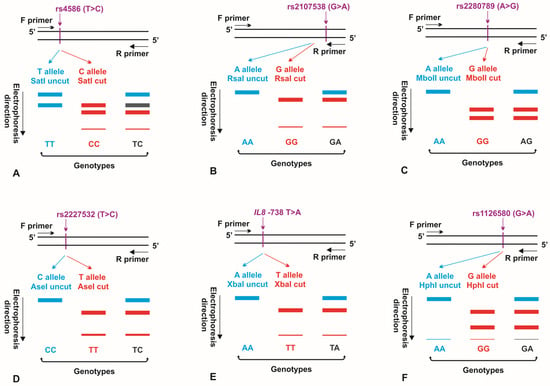
Figure 1.
Schematic representation of PCR-RFLP assays, used to determine genotypes for the CCL2 rs4586 (A), CCL5 rs2107538 (B), CCL5 rs2280789 (C), IL8 rs2227532 (D), IL8 −738 T>A (E), and CXCR2 rs1126580 (F) polymorphisms. The alleles of the polymorphic sites, not recognized by appropriate restriction enzymes, as well as DNA fragments and genotypes, obtained when no restriction digestion occurred in the polymorphism, are marked in blue. The alleles of the polymorphic regions, recognized by the endonucleases used in the research, as well as DNA fragments and genotypes, obtained as result of restriction digestion within the polymorphism, are shown in red. F primer: forward primer; R primer: reverse primer.

Table 2.
Oligonucleotides, annealing temperatures, and amplicon lengths in PCR assays for CCL2, CCL5, IL8, and CXCR2 polymorphisms.

Table 3.
Endonucleases and restriction profiles in PCR-RFLP assays.
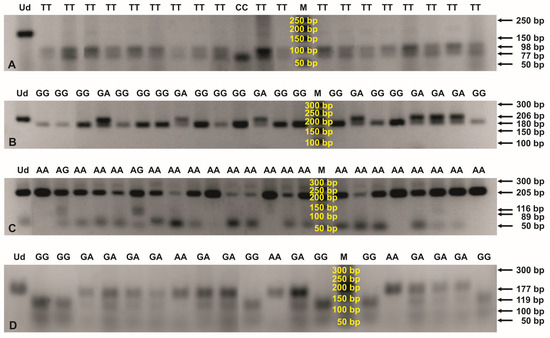
Figure 2.
PCR-RFLP profiles for genotyping the CCL2 rs4586 (A), CCL5 rs2107538 (B), CCL5 rs2280789 (C), and CXCR2 rs1126580 (D) polymorphisms. Digestions of the PCR products were performed with SatI (A), RsaI (B), MboII (C), and HphI (D) endonucleases and then separated in 2% agarose gels, stained with ethidium bromide. The numbers to the right of the electropherograms show the lengths of the separated DNA fragments. M: 50 bp DNA marker; Ud: undigested PCR product; AA, AG, CC, GA, GG, and TT: genotypes in the tested SNPs.
2.3. Statistical Analysis
The characteristics of both the women with endometrial cancer and the non-cancerous controls were compared, using the NCSS 2004 software. The Mann-Whitney U test was performed to compare the ages of the examined groups of women. Differences were estimated in the prevalence of diabetes mellitus, arterial hypertension, and endometrial thickness above 5 mm, and alleles in the tested polymorphisms of the examined women, using Pearson’s Chi-squared test. The distribution of genotypes and alleles was estimated within the analyzed SNPs and haplotypes for CCL2 and CCL5 polymorphisms in the studied groups of women by means of descriptive statistics, supported by the SNPStats software [25]. Hardy-Weinberg (H-W) equilibrium was analyzed for the genotypes of all the polymorphic sites, and linkage disequilibrium was estimated for the CCL2 and CCL5 SNPs. The H-W equilibrium test targeted the allele frequency rates, juxtaposing the values observed with the figures expected, assuming independence between the two, and a Chi-squared distribution had one degree of freedom. The associations of genotypes and haplotypes with the occurrence of endometrial cancer were determined, using a logistic regression model. Differences in genotype and allele prevalence rates were estimated among the various grades and stages of endometrial cancer, using Pearson’s Chi-squared test. Analyses were carried out for multiple SNPs and for haplotypes by the Expectation Maximization (EM) algorithm. First, the initial haplotype frequency values were provided. Passage E consisted then of the recalculation of the expected genotype frequency for the genotypes with haplotypes of uncertainty (in H-W equilibrium), using the haplotype frequency rates. Using the converted genotype frequency rates, the M run calculated each haplotype frequency. Consistent haplotypes were counted for each genotype. Finally, the algorithm converged to the desired haplotype frequency rates. The results were statistically significant at the significance level of p ≤ 0.050.
3. Results
3.1. Study Population
The women with endometrial cancer were significantly older than the non-cancerous control individuals (p ≤ 0.001, see Table 1). Diabetes mellitus, arterial hypertension, and endometrial thickness above 5 mm were much more common in the patients with endometrial cancer, compared to those in the control group (p ≤ 0.001). According to the FIGO grading, G1, G2, and G3 tumors were determined in 52.14% (61/117), 35.04% (41/117), and 12.82% (15/117) of the women diagnosed with endometrial cancer, respectively. Taking into account the FIGO staging rules, stages I, II, and III were found in 72.2% (83/115), 13.9% (16/115), and 13.9% of the endometrial cancer cases, respectively.
