A Comparative Analysis of Orthotopic and Subcutaneous Pancreatic Tumour Models: Tumour Microenvironment and Drug Delivery
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Tumour Cell Line and Tumour Implantations
2.3. Volume Growth Curves and Metastases
2.4. Contrast-Enhanced Ultrasound Imaging and Analysis
2.5. Tumour Sectioning for Microscopy
2.6. Histopathology
2.7. Confocal Laser Scanning Microscopy of the Functional Vessels
2.8. Collagen Imaging using Second-Harmonic Imaging Microscopy
2.9. Collagen Anisotropy Analysis
2.10. Stiffness Measurements by Macro-Indentation
2.11. Immunostaining and Flow Cytometry
2.12. Near-Infrared Whole Animal Fluorescence Imaging
2.13. Statistics
3. Results
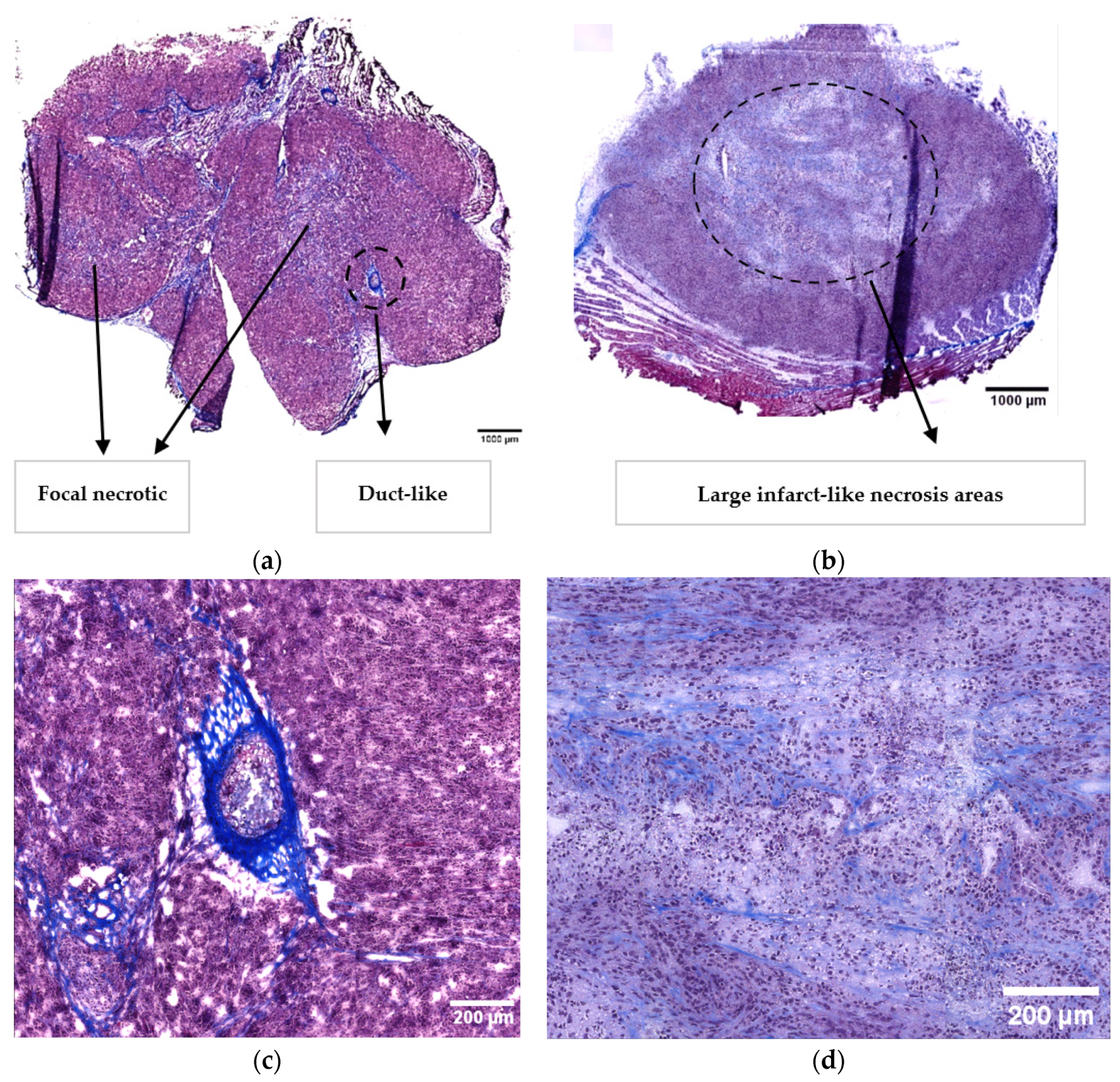
3.1. Histopathological Characterisation
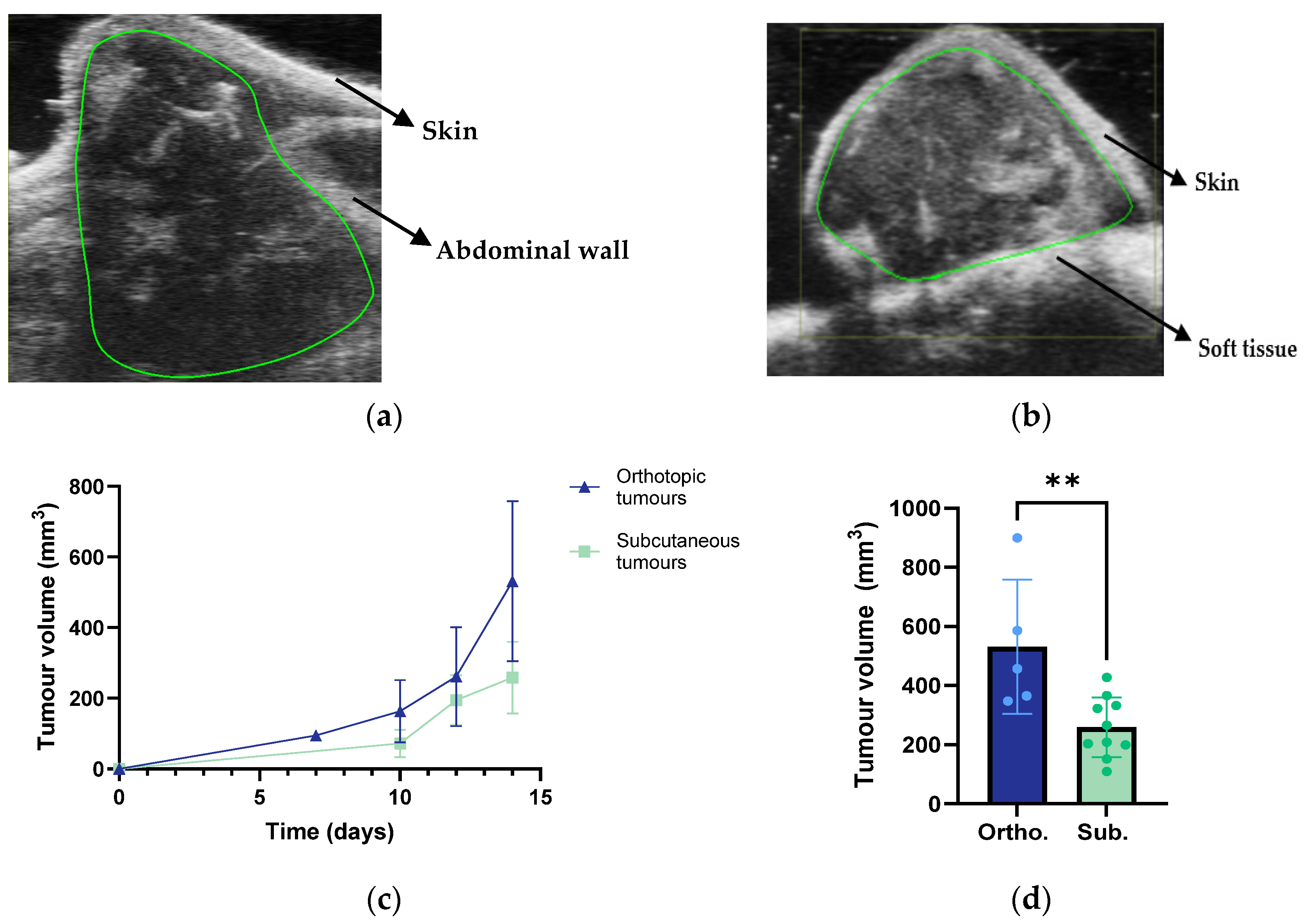
3.2. Tumour Growth and Metastases
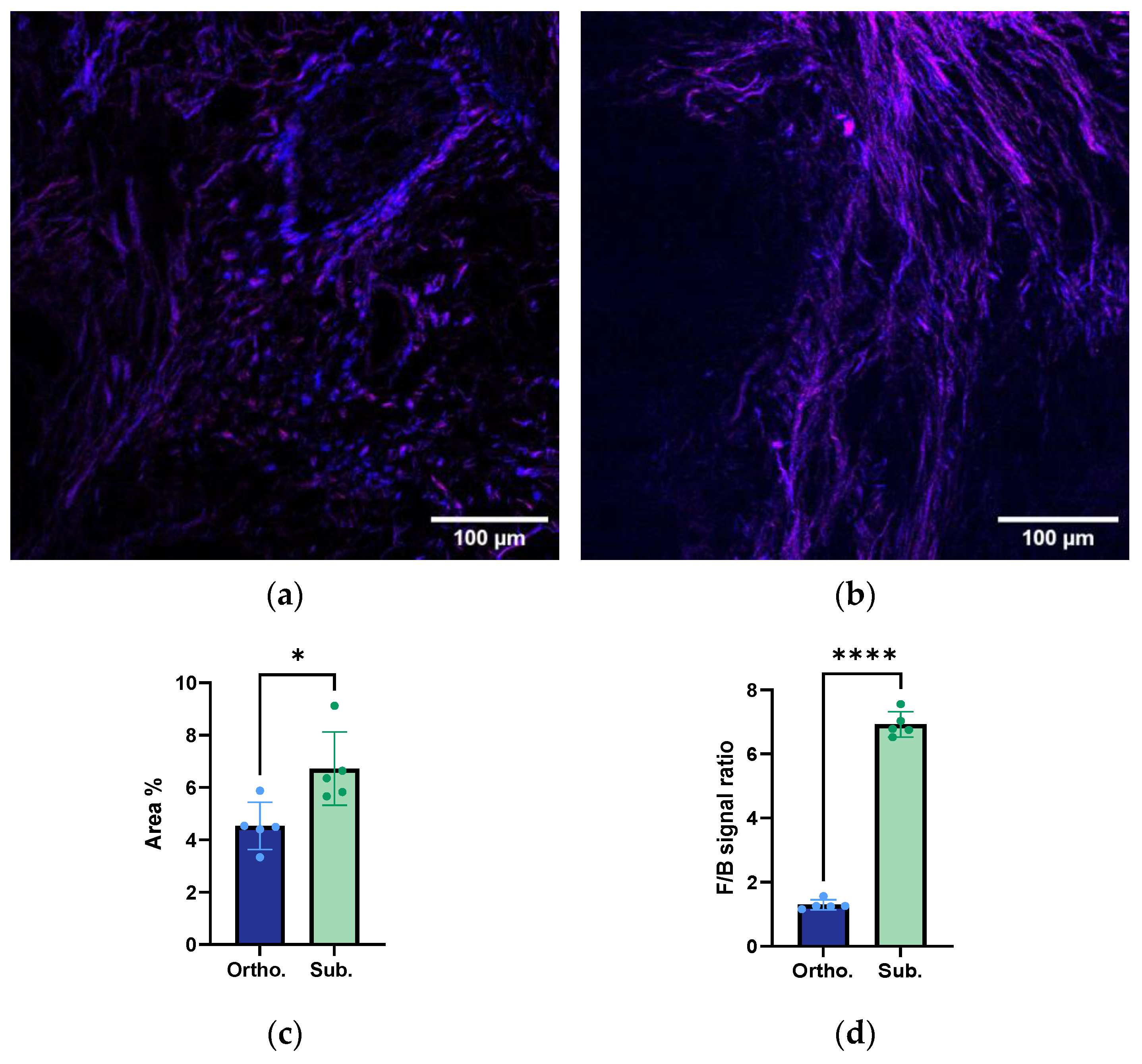
3.3. Collagen Fibre Estimation using Second-Harmonic Imaging Microscopy
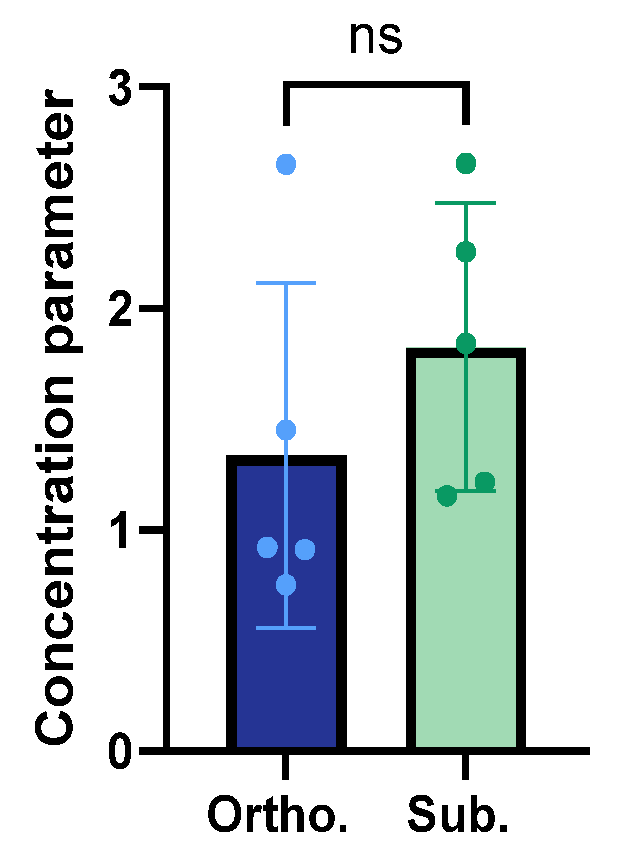
3.4. Structural Organisation and Alignment of the Collagen Fibres
3.5. Tumour Biomechanical Assessment Ex Vivo
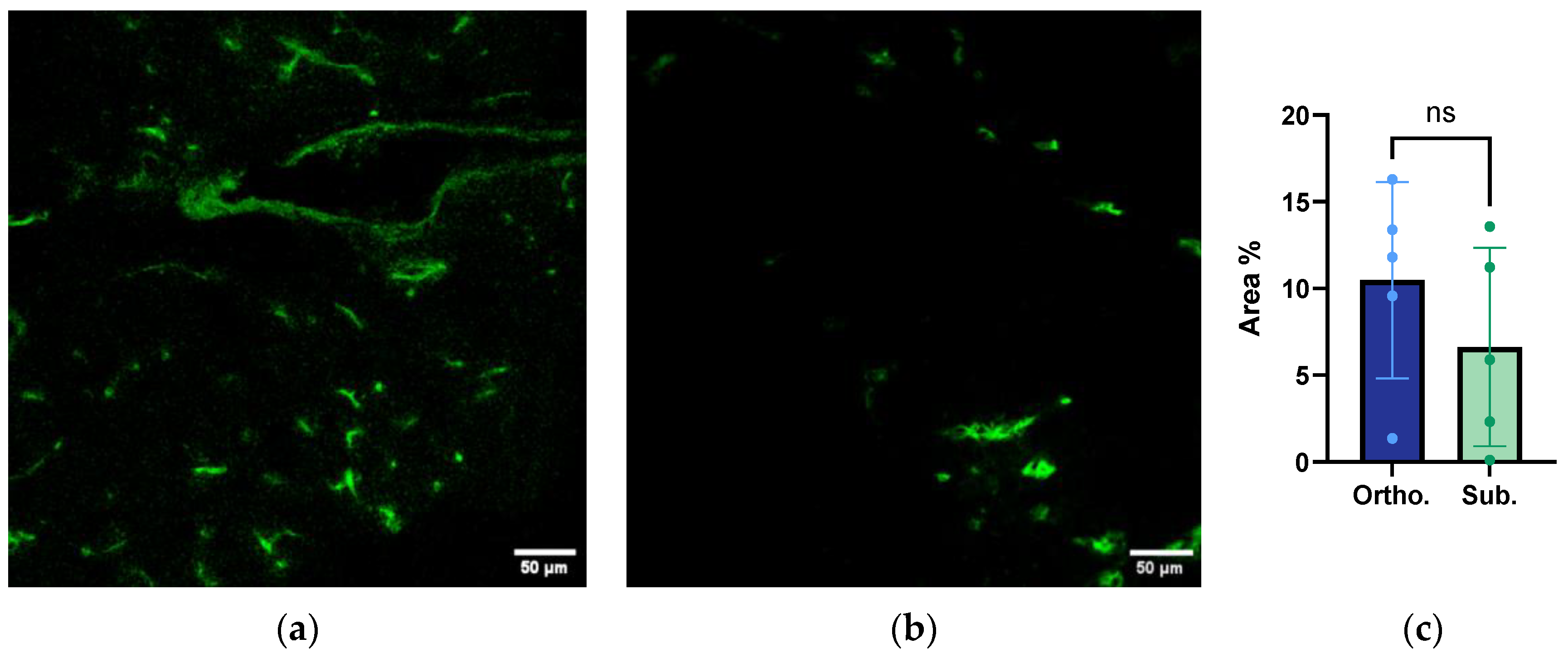
3.6. Functional Vessel Density Analysis from Confocal Laser Scanning Microscopy
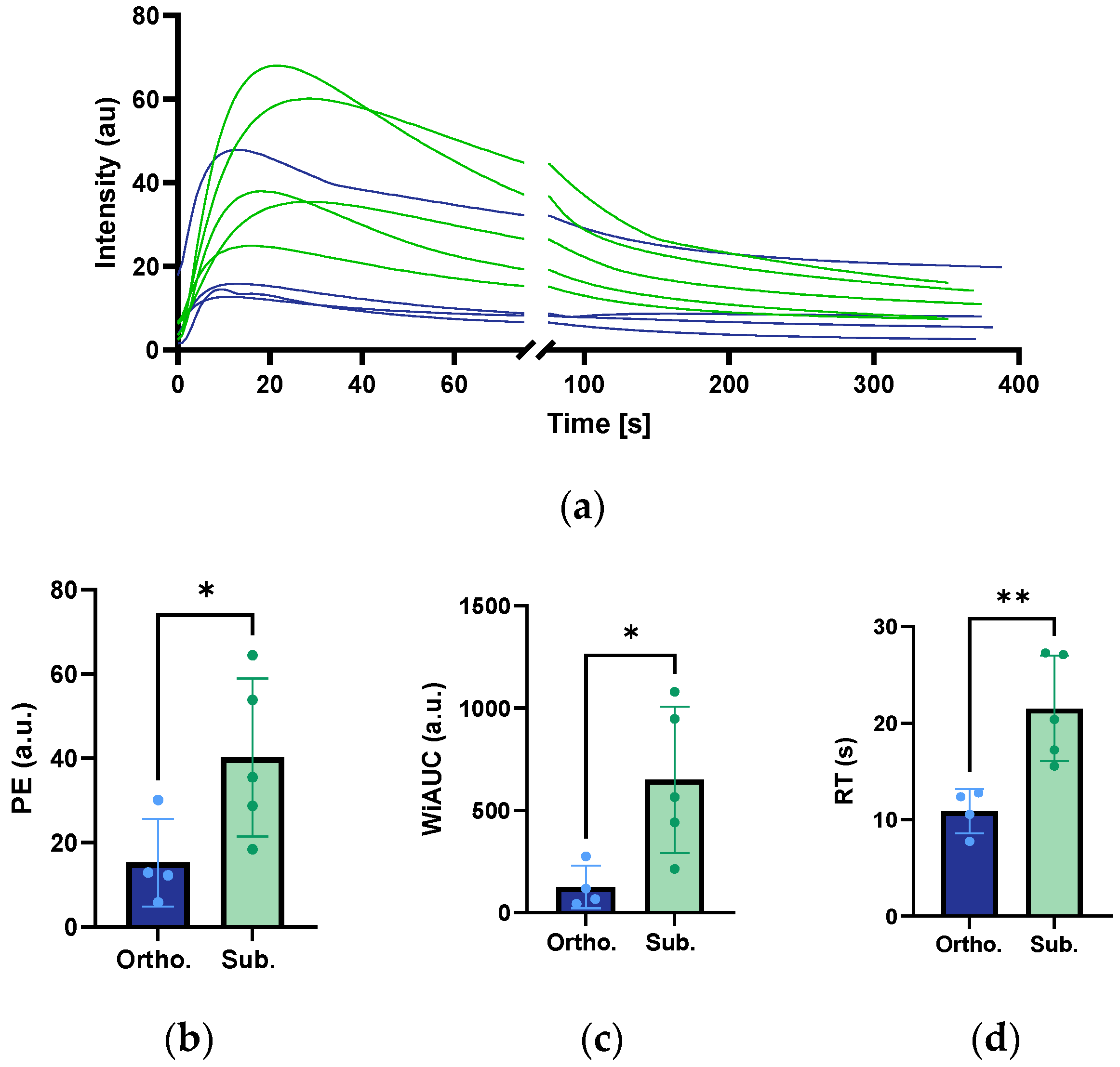
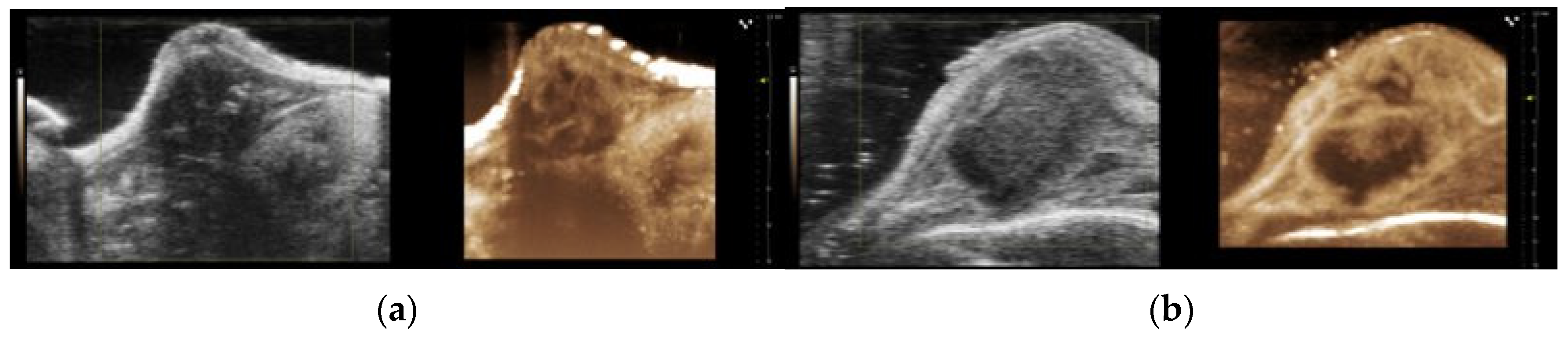
3.7. Perfusion Analysis from CEUS Imaging
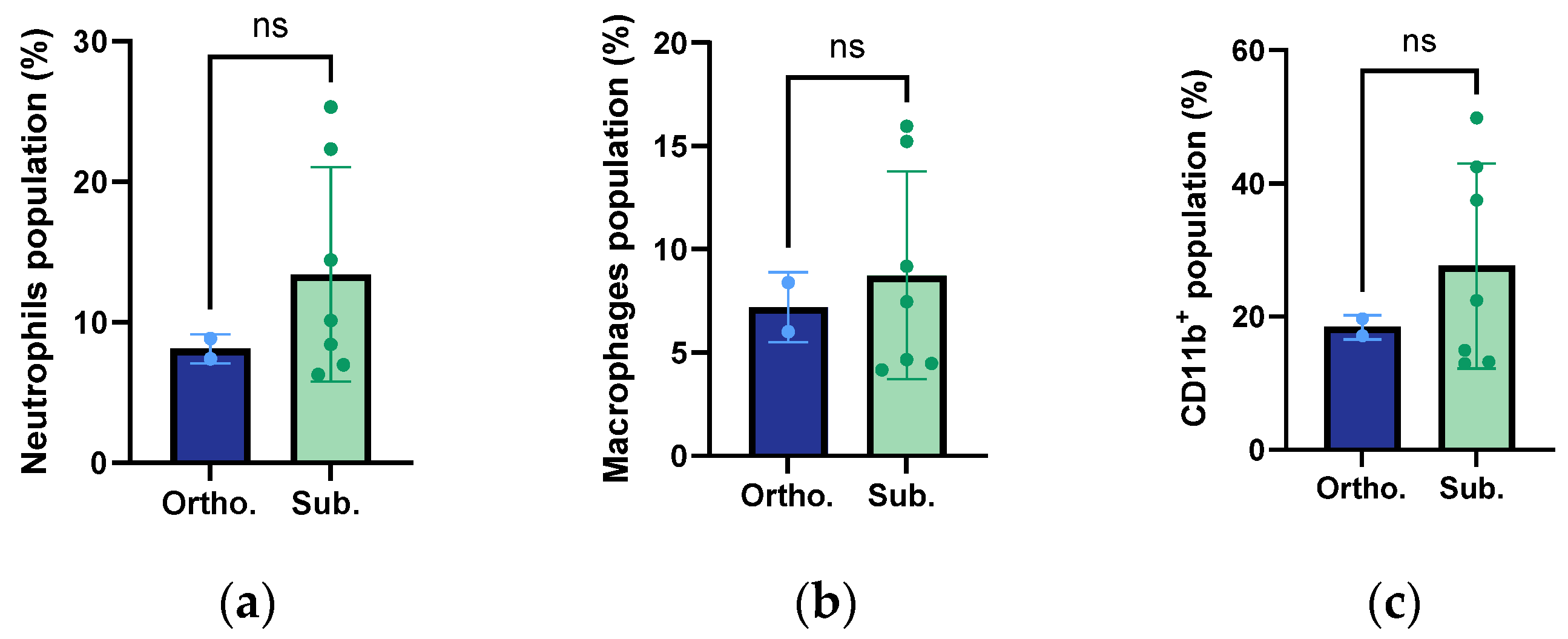
3.8. Infiltration of Macrophages, Neutrophils, and Innate Immune Cells
3.9. Kinetics of Accumulation of 800CW
3.10. Summarised Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kolbeinsson, H.M.; Chandana, S.; Wright, G.P.; Chung, M. Pancreatic Cancer: A Review of Current Treatment and Novel Therapies. J. Investig. Surg. 2023, 36, 2129884. [Google Scholar] [CrossRef]
