Simple Summary
Often in endometrial cancer (EC), the mismatch repair (MMR) system, which helps fix DNA mistakes, goes haywire because of MLH1 promoter hypermethylation (MLH1-PHM). We set out to find out how many ECs have MLH1-PHM, understand the impact of reflex MLH1-PHM testing and evaluate the associated costs within the publicly funded Canadian healthcare system. We looked at 2504 EC samples and found that in 534 of them (21.4%), the MMR system was deficient due to dual MLH1/PMS2-deficiency. Out of the 418 cases with available data, 404 (96.7%) had MLH1-PHM, while only 14 (3.3%) didn’t. Reflex MLH1-PHM tests cost CAD 231.90 per case, amounting to CAD 123,834.60 for 534 cases, with 30 tests needed per additional candidate for MLH1 germline analysis (CAD 6,957.00 per candidate). This raises a provocative question: can we assume that the majority of the MLH1-deficient ECs are due to PHM and forgo further testing in healthcare systems with finite resources?
Abstract
MLH1/PMS2 loss due to MLH1 promoter hypermethylation (MLH1-PHM) is the most common cause of mismatch repair (MMR) deficiency in endometrial cancer (EC). This study aimed to determine the proportion of MLH1-deficient EC with PHM, assess the impact of the reflex MLH1-PHM testing strategy, and evaluate the associated costs within the publicly funded Canadian healthcare system. In a cohort of 2504 EC samples, 534 (21.4%) exhibited dual MLH1/PMS2 loss, prompting MLH1-PHM testing. Among 418 cases with available testing results, 404 (96.7%) were MLH1-hypermethylated, while 14 (3.3%) were non-methylated. The incidence of MLH1 non-methylated cases in our cohort was 14/2504 (0.56%) of all ECs, underscoring the prevalence of hypermethylation-driven MLH1/PMS2 loss in ECs universally screened for MMR deficiency. Reflex MLH1-PHM testing incurs substantial costs and resource utilization. Assay cost is CAD 231.90 per case, amounting to CAD 123,834.60 for 534 cases, with 30 tests needed per additional candidate for MLH1 germline analysis (CAD 6957.00 per candidate). This raises a provocative question: can we assume that the majority of the MLH1-deficient ECs are due to PHM and forgo further testing in healthcare systems with finite resources? It is imperative to assess resource utilization efficiency and explore optimized approaches that encompass clinical correlation, family history and judicious utilization of methylation testing to ensure it is provided only to those who stand to benefit from it.
1. Introduction
Endometrial cancer (EC) is the second most prevalent gynecologic cancer worldwide, with an annual increase in morbidity of 0.6% and mortality of 2% [1,2]. While the majority of cases are sporadic, a subset of women, estimated at 2–5%, harbor an inherited DNA mismatch repair (MMR) mutation, resulting in Lynch syndrome (LS)-associated EC [3,4]. Loss-of-function pathogenic variants in one of the four MMR genes (MLH1, MSH2, MSH6, or PMS2), and occasionally the related gene EPCAM, result in the accumulation of single-nucleotide variants, insertions, and deletions, particularly in microsatellites, the repetitive regions of DNA [5]. Tumors with this phenotype are termed microsatellite unstable (MSI) or MMR-deficient (MMR-d).
As classification of EC transitions from histologic-based to molecular-based risk stratification, determining MMR/MSI status has a role beyond the identification of LS [6]. Accordingly, it has a fundamental role in the molecular classification of EC tumors, as defined by The Cancer Genome Atlas (TCGA), including POLE ultramutated, MMR-d, copy number high (p53 abnormal) and copy number low (no specific molecular profile) [7]. Molecular EC classification improves disease prognostication and provides predictive information that guides personalized therapy [8,9]. POLE ultramutated ECs have a favorable prognosis and may not require adjuvant treatment, while MMR-d and copy number low ECs have an intermediate prognosis and copy number high ECs experience the least favorable prognosis [10]. Notably, MMR-d and copy number low tumors express high levels of progesterone receptors, suggesting potential responsiveness to fertility-preserving hormonal therapy, which is particularly important for the quality of life and future prospects of young EC patients [11,12]. Recent analyses of prospective clinical trials showed no significant benefit from standard chemotherapy and persistent risk of recurrence following brachytherapy in MMR-d EC [8]. Moreover, MMR-d ECs have been shown to derive benefit from the addition of immunotherapies [13,14]. Current evidence shows no difference in risk stratification or treatment allocation between MMR-d caused by LS or sporadic promoter methylation, although such differences may emerge in the future when analyses of ongoing clinical trials become available [15].
