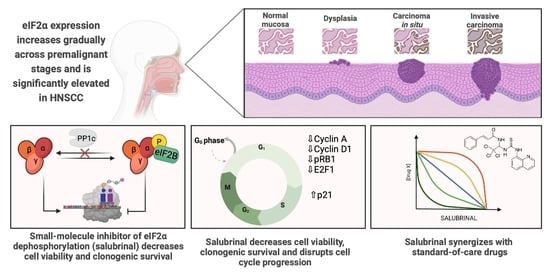
Inhibition of EIF2α Dephosphorylation Decreases Cell Viability and Synergizes with Standard-of-Care Chemotherapeutics in Head and Neck Squamous Cell Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Samples and Ethics Statement
2.2. Bioinformatic Analysis of EIF2S1 and EIF2α Expression
2.3. Immunohistochemistry (IHC)
2.4. Immunoblotting
2.5. Cell Culture and Preparation of Stock Solutions
2.6. Viability Testing and Calculation of Drug Synergies
2.7. Colony Formation Assay
2.8. Cell Death and Cell Cycle Analysis with Flow Cytometry
2.9. Chemosensitivity Testing of HNSCC Patient-Derived 3D Tumor Spheres
2.10. Statistical Analysis
3. Results
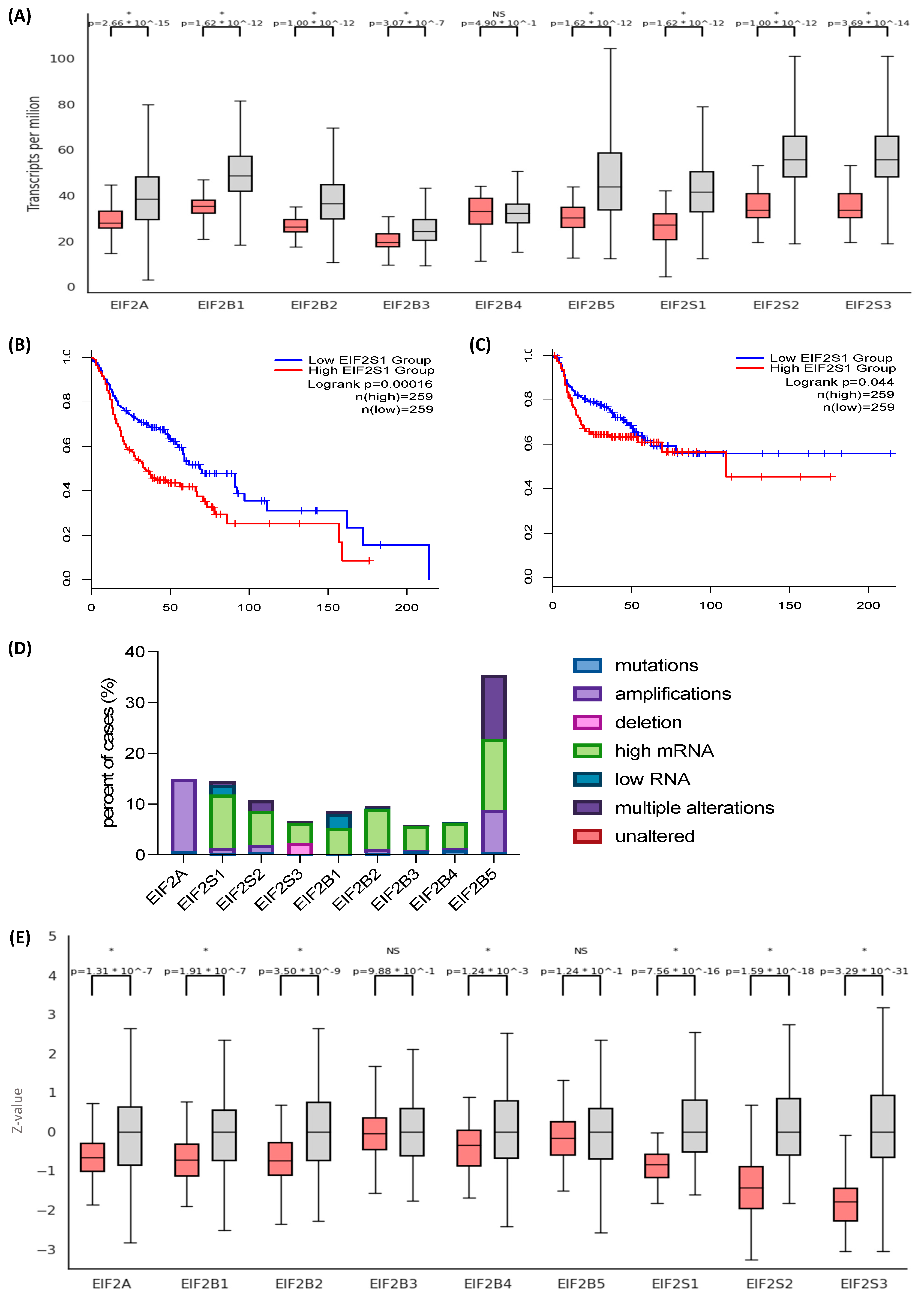
3.1. The Eukaryotic Initiation Factor 2 (EIF2) Genes and Their Protein Products Are Overexpressed in Head and Neck Squamous Cell Carcinoma (HNSCC)
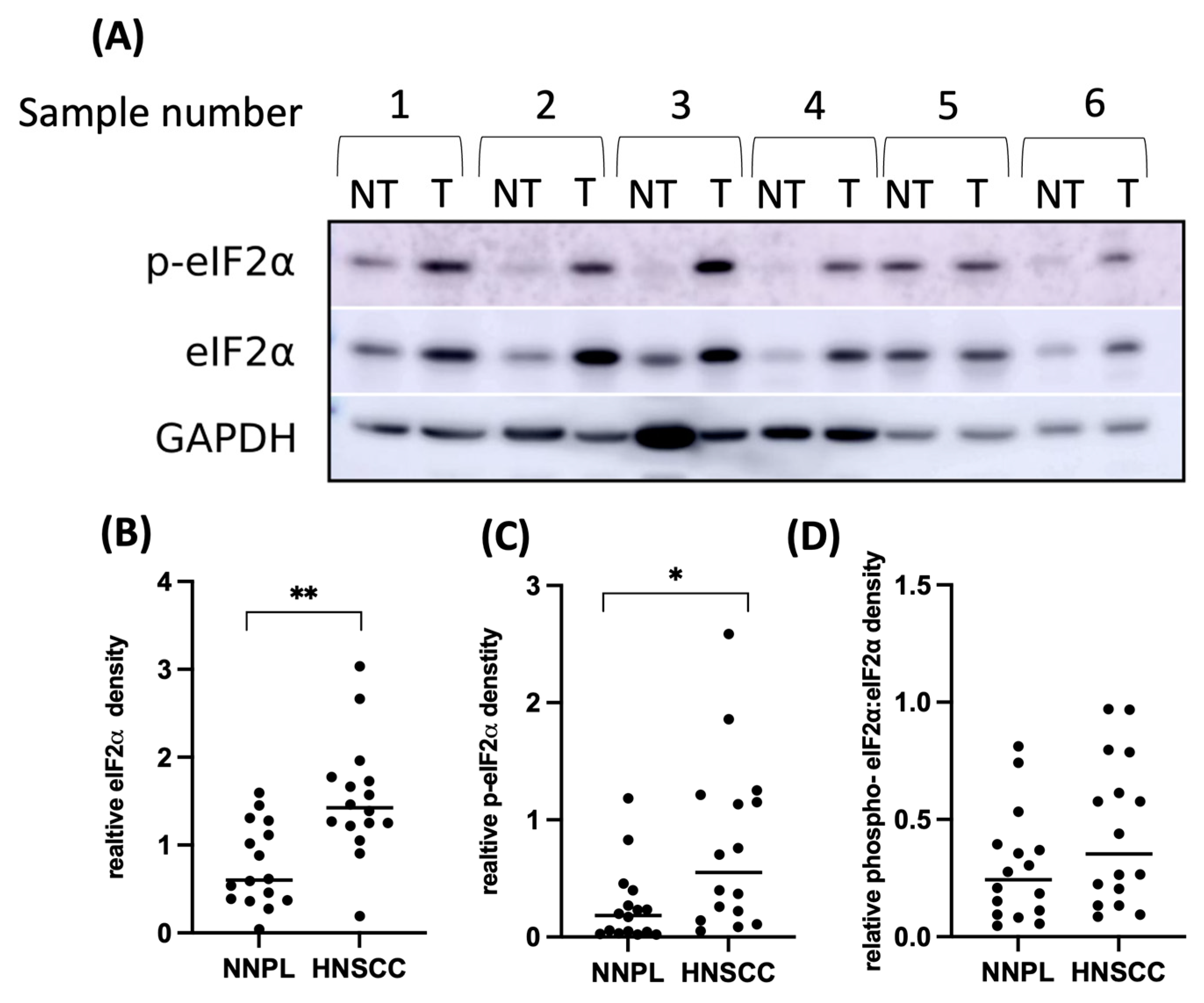
3.2. EIF2α Tissue Abundance and Phosphorylation Are Elevated in HNSCC
3.3. Differential EIF2α Expression in HPV-Positive and Negative HNSCC
3.4. Treatment with an EIF2α Dephosphorylation Inhibitor Decreases Cell Viability and Clonogenic Survival by Disrupting Cell Cycle Progression
3.5. Salubrinal Decreases the Viability of Patient-Derived 3D Tumor Spheres (PD3DS) and Enhances the Cytotoxicity of Selected Chemotherapeutics
4. Discussion
4.1. Pharmacological Modification of EIF2α Phosphorylation
4.2. The Effects of EIF2α Hyperphosphorylation on the Cell Cycle
4.3. Salubrinal-Induced Sensitization to Chemotherapeutics
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Gatta, G.; Botta, L.; Sánchez, M.J.; Anderson, L.A.; Pierannunzio, D.; Licitra, L. Prognoses and Improvement for Head and Neck Cancers Diagnosed in Europe in Early 2000s: The EUROCARE-5 Population-Based Study. Eur. J. Cancer 2015, 51, 2130–2143. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Ragin, C.C.R.; Taioli, E. Survival of Squamous Cell Carcinoma of the Head and Neck in Relation to Human Papillomavirus Infection: Review and Meta-Analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S.; et al. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodulation Photomed. Laser Surg. 2023, 41, 148–264. [Google Scholar] [CrossRef] [PubMed]