- Adamska, M.; Elaskalani, A.; Emmanouilidi, O. Molecular and cellular mechanisms of chemoresistance in pancreatic cancer. Adv. Biol. Regul. 2018, 68, 77–87. [Google Scholar] [CrossRef]
- Robin, F.; Angenard, G.; Cano, L.; Courtin-Tanguy, L.; Gaignard, E.; Khene, Z.E.; Bergeat, D.; Clement, B.; Boudjema, K.; Coulouarn, C.; et al. Molecular profiling of stroma highlights stratifin as a novel biomarker of poor prognosis in pancreatic ductal adenocarcinoma. Br. J. Cancer 2020, 123, 72–80. [Google Scholar] [CrossRef]
- Cannon, A.; Thompson, C.; Hall, B.R.; Jain, M.; Kumar, S.; Batra, S.K. Desmoplasia in pancreatic ductal adenocarcinoma: Insight into pathological function and therapeutic potential. Genes. Cancer 2018, 9, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Martin, J.D.; Liu, H.; Lacorre, D.A.; Jain, S.R.; Kozin, S.V.; Stylianopoulos, T.; Mousa, A.S.; Han, X.; Adstamongkonkul, P.; et al. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat. Commun. 2013, 4, 2516. [Google Scholar] [CrossRef]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar]
- Eikenes, L.; Bruland, O.S.; Brekken, C.; Davies Cde, L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004, 64, 4768–4773. [Google Scholar] [CrossRef]
- Wang, L.M.; Silva, M.A.; D’Costa, Z.; Bockelmann, R.; Soonawalla, Z.; Liu, S.; O’Neill, E.; Mukherjee, S.; McKenna, W.G.; Muschel, R.; et al. The prognostic role of desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 4183–4194. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Mortezaee, K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J. Biochem. Mol. Toxicol. 2021, 35, e22708. [Google Scholar] [CrossRef] [PubMed]
- Ramanujan, S.; Pluen, A.; McKee, T.D.; Brown, E.B.; Boucher, Y.; Jain, R.K. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys. J. 2002, 83, 1650–1660. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, G.; Huang, H.; Fu, Z.; Cao, Z.; Zheng, L.; You, L.; Zhang, T. Preclinical models of pancreatic ductal adenocarcinoma: Challenges and opportunities in the era of precision medicine. J. Exp. Clin. Cancer Res. 2021, 40, 8. [Google Scholar] [CrossRef]