Due to its dual purpose in LS screening and molecular classification, universal screening of ECs for MMR-d is recommended by NCCN guidelines and has become the standard-of-care in many jurisdictions, including Canada [16,17,18,19]. This is commonly achieved through the utilization of immunohistochemistry (IHC) for the detection of MMR protein status [18,20]. Notably, while only a small fraction (2–5%) of ECs are associated with LS, a cancer predisposition syndrome, a considerably larger proportion, approximately 30%, of universally screened tumors exhibit MMR-d [20]. While IHC loss of MSH2, MSH6, or isolated loss of PMS2 is highly associated with LS and points to a specific MMR gene for subsequent genomic sequencing, MLH1 loss is largely secondary to sporadic MLH1 CpG island promoter hypermethylation (MLH1-PHM), causing transcriptional silencing [21]. Recently, rare cases of LS-like MLH1-deficient (MLH1-d) cancers associated with high-risk constitutional MLH1 methylation (epimutation) have been described, with a prevalence of <1% of MLH1-d methylated tumors [22]. Therefore, in the context of LS screening, it has been recommended that if MLH1 protein loss is noted on IHC, MLH1-PHM testing is reflexively performed to differentiate between sporadic and hereditary MLH1 loss [6,23]. Germline MLH1 defects are rare in EC and the likelihood of a concurrent germline mutation and hypermethylation is generally thought to be relatively low [24]. Therefore, the presence of MLH1-PHM is considered a negative predictor of LS, limiting the need for consultations in cancer genetic clinics and germline analysis to exclude LS. As MLH1-d is detected in over 20% of unselected EC samples, reflex MLH1-PHM testing generates substantial costs and burdens on pathology laboratories, partly due to limited assay availability [24].
This study was designed to critically evaluate reflex MLH1-PHM testing strategy’s impact, determine the proportion of MLH1-d EC with PHM and assess the associated costs within the publicly funded Canadian healthcare system. We are raising a provocative question: can we assume that the majority of the MLH1-d ECs are due to PHM and forgo further testing in healthcare systems with finite resources? The data could be used to explore opportunities for process improvement, aiming to optimize its effectiveness in identifying individuals with LS, and constitutional hypermethylation syndromes versus those with sporadic MLH1 loss.
2. Materials and Methods
2.1. Study Population
This retrospective study was approved by the Sunnybrook Health Sciences Centre research ethics board (SUN-5582) and written informed consent was waived. The study included a universally screened population of patients diagnosed with EC at a tertiary-care hospital (Sunnybrook Health Sciences, June 2017–July 2022, n = 1295) and a major community laboratory in Ontario, Canada (LifeLabs, January 2018–December 2021, n = 1215). A query of the Sunnybrook and LifeLabs Pathology Department laboratory information system was conducted by searching for all EC specimens tested by IHC for MMR protein (MLH1, MSH2, MSH6, and PMS2) expression status followed by an MLH1-PHM test when appropriate. Pathology reports for 2510 EC specimens (including curettages, biopsies and hysterectomies), unselected for age, were retrieved. The following information was extracted from the pathology reports for all patients: age at diagnosis, date of primary diagnosis, histological classification and tumor grade, results of MMR IHC, ER-IHC, p53-IHC and POLE-mutation status (when available). For patients who had absent MLH1 expression, MLH1-PHM results were accessed and recorded.