3.2. Hardy-Weinberg Equilibrium and Linkage Disequilibrium
The H-W equilibrium was observed for IL8 −738 T>A and CCL5 351 A>G SNPs among all the studied women, as well as for CXCR2 1440 G>A and CCL5 −403 G>A polymorphisms, among the patients with endometrial cancer and non-cancerous individuals, respectively (p ≤ 0.050). Regarding IL8 −845 T>C, the analyzed groups were monomorphic, while H-W was not calculated. In the case of the remaining genotypes, H-W was not preserved (p ≤ 0.050). The genotypes within CCL2 and CCL5 SNPs were found in the linkage disequilibrium between the cancerous and non-cancerous women (p ≤ 0.050).
3.3. Genotypes in Chemokine and Chemokine Receptor Gene Polymorphisms
Among the patients with endometrial cancer, TT, TC, and CC genotypes in CCL2 903 T>C SNP, were determined in 86.2% (112/130), 3.1% (4/130), and 10.8% (14/130) of the patients, respectively (see Table 4). In the case of CCL5 −403 G>A polymorphism, GG, GA, and AA variants were found in 56.9% (70/123), 26.8% (33/123), and 16.3% (20/123) of the women, respectively. For CCL5 351 A>G SNP, AA, GA, and GG genotypes were observed in 85.0% (108/127), 14.2% (18/127), and 0.8% (1/127) of the patients, respectively. Regarding IL8 −738 T>A SNP, TT and TA genotypes were found in 99.2% (129/130) and 0.8% (1/130) of the cancerous individuals, respectively. Taking into account the monomorphic IL8 −845 T>C polymorphism, all the analyzed women carried the TT genotype. In the case of CXCR2 1440 G>A SNP, AA, GA, and GG genotypes, were determined in 44.4% (56/126), 35.7% (45/126), and 19.8% (25/126) of the patients, respectively.

Table 4.
Relationship between genotypes in CCL2, CCL5, IL8, and CXCR2 polymorphisms and the occurrence of endometrial cancer.
Considering the non-cancerous women, TT, CT, and CC genotypes within CCL2 SNP were observed in 89.3% (133/149), 2.0% (3/149), and 8.7% (13/149) of the patients, respectively. Taking into account CCL5 −403 G>A polymorphism, GG, GA, and AA genotypes were determined in 66.4% (99/149), 29.5% (44/149), and 4.0% (6/149) of the controls, respectively. In the case of CCL5 351 A>G SNP, AA and AG genotypes were found in 83.2% (124/149) and 16.8% (25/149) of the patients, respectively. For IL8 −738 T>A SNP, TT and TA variants were determined in 99.3% (149/150) and 0.7% (1/150) of the controls, respectively. Regarding CXCR2 polymorphism, AA, GA, and GG genotypes were observed in 55.7% (83/149), 14.8% (22/149), and 29.5% (44/149) of the patients, respectively.
A single-SNP statistical analysis showed more than a four times higher risk of endometrial cancer in the women with the AA homozygotic status within CCL5 −403 G>A polymorphism (OR 4.71 95% CI 1.80–12.34 in the codominant model and OR 4.63 95% CI 1.80–11.93 in the recessive model, p ≤ 0.050, see Table 4). Similarly, it was observed that the women who were GA heterozygous for the CXCR2 SNP had an approximately threefold higher risk of endometrial cancer (OR 3.03 95% CI 1.64–5.59 in the codominant model and OR 3.21 95% CI 1.79–5.73 in the overdominant model, p ≤ 0.001, see Table 4). The association also remained significant after the correction for age, diabetes mellitus, or arterial hypertension, both in codominant and overdominant models (p ≤ 0.050, see Table 5). When adjusted for endometrial thickness above 5 mm, GA heterozygotes in the CXCR2 polymorphism were associated with about double the risk of cancer in the overdominant model (OR 2.23 95% CI 1.03–4.83, p = 0.038).

Table 5.
Associations of CXCR2 1440 G>A polymorphism with endometrial cancer adjusted by selected covariates.
A multiple-SNP analysis determined A-A haplotypes, for both CCL5 polymorphisms and T-A-A haplotypes in the range of CCL2 and CCL5 SNPs to be associated with an approximately twice higher risk of endometrial cancer (OR 1.84 95% CI 1.21–2.81, and OR 1.71 95% CI 1.10–2.65, respectively, p ≤ 0.050, see Table 6 and Table 7).

Table 6.
Relationships between the haplotypes for CCL5 SNPs and the occurrence of endometrial cancer.

Table 7.
Associations of the haplotypes for CCL2 and CCL5 polymorphisms with the onset of endometrial cancer.
Taking into account the grades and stages of endometrial cancer, the genotypes for all the analyzed SNPs were similarly distributed among the analyzed groups of cancer (see Table S2).