- Kondo, J.; Ekawa, T.; Endo, H.; Yamazaki, K.; Tanaka, N.; Kukita, Y.; Okuyama, H.; Okami, J.; Imamura, F.; Ohue, M.; et al. High-throughput screening in colorectal cancer tissue-originated spheroids. Cancer Sci. 2019, 110, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Tiriac, H.; Sridharan, B.P.; Scampavia, L.; Madoux, F.; Seldin, J.; Souza, G.R.; Watson, D.; Tuveson, D.; Spicer, T.P. Advanced Development of Primary Pancreatic Organoid Tumor Models for High-Throughput Phenotypic Drug Screening. SLAS Discov. 2018, 23, 574–584. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, S.R.; Wang, L.; Multani, A.S.; Combs, C.; Deramaudt, T.B.; Hruban, R.H.; Rustgi, A.K.; Chang, S.; Tuveson, D.A. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005, 7, 469–483. [Google Scholar] [CrossRef]
- Lee, J.W.; Komar, C.A.; Bengsch, F.; Graham, K.; Beatty, G.L. Genetically Engineered Mouse Models of Pancreatic Cancer: The KPC Model (LSL-Kras(G12D/+); LSL-Trp53(R172H/+); Pdx-1-Cre), Its Variants, and Their Application in Immuno-oncology Drug Discovery. Curr. Protoc. Pharmacol. 2016, 73, 143911–143920. [Google Scholar] [CrossRef]
- Stribbling, S.M.; Ryan, A.J. The cell-line-derived subcutaneous tumor model in preclinical cancer research. Nat. Protoc. 2022, 17, 2108–2128. [Google Scholar] [CrossRef] [PubMed]
- Hiroshima, Y.; Maawy, A.; Zhang, Y.; Zhang, N.; Murakami, T.; Chishima, T.; Tanaka, K.; Ichikawa, Y.; Bouvet, M.; Endo, I.; et al. Patient-derived mouse models of cancer need to be orthotopic in order to evaluate targeted anti-metastatic therapy. Oncotarget 2016, 7, 71696–71702. [Google Scholar] [CrossRef]