2.2. Universal Tumor Screening Protocol
According to the universal LS screening protocol at the two laboratories, each EC is tested at the time of first available specimen using IHC to detect the expression of the four MMR proteins: MLH1, MSH2, MSH6 or PMS2. If loss of MLH1 is detected, reflex MLH1-PHM testing is performed. The presence of MLH1-PHM indicates likely non-inherited (sporadic) tumor development and germline genetic testing for LS is not indicated. Individuals with detected MLH1 IHC loss and absence of MLH1-PHM were eligible for germline genetic testing and genetic counselling. Consultations at the cancer genetic clinic were recorded, when available, for MLH1-d without PMH cases at Sunnybrook Health Sciences Centre.
2.3. Statistical Analysis
Statistical analysis was performed in SPSS 24.0 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were summarized by percentages for categorical variables and by mean/median and range for continuous variables.
2.4. Cost Analysis
In this study, MLH1-PHM assay was not available on sites and cases were tested at a central laboratory. Incremental burden included block retrieval, office handling for send out, transportation to an outside molecular pathology laboratory, molecular report uploading to the electronic medical record system, follow-up by pathologist, addendum to the original pathology report and follow-up by oncologist. When PHM is not detected, referral to the cancer genetic clinic was suggested.
3. Results
3.1. Cohort of Universally Screened Endometrial Carcinomas
The study cohort consisted of 2510 consecutive EC samples tested using MMR IHC, classified as MMR proficient (MMRp, n = 1844, 73.6%) and MMR-d (n = 660, 26.4%, Figure 1). In six cases, MMR protein status could not be determined, and these were excluded from further analysis. Among the MMR-d cases, MLH1/PMS2 loss was detected in 78.9% (521/660) of cases, dual loss of MLH1/PMS2 and MSH6 in 1.2% (8/660) and dual loss of MLH1/PMS2 and MSH2/MSH6 in 0.8% (5/660) of cases. Furthermore, MSH6 loss was identified in 9.1% (60/660) of cases, MSH2/MSH6 loss in 6.7% (44/660) of cases, isolated PMS2 loss in 3.0% (20/660) of cases and MSH6 and PMS2 loss in 0.3% (2/660) of cases.
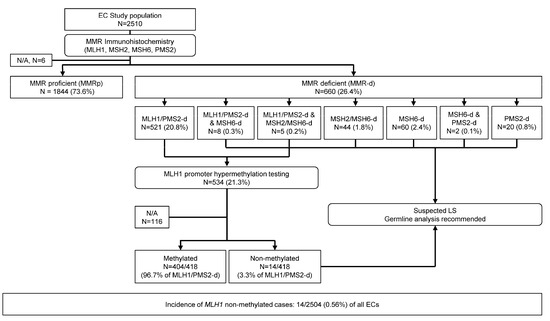
Figure 1.
Study Cohort diagram. This study cohort consisted of 2510 consecutive endometrial cancer samples tested by MMR IHC, classified as MMR-proficient and MMR-deficient. MLH1/PMS2-deficient endometrial cancers underwent MLH1 hypermethylation testing, classified as methylated and not methylated. Patients with loss of MSH2 and/or MSH6, isolated loss of PMS2 and non-methylated MLH1-deficient endometrial cancers were recommended to pursue germline testing for Lynch syndrome.
3.2. MLH1 Promoter Hypermethylation Testing Results
MLH1 (and PMS2) loss was detected in 534 cases (21.3%) that were candidates for reflex MLH1-PHM analysis. Testing could not be completed due to insufficient material in two cases. Further, testing results were inconclusive in three cases, could not be determined in five cases and were not available in 105 cases. Among these, 44 cases from a major community laboratory were not sent for MLH1-PHM testing as it was not routinely performed prior to June 2020. Additionally, 21 tertiary-care hospital cases were consults and their materials were returned following MMR IHC. In 18 MLH1-PHM testing was not conducted, but LS testing was undertaken. For 15 cases, MLH1-PHM testing was not ordered, while in four cases, it was ordered, but the addendum pathology report was unavailable. In two cases, material was sent to another institution for a clinical trial following MMR IHC, and in one case, MLH1-PHM testing was not ordered due to the patient’s age (85). These were excluded from further analysis (Figure 1). Of 418 cases with available testing results, in 404 (96.7%) MLH1-PHM was detected and in 14 (3.3%) it was not. Therefore, the incidence of MLH1 non-methylated cases in our cohort was 14/2504 (0.56%) of all ECs.