3.4. Allelic Variants within SNPs of the Chemokine and Chemokine Receptor Genes
Considering the women with endometrial cancer, C and T alleles in CCL2 polymorphism demonstrated prevalence rates of 12.3% (32/260) and 87.7% (228/260), respectively (see Table 8). In the case of CCL5 −403 G>A SNP, the prevalence rate of G and A alleles was 70.3% (173/246) and 29.7% (73/246), respectively. For CCL5 351 A>G polymorphism, the prevalence rate of A and G alleles was 92.1% (234/254) and 7.9% (20/254), respectively. In the case of IL8 −738 T>A SNP, T and A alleles revealed prevalence rates of 99.6% (259/260) and 0.4% (1/260), respectively. Regarding CXCR2 polymorphism, G and A alleles were found with prevalence rates of 37.7% (95/252) and 62.3% (157/252), respectively.

Table 8.
Distribution of the alleles, localized within CCL2, CCL5, IL8, and CXCR2 polymorphisms.
Among the non-cancerous controls, the prevalence rates of C and T alleles in CCL2 SNP were 9.7% (29/298) and 90.3% (269/298), respectively. Regarding CCL5 −403 G>A polymorphism, the prevalence rates of G and A alleles were 81.2% (242/298) and 18.8% (56/298), respectively. For CCL5 351 A>G SNP, A and G alleles were at the prevalence of 91.6% (273/298) and 8.4% (25/298), respectively. In the case of IL8 −738 T>A polymorphism, the prevalence rates of T and A alleles were 99.7% (299/300) and 0.3% (1/300), respectively. Taking into account CXCR2 SNP, the prevalence rates of G and A alleles were 36.9% (110/298) and 63.1% (188/298), respectively.
The differences in the distribution of the G and A alleles within CCL5 −403 G>A polymorphism were statistically significant when compared between the studied cancerous and non-cancerous groups (p = 0.003, see Table 8). For the CXCR2 SNP, the G and A alleles were differentially distributed among the various grades and stages of endometrial cancer (p = 0.043 and p = 0.034, respectively, see Table S2). In turn, all the other polymorphisms demonstrated similar prevalence rates of their alleles among the analyzed patient or cancer groups.
3.5. Sample Size Calculation
Given the prevalence rates of the alleles, determined for the polymorphisms analyzed in the reported study, the minimum required sample size should have been 157 women, with a 95% confidence level and a 5% margin of error. The value was obtained relative to the results for CXCR2 rs1126580.
4. Discussion
Our study demonstrated that both the AA homozygotes in CCL5 rs2107538 and the GA heterozygotes in CXCR2 rs1126580 had significantly been associated with an increased risk of endometrial cancer. The association of GA genotypes in the CXCR2 polymorphism with a higher risk of endometrial cancer also remained significant after the adjustments carried out for age, diabetes mellitus, arterial hypertension, and endometrial thickness above 5 mm, which had previously been reported as important risk factors for the cancer studied [26,27,28,29]. Moreover, the G and A alleles in rs1126580 were differentially distributed in various grades and stages of the cancer. An earlier study, which targeted women from the central coast of Tunisia, revealed that CCL5 rs2107538 had also been correlated with triple-negative breast cancer (TNBC) and hormone receptor-positive BC [30]. Similar to our outcomes, TT genotypes in CCL5 rs2107538 were associated with an increased risk of BC, and the T allele was more prevalent in the women with the disease when compared to the healthy controls [30]. Moreover, the T allele in rs2107538 was associated with low levels of CCL5 mRNA in the blood cells of BC patients and correlated, in a dose-dependent manner, with low serum levels of CCL5 among BC and TNBC patients [30]. In turn, a case-control study, conducted on the Southeastern Iranian population, showed that the GA and GA-AA genotypes in rs2107538 had been much more common in women with BC than in healthy subjects [31]. In addition, the A allele in the analyzed polymorphism was significantly more frequently observed in BC patients than in healthy controls, as was the case with the women with endometrial cancer, compared to the control subjects in our study [31]. It was also previously established that CCL5 rs2107538 was a risk factor for prostate cancer, hepatocellular carcinoma, oral cancer, and papillary thyroid cancer [32,33,34,35]. Regarding the functional significance of CCL5 rs2107538, this SNP was found to be localized at the binding site for the GATA binding protein 2 (GATA2) and was, thus, suggested to be involved in the regulation of CCL5 transcriptional activity [36]. Rs2107538 has been reported to be possibly associated with an altered binding affinity for GATA2, therefore affecting the CCL5 mRNA expression levels. An analysis of the GTEx database [37] also showed that the reduced expression levels of CCL5 mRNA had occurred with the rs2107538 G>A change in whole blood cells [36]. Based on the genetic results, obtained in our project and on the previously reported data on CCL5 levels in endometrial cancer, the A allele in rs2107538 may also contribute to altered CCL5 expression levels in the disease in question [8,38].