- Saluja, A.K.; Dudeja, V. Relevance of animal models of pancreatic cancer and pancreatitis to human disease. Gastroenterology 2013, 144, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.M. Patient-derived orthotopic xenografts: Better mimic of metastasis than subcutaneous xenografts. Nat. Rev. Cancer 2015, 15, 451–452. [Google Scholar] [CrossRef] [PubMed]
- Jehanno, C.; Vulin, M.; Richina, V.; Richina, F.; Bentires-Alj, M. Phenotypic plasticity during metastatic colonization. Trends Cell Biol. 2022, 32, 854–867. [Google Scholar] [CrossRef]
- Killion, J.J.; Radinsky, R.; Fidler, I.J. Orthotopic Models are Necessary to Predict Therapy of Transplantable Tumors in Mice. Cancer Metastasis Rev. 1998, 17, 279–284. [Google Scholar] [CrossRef]
- Erstad, D.J.; Sojoodi, M.; Taylor, M.S.; Ghoshal, S.; Razavi, A.A.; Graham-O’Regan, K.A.; Bardeesy, N.; Ferrone, C.R.; Lanuti, M.; Caravan, P.; et al. Orthotopic and heterotopic murine models of pancreatic cancer and their different responses to FOLFIRINOX chemotherapy. Dis. Model. Mech. 2018, 11, dmm034793. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.K.; Herner, A.; Neff, F.; Siveke, J.T. Current Methods in Mouse Models of Pancreatic Cancer; Humana Press: New York, NY, USA, 2015. [Google Scholar]
- Haram, M.; Snipstad, S.; Berg, S.; Mjones, P.; Ronne, E.; Lage, J.; Muhlenpfordt, M.; Davies, C.L. Ultrasound and Microbubbles Increase the Uptake of Platinum in Murine Orthotopic Pancreatic Tumors. Ultrasound Med. Biol. 2023, 49, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Erikson, A.; Ortegren, J.; Hompland, T.; de Lange Davies, C.; Lindgren, M. Quantification of the second-order nonlinear susceptibility of collagen I using a laser scanning microscope. J. Biomed. Opt. 2007, 12, 044002. [Google Scholar] [CrossRef]