3.3. Clinicopathologic Features of MLH1-Methylated and Non-Methylated Endometrial Carcinomas
Clinicopathologic features from all 418 cases with available MLH1-PHM testing results are summarized in Table 1. The median age of the 404 patients with confirmed MLH1 hypermethylation was 64 years (range, 39–94). The median age of the 13 non-methylated cases was significantly younger at 56 years (range, 37–71), p = 0.018.

Table 1.
MLH1-deficient cases with available MLH1 promoter methylation analysis results.
MLH1 methylated cases consisted predominantly of endometrioid tumors (323/404, 80.0%), followed by high-grade ECs (16/404, 4.0%), mixed histology ECs (11/404, 2.7%), dedifferentiated/undifferentiated carcinomas (7/404, 1.7%), carcinosarcomas (6/404, 1.5%), mucinous (3/404, 0.7%) and clear cell (2/404, 0.5%).
Non-methylated cases were also primarily endometrioid tumors (12/14, 85.7%), one (7.1%) dedifferentiated/undifferentiated carcinoma and one (7.1%) serous carcinoma. Statistical analysis revealed no significant differences, although its power was limited by the low number of non-methylated cases. MLH1 mutational analysis was available for three non-methylated cases, all found to harbor a germline mutation of MLH1. Clinical follow-up data as well as any personal or family cancer history data were not available for our cohort.
3.4. Cost Analysis of Reflex MLH1 Methylation Testing
The diagnostic process for MLH1-d cases in our cohort involved reflex MLH1-PHM analysis in 534 cases (21.3% of cohort). This diagnostic algorithm incites a process utilizing time, labor, and resources of the pathology laboratory.
Cost analysis estimated the expense of office handling, transportation, MLH1-PHM testing and molecular reporting to be CAD 231.90 per case, resulting in a total expenditure of CAD 123,834.60 for the 534 cases. Analysis of our cohort revealed that 30 cases need to be tested for MLH1-PHM for each additional candidate for MLH1 germline analysis, resulting in a cost of CAD 6957.00 per candidate.
4. Discussion
In this study, we analyzed 2504 EC samples tested with MMR IHC and identified 660 MMR protein deficient cases, with 534 of those showing MLH1/PMS2 loss (21.3%). Cost analysis estimated the expense of MLH1-PHM testing to be CAD 231.90 per case, totaling CAD 123,834.60 for the 534 cases, along with significant time and labor burden for the pathology laboratory. MLH1 non-methylated cases comprised 3.3% (14/418) of MLH1/PMS2-d cases and 0.56% (14/2504) of all cases; this was consistent with prior studies indicating DNA methylation accounts for the vast majority of MLH1/PMS2-d ECs [24]. MMR testing in EC is essential for molecular subtype classification and for LS screening. However, for molecular classification and selection of immunotherapy, current guidelines do not distinguish between MLH1-d with or without PHM; although, some future and ongoing clinical trials are planned to have a subgroup analysis to study its potential effect on response to treatment [15].
Given the rarity of germline MLH1 defects in EC, a careful scrutiny of reflexive MLH1-PHM testing in all MLH1/PMS2-d EC cases is warranted. The current practice entails methylation testing in a substantial number (20–30%) of ECs, leading to notable financial and resource implications [25]. However, the benefits of methylation testing are applicable to a limited number of EC patients, highlighting the importance of assessing resource utilization efficiency and exploring strategies to enhance the cost-effectiveness of MLH1-PHM testing. The identification of even a single MLH1-associated LS EC holds great importance, as it facilitates early detection, prevention of other cancer types and extends benefits to family members. Therefore, an optimized approach should be adopted incorporating clinical correlation, family history and judicious consideration of methylation testing to ensure its provision exclusively to those who stand to derive benefit from it.