- Hajmohammadi, E.; Molaei, T.; Mowlaei, S.H.; Alam, M.; Abbasi, K.; Khayatan, D.; Rahbar, M.; Tebyanian, H. Sonodynamic Therapy and Common Head and Neck Cancers: In Vitro and in Vivo Studies. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5113–5221. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tie, Y.; Alu, A.; Ma, X.; Shi, H. Targeted Therapy for Head and Neck Cancer: Signaling Pathways and Clinical Studies. Signal. Transduct. Target Ther. 2023, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The Molecular Landscape of Head and Neck Cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Nagel, R.; Semenova, E.A.; Berns, A. Drugging the Addict: Non-Oncogene Addiction as a Target for Cancer Therapy. EMBO Rep. 2016, 17, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Solimini, N.L.; Elledge, S.J. Principles of Cancer Therapy: Oncogene and Non-Oncogene Addiction. Cell 2009, 136, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Robichaud, N.; Hulea, L.; Sonenberg, N.; Pelletier, J.; Topisirovic, I. Targeting the Translation Machinery in Cancer. Nat. Rev. Drug Discov. 2015, 14, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Yu, J.; Ward, R.; Liu, Y.; Hao, Q.; An, S.; Xu, T. Eukaryotic Translation Initiation Factors as Promising Targets in Cancer Therapy. Cell Commun. Signal. 2020, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- Spilka, R.; Ernst, C.; Mehta, A.K.; Haybaeck, J. Eukaryotic Translation Initiation Factors in Cancer Development and Progression. Cancer Lett. 2013, 340, 9–21. [Google Scholar] [CrossRef]
- Joshi, M.; Kulkarni, A.; Pal, J.K. Small Molecule Modulators of Eukaryotic Initiation Factor 2α Kinases, the Key Regulators of Protein Synthesis. Biochimie 2013, 95, 1980–1990. [Google Scholar] [CrossRef] [PubMed]
- Wek, R.C. Role of EIF2α Kinases in Translational Control and Adaptation to Cellular Stress. Cold. Spring Harb. Perspect. Biol. 2018, 10, a032870. [Google Scholar] [CrossRef]
- Sharma, D.K.; Bressler, K.; Patel, H.; Balasingam, N.; Thakor, N. Role of Eukaryotic Initiation Factors during Cellular Stress and Cancer Progression. J. Nucleic. Acids 2016, 2016, 8235121. [Google Scholar] [CrossRef]