- Sadeghinia, M.J.; Aguilera, H.M.; Urheim, S.; Persson, R.M.; Ellensen, V.S.; Haaverstad, R.; Holzapfel, G.A.; Skallerud, B.; Prot, V. Mechanical behavior and collagen structure of degenerative mitral valve leaflets and a finite element model of primary mitral regurgitation. Acta Biomater. 2023, 164, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; An, Y.H.; Wu, Y.D.; Song, Y.C.; Chao, Y.J.; Chien, C.H. Microindentation test for assessing the mechanical properties of cartilaginous tissues. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 25–31. [Google Scholar] [CrossRef]
- Haeberle, L.; Esposito, I. Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Valcourt, D.M.; Kapadia, C.H.; Scully, M.A.; Dang, M.N.; Day, E.S. Best Practices for Preclinical In Vivo Testing of Cancer Nanomedicines. Adv. Healthc. Mater. 2020, 9, e2000110. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Su, G.H. Development of orthotopic pancreatic tumor mouse models. Methods Mol. Biol. 2013, 980, 215–223. [Google Scholar]
- Liu, W.; Zhu, Y.; Ye, L.; Zhu, Y.; Wang, Y. Comparison of tumor angiogenesis in subcutaneous and orthotopic LNCaP mouse models using contrast-enhanced ultrasound imaging. Transl. Cancer Res. 2021, 10, 3268–3277. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Hu, J.; Ferguson, M.; Adighibe, O.; Snell, C.; Harris, A.L.; Gatter, K.C.; Pezzella, F. Vessel co-option in primary human tumors and metastases: An obstacle to effective anti-angiogenic treatment? Cancer Med. 2013, 2, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Tuma, R.F.; Durán, W.N.; Ley, K. Handbook of Physiology: Microcirculation, 2nd ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Amsterdam, The Netherlands, 2008; pp. 113–137. [Google Scholar]
- Guo, S.; Deng, C.X. Effect of Stromal Cells in Tumor Microenvironment on Metastasis Initiation. Int. J. Biol. Sci. 2018, 14, 2083–2093. [Google Scholar] [CrossRef]
- Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. The role of collagen in cancer: From bench to bedside. J. Transl. Med. 2019, 17, 309. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Molecular pathways: Connecting fibrosis and solid tumor metastasis. Clin. Cancer Res. 2014, 20, 3637–3643. [Google Scholar] [CrossRef]
- Olive, K.P.; Jacobetz, M.A.; Davidson, C.J.; Gopinathan, A.; McIntyre, D.; Honess, D.; Madhu, B.; Goldgraben, M.A.; Caldwell, M.E.; Allard, D.; et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009, 324, 1457–1461. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Martin, J.D.; Chauhan, V.P.; Jain, S.R.; Diop-Frimpong, B.; Bardeesy, N.; Smith, B.L.; Ferrone, C.R.; Hornicek, F.J.; Boucher, Y.; et al. Causes, consequences, and remedies for growth-induced solid stress in murine and human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 15101–15108. [Google Scholar] [CrossRef] [PubMed]
- Levental, K.R.; Yu, H.; Kass, L.; Lakins, J.N.; Egeblad, M.; Erler, J.T.; Fong, S.F.; Csiszar, K.; Giaccia, A.; Weninger, W.; et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 2009, 139, 891–906. [Google Scholar] [CrossRef]
- Hu, J.; Guo, J.; Pei, Y.; Hu, P.; Li, M.; Sack, I.; Li, W. Rectal Tumor Stiffness Quantified by In Vivo Tomoelastography and Collagen Content Estimated by Histopathology Predict Tumor Aggressiveness. Front. Oncol. 2021, 11, 701336. [Google Scholar] [CrossRef] [PubMed]
- Tjomsland, V.; Niklasson, L.; Sandstrom, P.; Borch, K.; Druid, H.; Bratthall, C.; Messmer, D.; Larsson, M.; Spangeus, A. The desmoplastic stroma plays an essential role in the accumulation and modulation of infiltrated immune cells in pancreatic adenocarcinoma. Clin. Dev. Immunol. 2011, 2011, 212810. [Google Scholar] [CrossRef]