Considering the CXCR2 rs1126580 polymorphism, its involvement has also been reported in several cancer types, including diffuse large B-cell lymphoma (DLBCL), bile duct cancer, and classic Kaposi sarcoma (CKS) [39,40,41]. A population-based case-control study, conducted in patients with non-Hodgkin lymphoma and in healthy individuals, showed that CXCR2 rs1126580, as well as IL1A rs1800587, TNF rs1800629, and IL4R rs2107356 polymorphisms, were the strongest predictors of the overall survival (OS) in the patients with DLBCL [41]. In the case of CKS, a lower risk of cancer was found in T-G haplotype carriers for the CXCR2 rs1126579-rs1126580 polymorphisms [39]. In turn, GA heterozygotes in rs1126580 were associated with an increased risk of periodontitis, although that effect was not observed when adjustments were run for covariates, including age, sex, skin color, and smoking habit [42]. In white non-smokers, the C-T-G/T-C-A haplotype for the CXCR2 rs2230054-rs1126579-rs1126580 polymorphisms correlated with an increased susceptibility to periodontitis, while the C-T-G/T-C-G haplotype protected against the disease [42]. So far, neither the functional importance of rs1126580 for CXCR2 expression nor the ability of CXCR2 to bind IL8 has been reported [42,43]. Of note, an increased expression of CXCR2 was observed in endometrial cancer tissues compared to non-cancerous endometrial controls [3,44]. Moreover, the CXCR2 expression was positively associated with the endometrial cancer grade, while being inversely associated with disease-free survival (DFS) [3]. Further research would be justified, regarding the involvement of the rs1126580 polymorphism in the altered CXCR2 expression in endometrial cancer and in the disease prognosis, while its outcomes could bring highly promising results.
In the analyzed cohort of women, we additionally found that the A-A haplotypes for the rs2107538-rs2280789 polymorphisms localized in the CCL5 gene were associated with an approximately twofold increase in the risk of endometrial cancer. Previously, those two CCL5 SNPs were also reported to be associated with a higher risk of TNBC [30], as well as having a detrimental effect on the cumulative risk of the acute graft-versus-host disease and on relapse-free survival from human HLA-matched allo-HSCT (allogeneic hematopoietic stem cell transplantation) [30,45]. Similarly, the T allele in CCL5 rs2107538, and the CCL5 rs2280789-G, BC risk allele were also correlated with the low levels of CCL5 mRNA in the blood cells of the BC patients and, in a dose-dependent manner, with the low amounts of CCL5 in the sera of the BC and TNBC patients [30]. It is noteworthy to mention that the CCL5 transcription was previously reported to be regulated mainly by rs2280789, localized in its promoter region, while severely decreasing when the G allele was present [46,47,48]. In African-American, European-American, and combined cohorts, infected with HIV-1, the C allele was also shown to reduce CCL5 transcription, increasing the progression rate of AIDS [46]. Among the subjects with metastatic colorectal cancer (mCRC), the carriers of any G allele in CCL5 rs2280789, who had received cetuximab plus FOLFIRI, demonstrated shorter OS when compared to AA homozygotes [47]. Japanese mCRC patients, treated with regorafenib, demonstrated a higher incidence of grade 3+ hand-foot skin reactions, observed in the subjects with GG genotypes for rs2280789 than in the carriers of any A allele [49]. We suggest that, in the case of endometrial cancer, the A allele in rs2280789 may be a significant variant, associated with an increased CCL5 transcription, possibly resulting in higher CCL5 levels, also previously reported for fibroblast cell populations derived from human endometrial cancer tissues [38].
A further multiple-SNP analysis in our study also showed that the T-A-A haplotypes for the CCL2 rs4586-CCL5 rs2107538-CCL5 rs2280789 polymorphisms had correlated with an increased risk of endometrial cancer. CCL2 rs4586 was previously shown to be involved in several other cancer types, including CRC, locoregional gastric cancer (LRGC), and BC [50,51,52,53]. Regarding the KRAS wild-type mCRC patients, treated with FOLFIRI/bevacizumab, CCL2 rs4586 was identified as a major OS predictor [51]. In turn, KRAS mutant tumors of the mCRC patients with C alleles of both CCL2 rs4586 and IRF3 rs2304205 were associated with better PFS [54]. Among Korean patients with CRC, the T allele in rs4586 correlated with favorable OS, observed in both univariate and multivariate analyses [53]. Taking into account LRGC, US patients with at least one T allele in CCL2 rs4586 demonstrated significantly shorter OS than CC homozygotes, while longer OS was observed in the Japanese cohort [52].
In premenopausal European American women, CCL2 rs4586 was also correlated with an increased risk of BC, although the effect disappeared when the analysis was conducted on a larger cohort of participants in the Women’s Circle of Health Study [50]. Given the functional importance of CCL2 rs4586, an analysis, performed using the GTEx database, showed that the SNP was an expression quantitative trait locus (eQTL) and correlated with higher levels of CCL13 mRNA in various human tissues [55]. In the Chinese population, the minor T allele in CCL2 rs4586 was found to be associated with significantly elevated serum CCL2 levels, which also correlated with a higher risk of chronic obstructive pulmonary disease [56]. On the other hand, a case-control study, conducted on a Chinese Han population, showed that the CC genotype in rs4586 had been associated with elevated plasma CCL2 levels compared to the TT genotype [57]. An ethnicity-dependent function of the rs4586 polymorphism was then suggested [57]. In endometrial cancer, the T allele in CCL2 rs4586 may also be involved in the elevated CCL2 production previously established for this type of malignancy [58,59].