- Thakor, N.; Holcik, M. IRES-Mediated Translation of Cellular Messenger RNA Operates in EIF2alpha- Independent Manner during Stress. Nucleic. Acids Res. 2012, 40, 541–552. [Google Scholar] [CrossRef]
- Koromilas, A.E. Roles of the Translation Initiation Factor EIF2α Serine 51 Phosphorylation in Cancer Formation and Treatment. Biochim. Et Biophys. Acta Gene Regul. Mech. 2015, 1849, 871–880. [Google Scholar] [CrossRef]
- Novoa, I.; Zhang, Y.; Zeng, H.; Jungreis, R.; Harding, H.P.; Ron, D. Stress-Induced Gene Expression Requires Programmed Recovery from Translational Repression. EMBO J. 2003, 22, 1180–1187. [Google Scholar] [CrossRef]
- Kojima, E.; Takeuchi, A.; Haneda, M.; Yagi, F.; Hasegawa, T.; Yamaki, K.-I.; Takeda, K.; Akira, S.; Shimokata, K.; Isobe, K.-I. The Function of GADD34 Is a Recovery from a Shutoff of Protein Synthesis Induced by ER Stress—Elucidation by GADD34-Deficient Mice. FASEB J. 2003, 17, 1573–1575. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Sougnez, C.; Lichtenstein, L.; Cancer Genome Atlas Network. Comprehensive Genomic Characterization of Head and Neck Squamous Cell Carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An Update to the Integrated Cancer Data Analysis Platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D.; Yau, C.; Bowlby, R. Genomic, Pathway Network, and Immunologic Features Distinguishing Squamous Carcinomas. Cell Rep. 2018, 23, 194–212.e6. [Google Scholar] [CrossRef] [PubMed]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A Web Server for Cancer and Normal Gene Expression Profiling and Interactive Analyses. Nucleic. Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef]
- Edwards, N.J.; Oberti, M.; Thangudu, R.R.; Cai, S.; McGarvey, P.B.; Jacob, S.; Madhavan, S.; Ketchum, K.A. The CPTAC Data Portal: A Resource for Cancer Proteomics Research. J. Proteome Res. 2015, 14, 2707–2713. [Google Scholar] [CrossRef]
- Ianevski, A.; Giri, A.K.; Aittokallio, T. SynergyFinder 3.0: An Interactive Analysis and Consensus Interpretation of Multi-Drug Synergies across Multiple Samples. Nucleic. Acids Res. 2022, 50, W739–W743. [Google Scholar] [CrossRef]