- Leddy, H.A.; Haider, M.A.; Guilak, F. Diffusional anisotropy in collagenous tissues: Fluorescence imaging of continuous point photobleaching. Biophys. J. 2006, 91, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, D.; Ban, E.; Toussaint, K.C.; Janmey, P.A.; Wells, R.G.; Shenoy, V.B. Glycosaminoglycans modulate long-range mechanical communication between cells in collagen networks. Proc. Natl. Acad. Sci. USA 2022, 119, e2116718119. [Google Scholar] [CrossRef] [PubMed]
- Kerbel, R.S. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: Better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2003, 2, S134–S139. [Google Scholar] [CrossRef] [PubMed]



| Metastatic and Infiltration Events | Orthotopic Tumours | Subcutaneous Tumours |
|---|---|---|
| Infiltration | ✓ (Adjacent tissue) | ✓ (Skeletal muscle) |
| Metastasis in the spleen | ✓ | - |
| Metastasis in the intestines | ✓ | - |
| Metastasis in the kidneys | ✓ | - |
| Metastasis in the liver | ✓ | - |
| CEUS Parameters | Orthotopic Tumours | Subcutaneous Tumours | p-Values |
|---|---|---|---|
| PE (a.u) | 15.3 ±10.4 | 40.2 ± 18.7 | 0.050 |
| WiAUC (a.u) | 126.2 ± 103.8 | 650.0 ± 358.5 | 0.027 |
| RT (s) | 10.9 ± 2.3 | 21.5 ± 5.5 | 0.009 |
| Tumour area (mm2) | 74.7 ± 18.3 | 50.3 ± 7.9 | 0.029 |
| Characterisation | Orthotopic Tumours | Subcutaneous Tumours |
|---|---|---|
| Histopathological characterisation | Focal necrotic regions Glandular structures Infiltration of pancreatic tissue | Extended necrotic regions |
| Tumour growth and metastases | Larger volumes Higher growth rate Infiltration into healthy pancreatic tissue Metastasis to the spleen, intestines, kidneys, and liver. | Infiltration into skeletal muscle |
| Collagen characterisation | Lower amount of collagen Less aligned collagen fibres | Higher amount of collagen More aligned collagen fibres |
| Biomechanical characterisation | Less stiff tumours (Lower Young’s modulus) | Stiffer tumours (Higher Young’s modulus) |