5. Conclusions
CCL2 rs4586, CCL5 rs2107538 and rs2280789, as well as CXCR2 rs1126580, seem to be significantly associated with an increased risk of endometrial cancer in the population of Polish women. It may also be suggested that the T allele in CCL2 rs4586 and the A allele in CCL5 rs2280789 are likely involved in the increased levels of CCL2 and CCL5, respectively. However, further research projects would be beneficial to unveil in detail the role of the reported polymorphisms in the altered function of the encoded molecules and signaling pathways affected in endometrial cancer. The presented results may also be useful for the development of new therapeutic strategies in endometrial cancer, based on molecular/genetic profiling.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers15225416/s1. Table S1A. Densitometric analysis of the PCR-RFLP products obtained for CCL2 rs4586 genotyping, shown in Figure S1A; Table S1B. Densitometric analysis of the PCR-RFLP products obtained for CCL5 rs2107538 genotyping, shown in Figure S1B; Table S1C. Densitometric analysis of the PCR-RFLP products obtained for CCL5 rs2280789 genotyping, shown in Figure S1C; Table S1D. Densitometric analysis of the PCR-RFLP products obtained for CXCR2 rs1126580 genotyping, shown in Figure S1D; Table S2. Distribution of the genotypes and alleles of CCL2, CCL5 and CXCR2 polymorphisms among the grades and stages of endometrial cancer; Figure S1. Full blot images.
Author Contributions
Conceptualization, W.I.W.; data curation, W.I.W., H.R., B.S. and G.S.; formal analysis, W.I.W., H.R. and G.S.; funding acquisition, W.I.W.; investigation, W.I.W.; methodology, W.I.W., A.Z., K.S., H.R. and G.S.; project administration, W.I.W.; resources, A.Z., K.S., H.R. and G.S.; supervision, W.I.W.; visualization, W.I.W.; writing—original draft, W.I.W.; writing—review and editing, W.I.W., A.Z., K.S., H.R., B.S. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science & Higher Education, Polish Mother’s Memorial Hospital—Research Institute (the funds supporting statutory research projects).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Research Ethics Committee of the Polish Mother’s Memorial Hospital—Research Institute (protocol code: 42/2018; date of approval: 22 May 2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, B.; Chen, P.; Xi, D.; Zhu, H.; Gao, Y. ATF4 regulates CCL2 expression to promote endometrial cancer growth by controlling macrophage infiltration. Exp. Cell Res. 2017, 360, 105–112. [Google Scholar] [CrossRef]
- Yu, X.; Zhou, B.; Zhang, Z.; Lan, Z.; Chen, P.; Duan, R.; Zhang, L.; Xi, M. Insertion/deletion polymorphism in IL1A 3′-UTR is associated with susceptibility to endometrial cancer in Chinese Han women. J. Obstet. Gynaecol. Res. 2016, 42, 983–989. [Google Scholar] [CrossRef]
- Ewington, L.; Taylor, A.; Sriraksa, R.; Horimoto, Y.; Lam, E.W.; El-Bahrawy, M.A. The expression of interleukin-8 and interleukin-8 receptors in endometrial carcinoma. Cytokine 2012, 59, 417–422. [Google Scholar] [CrossRef]
- Smith, H.O.; Stephens, N.D.; Qualls, C.R.; Fligelman, T.; Wang, T.; Lin, C.Y.; Burton, E.; Griffith, J.K.; Pollard, J.W. The clinical significance of inflammatory cytokines in primary cell culture in endometrial carcinoma. Mol. Oncol. 2013, 7, 41–54. [Google Scholar] [CrossRef]
- Tong, H.; Ke, J.Q.; Jiang, F.Z.; Wang, X.J.; Wang, F.Y.; Li, Y.R.; Lu, W.; Wan, X.P. Tumor-associated macrophage-derived CXCL8 could induce ERα suppression via HOXB13 in endometrial cancer. Cancer Lett. 2016, 376, 127–136. [Google Scholar] [CrossRef]
- Attar, R.; Agachan, B.; Kuran, S.B.; Cacina, C.; Sozen, S.; Yurdum, L.M.; Attar, E.; Isbir, T. Association of CCL2 and CCR2 gene variants with endometrial cancer in Turkish women. In Vivo 2010, 24, 243–248. [Google Scholar]
- Brooks, N.; Stojanovska, L.; Grant, P.; Apostolopoulos, V.; McDonald, C.F.; Pouniotis, D.S. Characterization of blood monocyte phenotype in patients with endometrial cancer. Int. J. Gynecol. Cancer 2012, 22, 1500–1508. [Google Scholar] [CrossRef]