- Boehnke, K.; Iversen, P.W.; Schumacher, D.; Lallena, M.J.; Haro, R.; Amat, J.; Haybaeck, J.; Liebs, S.; Lange, M.; Schäfer, R.; et al. Assay Establishment and Validation of a High-Throughput Screening Platform for Three-Dimensional Patient-Derived Colon Cancer Organoid Cultures. J. Biomol. Screen 2016, 21, 931–941. [Google Scholar] [CrossRef]
- Komar, A.A.; Merrick, W.C. A Retrospective on EIF2A-and Not the Alpha Subunit of EIF2. Int. J. Mol. Sci. 2020, 21, 2054. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human Papillomavirus as a Driver of Head and Neck Cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Boyce, M.; Bryant, K.F.; Jousse, C.; Long, K.; Harding, H.P.; Scheuner, D.; Kaufman, R.J.; Ma, D.; Coen, D.M.; Ron, D.; et al. A Selective Inhibitor of EIF2alpha Dephosphorylation Protects Cells from ER Stress. Science 2005, 307, 935–939. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell Cycle Control in Cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, A.L.; Osman, A.A.; Xie, T.-X.; Patel, A.; Skinner, H.; Sandulache, V.; Myers, J.N. Reactive Oxygen Species and P21Waf1/Cip1 Are Both Essential for P53-Mediated Senescence of Head and Neck Cancer Cells. Cell Death Dis. 2015, 6, e1678. [Google Scholar] [CrossRef] [PubMed]
- Schewe, D.M.; Aguirre-Ghiso, J.A. Inhibition of EIF2alpha Dephosphorylation Maximizes Bortezomib Efficiency and Eliminates Quiescent Multiple Myeloma Cells Surviving Proteasome Inhibitor Therapy. Cancer Res. 2009, 69, 1545–1552. [Google Scholar] [CrossRef]
- Drexler, H.C.A. Synergistic Apoptosis Induction in Leukemic Cells by the Phosphatase Inhibitor Salubrinal and Proteasome Inhibitors. PLoS ONE 2009, 4, e4161. [Google Scholar] [CrossRef]
- Lobo, M.V.T.; Martín, M.E.; Pérez, M.I.; Alonso, F.J.M.; Redondo, C.; Álvarez, M.I.; Salinas, M. Levels, Phosphorylation Status and Cellular Localization of Translational Factor EIF2 in Gastrointestinal Carcinomas. Histochem. J. 2000, 32, 139–150. [Google Scholar] [CrossRef]