An additional crucial aspect of current practice that requires scrutiny is how clinicians navigate and analyze MLH1-PHM test results. By conducting reflexive MLH1-PHM testing on all MLH1-d ECs, a substantial number of cases with MLH1 hypermethylation can be identified. Consequently, given the prevalence of promoter hypermethylation in MLH1-d cases, clinicians may develop an implicitly overlearned association between MLH1-d and MLH1 promoter methylation, leading them to assume MLH1-d is a sporadic loss [26]. Further, clinicians can initiate treatment without waiting for MLH1-PHM results since pembrolizumab has been approved for the treatment of any MMR-d solid tumor, and dostarlimab for MMR-d ECs that have progressed on or after prior therapy, irrespective of the underlying cause being germline or sporadic [27,28,29].
The current MLH1-PHM testing approach relies on MLH1-hypermethylation as a “hard-stop” in the LS work-up, which can obscure underlying PMS2 mutations and/or MLH1 epimutations [22,30]. PMS2 pathogenic variants underlie a distinct subset of LS-associated ECs that can be masked by MLH1 hypermethylation due to the current LS screening approach that associates the simultaneous loss of MLH1 and PMS2 with MLH1-d and reflex testing for MLH1-PHM [30]. Additionally, the MLH1-PHM testing approach overlooks the significance of MLH1 epimutations, an alternative mechanism for MLH1-d in EC [22,31]. Constitutional MLH1 epimutation carriers have a predisposition to an early-onset and/or multiple cancers resembling the MLH1-d LS-associated EC phenotype. The exact prevalence of constitutional MLH1 epimutation in MMR-d ECs is uncertain. Previous studies, primarily focused on colorectal cancer, reported a prevalence of 3–9% among patients meeting the revised Bethesda Guidelines for MSI testing with an MLH1-d tumor, and negative germline genetic test [22,32]. Including tumor MLH1 methylation as part of the selection criteria slightly increased these frequencies to 3.5–15.6% [22]. A single study of EC reported a frequency of 0.49% (1/206) for constitutional MLH1 methylation in a hospital-based unselected, consecutive series of EC cases in Japan [33,34]. This underscores the need for the incorporation of clinical correlation and the consideration of germline testing or MLH1-PHM testing if the clinical scenario is sufficiently concerning. This prompts further a questioning of the existing threshold for overlooking PMS2 mutations and MLH1 epimutations while performing MLH1-PHM testing on all MLH1-d ECs to ensure accurate detection of LS-associated EC as well as the judicious use of germline and MLH1-PHM testing. Another option is to explore a less technically demanding and more cost-effective assay than MLH1-PHM testing, such as EMP2AIP1 IHC, which exhibits high concordance with MLH1-PHM (95% concordance, 94.5% sensitivity, 98.1% positive predictive value), showing promise as a surrogate marker [35].
Another aspect to consider when EC premenopausal patients are triaged out of LS screening based on MLH1-PHM or not tested for MLH1 epimutations are the implications on fertility and ovarian preservation approaches to therapy. According to the NCCN guidelines ovarian preservation can be considered in selected low risk EC patients without LS [11]. Of the 534 patients with MLH1-d tumors in our audit, there were three aged 34–39 and sixteen aged 40–49 to whom this consideration would apply.
The study’s limitations lie in its retrospective design and being confined to two institutions. Nevertheless, our findings provide valuable insights into real-world outcomes of MLH1 PHM testing, aiding the identification of an optimal screening strategy for clinical practice.