- Doster, A.; Schwarzig, U.; Zygmunt, M.; Rom, J.; Schutz, F.; Fluhr, H. Unfractionated Heparin Selectively Modulates the Expression of CXCL8, CCL2 and CCL5 in Endometrial Carcinoma Cells. Anticancer. Res. 2016, 36, 1535–1544. [Google Scholar]
- Pena, C.G.; Nakada, Y.; Saatcioglu, H.D.; Aloisio, G.M.; Cuevas, I.; Zhang, S.; Miller, D.S.; Lea, J.S.; Wong, K.K.; DeBerardinis, R.J.; et al. LKB1 loss promotes endometrial cancer progression via CCL2-dependent macrophage recruitment. J. Clin. Investig. 2015, 125, 4063–4076. [Google Scholar] [CrossRef]
- Zhang, W.; Hou, F.; Zhang, Y.; Tian, Y.; Jiao, J.; Ma, D.; Kong, B.; Cui, B. Changes of Th17/Tc17 and Th17/Treg cells in endometrial carcinoma. Gynecol. Oncol. 2014, 132, 599–605. [Google Scholar] [CrossRef]
- Berry, K.K.; Varney, M.L.; Dave, B.J.; Bucana, C.D.; Fidler, I.J.; Singh, R.K. Expression of interleukin-8 in human metastatic endometrial carcinoma cells and its regulation by inflammatory cytokines. Int. J. Gynecol. Cancer 2001, 11, 54–60. [Google Scholar] [CrossRef]
- Di Donato, V.; Giannini, A.; Bogani, G. Recent Advances in Endometrial Cancer Management. J. Clin. Med. 2023, 12, 2241. [Google Scholar] [CrossRef]
- Golia D’Augè, T.; Cuccu, I.; Santangelo, G.; Muzii, L.; Giannini, A.; Bogani, G.; Di Donato, V. Novel Insights into Molecular Mechanisms of Endometrial Diseases. Biomolecules 2023, 13, 499. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar]
- Dinkic, C.; Kruse, A.; Zygmunt, M.; Schuetz, F.; Brucker, J.; Rom, J.; Sohn, C.; Fluhr, H. Influence of Paclitaxel and Heparin on Vitality, Proliferation and Cytokine Production of Endometrial Cancer Cells. Geburtshilfe Frauenheilkd. 2017, 77, 1104–1110. [Google Scholar] [CrossRef]
- He, S.; Zhang, X. The rs1024611 in the CCL2 gene and risk of gynecological cancer in Asians: A meta-analysis. World J. Surg. Oncol. 2018, 16, 34. [Google Scholar] [CrossRef]
- Jakóbisiak, M.; Lasek, W. Immunologia nowotworów. In Immunologia; Gołąb, J., Jakóbisiak, M., Lasek, W., Stokłosa, T., Eds.; Wydawnictwo Naukowe PWN SA: Warszawa, Poland, 2012; pp. 450–467. [Google Scholar]
- Wallace, A.E.; Sales, K.J.; Catalano, R.D.; Anderson, R.A.; Williams, A.R.; Wilson, M.R.; Schwarze, J.; Wang, H.; Rossi, A.G.; Jabbour, H.N. Prostaglandin F2alpha-F-prostanoid receptor signaling promotes neutrophil chemotaxis via chemokine (C-X-C motif) ligand 1 in endometrial adenocarcinoma. Cancer Res. 2009, 69, 5726–5733. [Google Scholar] [CrossRef]
- Wang, J.; Taylor, A.; Showeil, R.; Trivedi, P.; Horimoto, Y.; Bagwan, I.; Ewington, L.; Lam, E.W.; El-Bahrawy, M.A. Expression profiling and significance of VEGF-A, VEGFR2, VEGFR3 and related proteins in endometrial carcinoma. Cytokine 2014, 68, 94–100. [Google Scholar] [CrossRef]
- FIGO Staging of Endometrial Cancer: 2023. Available online: https://www.figo.org/news/figo-staging-endometrial-cancer-2023 (accessed on 26 September 2023).
- Dan, H.; Liu, W.; Zhou, Y.; Wang, J.; Chen, Q.; Zeng, X. Association of interleukin-8 gene polymorphisms and haplotypes with oral lichen planus in a Chinese population. Inflammation 2010, 33, 76–81. [Google Scholar] [CrossRef]
- Mhmoud, N.; Fahal, A.; Wendy van de Sande, W.J. Association of IL-10 and CCL5 single nucleotide polymorphisms with tuberculosis in the Sudanese population. Trop. Med. Int. Health 2013, 18, 1119–1127. [Google Scholar] [CrossRef]
- Singh, B.; Chitra, J.; Selvaraj, P. CCL2, CCL3 and CCL4 gene polymorphisms in pulmonary tuberculosis patients of South India. Int. J. Immunogenet. 2014, 41, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Viana, A.C.; Kim, Y.J.; Cirelli, J.A.; Orrico, S.R.; Curtis, K.C.; Cano, V.S.; Valentini, S.R.; Scarel-Caminaga, R.M. A novel PCR-RFLP assay for the detection of the single nucleotide polymorphism at position +1440 in the human CXCR2 gene. Biochem. Genet. 2007, 45, 737–741. [Google Scholar] [CrossRef]
- SNPStats Software. 2023. Available online: https://www.snpstats.net/start.htm (accessed on 26 September 2023).