- Guo, L.; Chi, Y.; Xue, J.; Ma, L.; Shao, Z.; Wu, J. Phosphorylated EIF2α Predicts Disease-Free Survival in Triple-Negative Breast Cancer Patients. Sci. Rep. 2017, 7, 44674. [Google Scholar] [CrossRef]
- Wang, E.M.; Akasaka, H.; Zhao, J.; Varadhachary, G.R.; Lee, J.E.; Maitra, A.; Fleming, J.B.; Hung, M.-C.; Wang, H.; Katz, M.H.G. Expression and Clinical Significance of Protein Kinase RNA-Like Endoplasmic Reticulum Kinase and Phosphorylated Eukaryotic Initiation Factor 2α in Pancreatic Ductal Adenocarcinoma. Pancreas 2019, 48, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.G.; Conn, C.S.; Kye, Y.; Xue, L.; Forester, C.M.; Cowan, J.E.; Hsieh, A.C.; Cunningham, J.T.; Truillet, C.; Tameire, F.; et al. Development of a Stress Response Therapy Targeting Aggressive Prostate Cancer. Sci. Transl. Med. 2018, 10, eaar2036. [Google Scholar] [CrossRef]
- Sabharwal, R.; Mahendra, A.; Moon, N.J.; Gupta, P.; Jain, A.; Gupta, S. Genetically Altered Fields in Head and Neck Cancer and Second Field Tumor. South Asian J. Cancer 2014, 3, 151–153. [Google Scholar] [CrossRef]
- Economopoulou, P.; Kotsantis, I.; Psyrri, A. Special Issue about Head and Neck Cancers: HPV Positive Cancers. Int. J. Mol. Sci. 2020, 21, 3388. [Google Scholar] [CrossRef]
- Kazemi, S.; Papadopoulou, S.; Li, S.; Su, Q.; Wang, S.; Yoshimura, A.; Matlashewski, G.; Dever, T.E.; Koromilas, A.E. Control of Alpha Subunit of Eukaryotic Translation Initiation Factor 2 (EIF2 Alpha) Phosphorylation by the Human Papillomavirus Type 18 E6 Oncoprotein: Implications for EIF2 Alpha-Dependent Gene Expression and Cell Death. Mol. Cell Biol. 2004, 24, 3415–3429. [Google Scholar] [CrossRef]
- Walczak, A.; Gradzik, K.; Kabzinski, J.; Przybylowska-Sygut, K.; Majsterek, I. The Role of the ER-Induced UPR Pathway and the Efficacy of Its Inhibitors and Inducers in the Inhibition of Tumor Progression. Oxid. Med. Cell Longev. 2019, 2019, 5729710. [Google Scholar] [CrossRef]
- Donzé, O.; Jagus, R.; Koromilas, A.E.; Hershey, J.W.; Sonenberg, N. Abrogation of Translation Initiation Factor EIF-2 Phosphorylation Causes Malignant Transformation of NIH 3T3 Cells. EMBO J. 1995, 14, 3828–3834. [Google Scholar] [CrossRef]