5. Conclusions
This study analyzed a large series of universally screened ECs and found that MLH1/PMS2 loss due to hypermethylation is the predominant cause of MMR-d. Therefore, the cost and significant resource implications of reflexive MLH1-PHM testing in all MLH1/PMS2-d ECs need to be carefully evaluated. Clinicians should consider the limited benefits of methylation testing in the majority of MLH1/PMS2-d ECs and consider adopting an optimized approach that incorporates clinical correlation, family history and judicious use of methylation testing to ensure it is provided only to those who would benefit.
Author Contributions
Conceptualization, A.P. and S.N.-M.; methodology, A.P., E.O.-M. and S.N.-M.; formal analysis, A.P., E.O.-M. and S.N.-M.; data curation, A.P., E.O.-M. and S.N.-M.; writing—original draft preparation, E.O.-M.; writing—review and editing, A.P., E.O.-M. and S.N.-M.; supervision, A.P. and S.N.-M.; project administration, E.O.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Research Ethics Board of Sunnybrook Health Sciences Centre (SUN-5582) approved 31 October 2022.
Informed Consent Statement
Patient consent was waived because this research study satisfied all the conditions in Chapter 5, “Privacy and Confidentiality, D. Consent and Secondary Use of Information for Research Purposes”, Article 5.5A (a) to (f) of the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. (http://pre.ethics.gc.ca/eng/policy-politique_tcps2-eptc2_2018.html, accessed on 5 October 2022).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, A.P.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Thompson, E.F.; Leung, S.; Chiu, D.; Lum, A.; McConechy, M.; Grondin, K.; Aguirre-Hernandez, R.; Salvador, S.; et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol. Oncol. 2022, 165, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Post, C.C.B.; Stelloo, E.; Smit, V.; Ruano, D.; Tops, C.M.; Vermij, L.; Rutten, T.A.; Jurgenliemk-Schulz, I.M.; Lutgens, L.; Jobsen, J.J.; et al. Prevalence and Prognosis of Lynch Syndrome and Sporadic Mismatch Repair Deficiency in Endometrial Cancer. J. Natl. Cancer Inst. 2021, 113, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Broaddus, R.R.; Lu, K.H. Endometrial cancer and Lynch syndrome: Clinical and pathologic considerations. Cancer Control 2009, 16, 14–22. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Consortium, R.R. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, I.; D’Oria, O.; Sgamba, L.; De Angelis, E.; Golia D’Augè, T.; Turetta, C.; Di Dio, C.; Scudo, M.; Bogani, G.; Di Donato, V.; et al. Role of Genomic and Molecular Biology in the Modulation of the Treatment of Endometrial Cancer: Narrative Review and Perspectives. Healthcare 2023, 11, 571. [Google Scholar] [CrossRef]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur. J. Contracept. Reprod. Health Care 2022, 27, 335–340. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef]
- Bellone, S.; Roque, D.M.; Siegel, E.R.; Buza, N.; Hui, P.; Bonazzoli, E.; Guglielmi, A.; Zammataro, L.; Nagarkatti, N.; Zaidi, S.; et al. A phase II evaluation of pembrolizumab in recurrent microsatellite instability-high (MSI-H) endometrial cancer patients with Lynch-like versus MLH-1 methylated characteristics (NCT02899793). Ann. Oncol. 2021, 32, 1045–1046. [Google Scholar] [CrossRef]