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474. [Google Scholar] [PubMed]
- Gentry-Maharaj, A.; Karpinskyj, C. Current and future approaches to screening for endometrial cancer. Best. Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, C.; Cheng, J. The relationship between endometrial thickening and endometrial lesions in postmenopausal women. Arch. Gynecol. Obstet. 2022, 306, 2047–2054. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Qian, G.; Su, T.; Xu, H. Value of endometrial thickness for the detection of endometrial cancer and atypical hyperplasia in asymptomatic postmenopausal women. BMC Womens Health 2022, 22, 517. [Google Scholar] [CrossRef]
- Shan, J.; Chouchane, A.; Mokrab, Y.; Saad, M.; Boujassoum, S.; Sayaman, R.W.; Ziv, E.; Bouaouina, N.; Remadi, Y.; Gabbouj, S.; et al. Genetic Variation in CCL5 Signaling Genes and Triple Negative Breast Cancer: Susceptibility and Prognosis Implications. Front. Oncol. 2019, 9, 1328. [Google Scholar] [CrossRef] [PubMed]
- Eskandari-Nasab, E.; Hashemi, M.; Ebrahimi, M.; Amininia, S.; Bahari, G.; Mashhadi, M.A.; Taheri, M. Evaluation of CCL5 -403 G>A and CCR5 Δ32 gene polymorphisms in patients with breast cancer. Cancer Biomark. 2014, 14, 343–351. [Google Scholar] [CrossRef]
- Charni, F.; Sutton, A.; Rufat, P.; Laguillier, C.; Mansouri, A.; Moreau, R.; Ganne-Carrié, N.; Trinchet, J.C.; Beaugrand, M.; Charnaux, N.; et al. Chemokine RANTES promoter dimorphisms and hepatocellular carcinoma occurrence in patients with alcoholic or hepatitis C virus-related cirrhosis. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1439–1446. [Google Scholar] [CrossRef]
- Kidd, L.R.; Jones, D.Z.; Rogers, E.N.; Kidd, N.C.; Beache, S.; Rudd, J.E.; Ragin, C.; Jackson, M.; McFarlane-Anderson, N.; Tulloch-Reid, M.; et al. Chemokine Ligand 5 (CCL5) and chemokine receptor (CCR5) genetic variants and prostate cancer risk among men of African Descent: A case-control study. Hered. Cancer Clin. Pract. 2012, 10, 16. [Google Scholar] [CrossRef]
- Kwon, K.H.; Lee, Y.C.; Chung, J.H.; Eun, Y.G. Association study of chemokine (C-C motif) ligand 5 gene polymorphism and papillary thyroid cancer. J. Investig. Surg. 2013, 26, 319–324. [Google Scholar] [CrossRef]
- Weng, C.J.; Chien, M.H.; Lin, C.W.; Chung, T.T.; Zavras, A.I.; Tsai, C.M.; Chen, M.K.; Yang, S.F. Effect of CC chemokine ligand 5 and CC chemokine receptor 5 genes polymorphisms on the risk and clinicopathological development of oral cancer. Oral. Oncol. 2010, 46, 767–772. [Google Scholar] [CrossRef]
- Pang, Y.; Li, H.; Gong, Y.; Jing, S.; Peng, C.; Liu, W.; Zhao, Y.; Wang, H.; Kaushik, D.; Rodriguez, R.; et al. Association of CCL2, CCR2 and CCL5 genetic polymorphisms with the development and progression of benign prostatic hyperplasia. Oncol. Rep. 2019, 41, 2491–2501. [Google Scholar] [CrossRef] [PubMed]
- GTEx Portal. 2018. Available online: http://www.gtexportal.org/home/ (accessed on 26 September 2023).
- Subramaniam, K.S.; Tham, S.T.; Mohamed, Z.; Woo, Y.L.; Mat Adenan, N.A.; Chung, I. Cancer-associated fibroblasts promote proliferation of endometrial cancer cells. PLoS ONE 2013, 8, e68923. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; Fallin, D.; Ruczinski, I.; Hutchinson, A.; Staats, B.; Vitale, F.; Lauria, C.; Serraino, D.; Rezza, G.; Mbisa, G.; et al. Associations of classic Kaposi sarcoma with common variants in genes that modulate host immunity. Cancer Epidemiol. Biomark. Prev. 2006, 15, 926–934. [Google Scholar] [CrossRef]
- Hsing, A.W.; Sakoda, L.C.; Rashid, A.; Andreotti, G.; Chen, J.; Wang, B.S.; Shen, M.C.; Chen, B.E.; Rosenberg, P.S.; Zhang, M.; et al. Variants in inflammation genes and the risk of biliary tract cancers and stones: A population-based study in China. Cancer Res. 2008, 68, 6442–6452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, Y.; Zheng, T.; Ma, S. Risk Factors of Non-Hodgkin Lymphoma. Expert Opin. Med. Diagn. 2011, 5, 539–550. [Google Scholar] [CrossRef]
- Viana, A.C.; Kim, Y.J.; Curtis, K.M.; Renzi, R.; Orrico, S.R.; Cirelli, J.A.; Scarel-Caminaga, R.M. Association of haplotypes in the CXCR2 gene with periodontitis in a Brazilian population. DNA Cell Biol. 2010, 29, 191–200. [Google Scholar] [CrossRef]
- Lupiañez, C.B.; Canet, L.M.; Carvalho, A.; Alcazar-Fuoli, L.; Springer, J.; Lackner, M.; Segura-Catena, J.; Comino, A.; Olmedo, C.; Ríos, R.; et al. Polymorphisms in Host Immunity-Modulating Genes and Risk of Invasive Aspergillosis: Results from the AspBIOmics Consortium. Infect. Immun. 2015, 84, 643–657. [Google Scholar] [CrossRef]