| Functional vasculature | Trend—less functional microvasculature | Trend—more functional microvasculature |
| Perfusion Rise time | Smaller overall vascular volume Lower | Larger vascular volume Higher |
| Immune cells | Similar levels of neutrophils and macrophages | Similar levels of neutrophils and macrophages |
| Accumulation of 800CW | Lower and slower accumulation | Higher and faster accumulation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez, J.L.; Årbogen, S.; Sadeghinia, M.J.; Haram, M.; Snipstad, S.; Torp, S.H.; Einen, C.; Mühlenpfordt, M.; Maardalen, M.; Vikedal, K.; et al. A Comparative Analysis of Orthotopic and Subcutaneous Pancreatic Tumour Models: Tumour Microenvironment and Drug Delivery. Cancers 2023, 15, 5415. https://doi.org/10.3390/cancers15225415
Fernandez JL, Årbogen S, Sadeghinia MJ, Haram M, Snipstad S, Torp SH, Einen C, Mühlenpfordt M, Maardalen M, Vikedal K, et al. A Comparative Analysis of Orthotopic and Subcutaneous Pancreatic Tumour Models: Tumour Microenvironment and Drug Delivery. Cancers. 2023; 15(22):5415. https://doi.org/10.3390/cancers15225415
Chicago/Turabian StyleFernandez, Jessica Lage, Sara Årbogen, Mohammad Javad Sadeghinia, Margrete Haram, Sofie Snipstad, Sverre Helge Torp, Caroline Einen, Melina Mühlenpfordt, Matilde Maardalen, Krister Vikedal, and et al. 2023. "A Comparative Analysis of Orthotopic and Subcutaneous Pancreatic Tumour Models: Tumour Microenvironment and Drug Delivery" Cancers 15, no. 22: 5415. https://doi.org/10.3390/cancers15225415
APA StyleFernandez, J. L., Årbogen, S., Sadeghinia, M. J., Haram, M., Snipstad, S., Torp, S. H., Einen, C., Mühlenpfordt, M., Maardalen, M., Vikedal, K., & Davies, C. d. L. (2023). A Comparative Analysis of Orthotopic and Subcutaneous Pancreatic Tumour Models: Tumour Microenvironment and Drug Delivery. Cancers, 15(22), 5415. https://doi.org/10.3390/cancers15225415