- Buchanan, D.D.; Tan, Y.Y.; Walsh, M.D.; Clendenning, M.; Metcalf, A.M.; Ferguson, K.; Arnold, S.T.; Thompson, B.A.; Lose, F.A.; Parsons, M.T.; et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J. Clin. Oncol. 2014, 32, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Batte, B.A.; Bruegl, A.S.; Daniels, M.S.; Ring, K.L.; Dempsey, K.M.; Djordjevic, B.; Luthra, R.; Fellman, B.M.; Lu, K.H.; Broaddus, R.R. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecol. Oncol. 2014, 134, 319–325. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Favier, A.; Varinot, J.; Uzan, C.; Duval, A.; Brocheriou, I.; Canlorbe, G. The Role of Immunohistochemistry Markers in Endometrial Cancer with Mismatch Repair Deficiency: A Systematic Review. Cancers 2022, 14, 3783. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, P.J.; Billingsley, C.C.; Lankes, H.A.; Ali, S.; Cohn, D.E.; Broaddus, R.J.; Ramirez, N.; Pritchard, C.C.; Hampel, H.; Chassen, A.S.; et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 4301–4308. [Google Scholar] [CrossRef]
- Hitchins, M.P.; Alvarez, R.; Zhou, L.; Aguirre, F.; Damaso, E.; Pineda, M.; Capella, G.; Wong, J.J.; Yuan, X.; Ryan, S.R.; et al. MLH1-methylated endometrial cancer under 60 years of age as the “sentinel” cancer in female carriers of high-risk constitutional MLH1 epimutation. Gynecol. Oncol. 2023, 171, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Richer, L.; Arseneau, J.; Zeng, X.; Chong, G.; Weber, E.; Foulkes, W.; Palma, L. Mismatch Repair Universal Screening of Endometrial Cancers (MUSE) in a Canadian Cohort. Curr. Oncol. 2021, 28, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Kurpiel, B.; Thomas, M.S.; Mubeen, M.; Ring, K.L.; Modesitt, S.C.; Moskaluk, C.A.; Mills, A.M. MLH1/PMS2-deficient Endometrial Carcinomas in a Universally Screened Population: MLH1 Hypermethylation and Germline Mutation Status. Int. J. Gynecol. Pathol. 2022, 41, 1–11. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Kaikkonen, E.; Olkinuora, A.; Pylvanainen, K.; Alhopuro, P.; Peltomaki, P.; Mecklin, J.P.; Butzow, R. Testing for Lynch Syndrome in Endometrial Carcinoma: From Universal to Age-Selective MLH1 Methylation Analysis. Cancers 2022, 14, 1348. [Google Scholar] [CrossRef]
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. In Improving Diagnosis in Health Care; Balogh, E.P., Miller, B.T., Ball, J.R., Eds.; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouelian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Vale, N. Dostarlimab: A Review. Biomolecules 2022, 12, 1031. [Google Scholar] [CrossRef]
- Mills, A.M.; Longacre, T.A. Lynch Syndrome Screening in the Gynecologic Tract: Current State of the Art. Am. J. Surg. Pathol. 2016, 40, e35–e44. [Google Scholar] [CrossRef]
- Zyla, R.; Graham, T.; Aronson, M.; Velsher, L.; Mrkonjic, M.; Turashvili, G. MLH1 epimutation is a rare mechanism for Lynch syndrome: A case report and review of the literature. Genes Chromosomes Cancer 2021, 60, 635–639. [Google Scholar] [CrossRef]
- Hitchins, M.P. Finding the needle in a haystack: Identification of cases of Lynch syndrome with MLH1 epimutation. Fam. Cancer 2016, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Pinto, C.; Guerra, J.; Pinheiro, M.; Santos, R.; Vedeld, H.M.; Yohannes, Z.; Peixoto, A.; Santos, C.; Pinto, P.; et al. Contribution of MLH1 constitutional methylation for Lynch syndrome diagnosis in patients with tumor MLH1 downregulation. Cancer Med. 2018, 7, 433–444. [Google Scholar] [CrossRef]
- Takeda, T.; Banno, K.; Yanokura, M.; Adachi, M.; Iijima, M.; Kunitomi, H.; Nakamura, K.; Iida, M.; Nogami, Y.; Umene, K.; et al. Methylation Analysis of DNA Mismatch Repair Genes Using DNA Derived from the Peripheral Blood of Patients with Endometrial Cancer: Epimutation in Endometrial Carcinogenesis. Genes 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Mrkonjic, M.; Turashvili, G. EPM2AIP1 Immunohistochemistry Can Be Used as Surrogate Testing for MLH1 Promoter Methylation in Endometrial Cancer. Am. J. Surg. Pathol. 2022, 46, 376–382. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).