- Kulinczak, M.; Sromek, M.; Panek, G.; Zakrzewska, K.; Lotocka, R.; Szafron, L.M.; Chechlinska, M.; Siwicki, J.K. Endometrial Cancer-Adjacent Tissues Express Higher Levels of Cancer-Promoting Genes than the Matched Tumors. Genes 2022, 13, 1611. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, I.; Kim, J.H.; Lee, Y.G.; Kang, E.J.; Cho, H.J.; Lee, K.H.; Kim, H.J.; Park, E.H.; Lee, J.E.; et al. RANTES polymorphisms and the risk of graft-versus-host disease in human leukocyte antigen-matched sibling allogeneic hematopoietic stem cell transplantation. Acta Haematol. 2013, 129, 137–145. [Google Scholar] [CrossRef]
- An, P.; Nelson, G.W.; Wang, L.; Donfield, S.; Goedert, J.J.; Phair, J.; Vlahov, D.; Buchbinder, S.; Farrar, W.L.; Modi, W.; et al. Modulating influence on HIV/AIDS by interacting RANTES gene variants. Proc. Natl. Acad. Sci. USA 2002, 99, 10002–10007. [Google Scholar] [CrossRef] [PubMed]
- Suenaga, M.; Stintzing, S.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Schirripa, M.; et al. Role of CCL5 and CCR5 gene polymorphisms in epidermal growth factor receptor signalling blockade in metastatic colorectal cancer: Analysis of the FIRE-3 trial. Eur. J. Cancer 2019, 107, 100–114. [Google Scholar] [CrossRef]
- Suenaga, M.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Okazaki, S.; Berger, M.D.; Miyamoto, Y.; Schirripa, M.; Soni, S.; et al. Genetic variants in CCL5 and CCR5 genes and serum VEGF-A levels predict efficacy of bevacizumab in metastatic colorectal cancer patients. Int. J. Cancer 2019, 144, 2567–2577. [Google Scholar] [CrossRef]
- Suenaga, M.; Schirripa, M.; Cao, S.; Zhang, W.; Yang, D.; Ning, Y.; Cremolini, C.; Antoniotti, C.; Borelli, B.; Mashima, T.; et al. Gene Polymorphisms in the CCL5/CCR5 Pathway as a Genetic Biomarker for Outcome and Hand-Foot Skin Reaction in Metastatic Colorectal Cancer Patients Treated With Regorafenib. Clin. Color. Cancer 2018, 17, e395–e414. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Quan, L.; Yao, S.; Zirpoli, G.; Bandera, E.V.; Roberts, M.; Coignet, J.G.; Cabasag, C.; Sucheston, L.; Hwang, H.; et al. Innate immunity pathways and breast cancer Risk in African American and European-American women in the Women’s Circle of Health Study (WCHS). PLoS ONE 2013, 8, e72619. [Google Scholar] [CrossRef]
- Naseem, M.; Cao, S.; Yang, D.; Millstein, J.; Puccini, A.; Loupakis, F.; Stintzing, S.; Cremolini, C.; Tokunaga, R.; Battaglin, F.; et al. Random survival forests identify pathways with polymorphisms predictive of survival in KRAS mutant and KRAS wild-type metastatic colorectal cancer patients. Sci. Rep. 2021, 11, 12191. [Google Scholar] [CrossRef] [PubMed]
- Sunakawa, Y.; Stremitzer, S.; Cao, S.; Zhang, W.; Yang, D.; Wakatsuki, T.; Ning, Y.; Yamauchi, S.; Stintzing, S.; Sebio, A.; et al. Association of variants in genes encoding for macrophage-related functions with clinical outcome in patients with locoregional gastric cancer. Ann. Oncol. 2015, 26, 332–339. [Google Scholar] [CrossRef]
- Yoon, S.; Kang, B.W.; Park, S.Y.; Kim, H.J.; Park, J.S.; Choi, G.S.; Kim, J.G. Prognostic relevance of genetic variants involved in immune checkpoints in patients with colorectal cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1775–1780. [Google Scholar] [CrossRef]
- Sunakawa, Y.; Stintzing, S.; Cao, S.; Heinemann, V.; Cremolini, C.; Falcone, A.; Yang, D.; Zhang, W.; Ning, Y.; Stremitzer, S.; et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: Results from TRIBE and FIRE3 trials. Ann. Oncol. 2015, 26, 2450–2456. [Google Scholar] [CrossRef]
- GTEx Consortium. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Lin, C.; Wang, Z.; Shen, L.; Yi, G.; Li, M.; Li, D. Genetic Variants, Circulating Level of MCP1 with Risk of Chronic Obstructive Pulmonary Disease: A Case-Control Study. Pharmgenom. Pers. Med. 2021, 14, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, J.; Yang, H.; Jiang, L.; Zhou, X.; Huang, Y.; Xu, N. Association of CCL2 Gene Variants with Osteoarthritis. Arch. Med. Res. 2019, 50, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Dobroch, J.; Bojczuk, K.; Kołakowski, A.; Baczewska, M.; Knapp, P. The Exploration of Chemokines Importance in the Pathogenesis and Development of Endometrial Cancer. Molecules 2022, 27, 2041. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, W.; Xing, G.Z.; Xiang, L.; Zheng, W.M.; Ma, Z.L. Role of CC-chemokine ligand 2 in gynecological cancer. Cancer Cell Int. 2022, 22, 361. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).