- Ranganathan, A.C.; Ojha, S.; Kourtidis, A.; Conklin, D.S.; Aguirre-Ghiso, J.A. Dual Function of Pancreatic Endoplasmic Reticulum Kinase in Tumor Cell Growth Arrest and Survival. Cancer Res. 2008, 68, 3260–3268. [Google Scholar] [CrossRef]
- Jousse, C.; Oyadomari, S.; Novoa, I.; Lu, P.; Zhang, Y.; Harding, H.P.; Ron, D. Inhibition of a Constitutive Translation Initiation Factor 2alpha Phosphatase, CReP, Promotes Survival of Stressed Cells. J. Cell Biol. 2003, 163, 767–775. [Google Scholar] [CrossRef]
- Harding, H.P.; Zhang, Y.; Scheuner, D.; Chen, J.J.; Kaufman, R.J.; Ron, D. Ppp1r15 Gene Knockout Reveals an Essential Role for Translation Initiation Factor 2 Alpha (EIF2α) Dephosphorylation in Mammalian Development. Proc. Natl. Acad. Sci. USA 2009, 106, 1832–1837. [Google Scholar] [CrossRef]
- Dempster, J.M.; Boyle, I.; Vazquez, F.; Root, D.; Boehm, J.S.; Hahn, W.C.; Tsherniak, A.; McFarland, J.M. Chronos: A CRISPR Cell Population Dynamics Model. bioRxiv 2021. [Google Scholar] [CrossRef]
- Pacini, C.; Dempster, J.M.; Boyle, I.; Gonçalves, E.; Najgebauer, H.; Karakoc, E.; van der Meer, D.; Barthorpe, A.; Lightfoot, H.; Jaaks, P.; et al. Integrated Cross-Study Datasets of Genetic Dependencies in Cancer. Nat. Commun. 2021, 12, 1661. [Google Scholar] [CrossRef]
- Dempster, J.M.; Rossen, J.; Kazachkova, M.; Pan, J.; Kugener, G.; Root, D.E.; Tsherniak, A. Extracting Biological Insights from the Project Achilles Genome-Scale CRISPR Screens in Cancer Cell Lines. bioRxiv. 2019. [Google Scholar] [CrossRef]
- Meyers, R.M.; Bryan, J.G.; McFarland, J.M.; Weir, B.A.; Sizemore, A.E.; Xu, H.; Dharia, N.V.; Montgomery, P.G.; Cowley, G.S.; Pantel, S.; et al. Computational Correction of Copy Number Effect Improves Specificity of CRISPR-Cas9 Essentiality Screens in Cancer Cells. Nat. Genet. 2017, 49, 1779–1784. [Google Scholar] [CrossRef]
- Dadey, D.Y.A.; Kapoor, V.; Khudanyan, A.; Thotala, D.; Hallahan, D.E. PERK Regulates Glioblastoma Sensitivity to ER Stress Although Promoting Radiation Resistance. Mol. Cancer Res. 2018, 16, 1447–1453. [Google Scholar] [CrossRef]
- Sengupta, S.; Sevigny, C.M.; Bhattacharya, P.; Jordan, V.C.; Clarke, R. Estrogen-Induced Apoptosis in Breast Cancers Is Phenocopied by Blocking Dephosphorylation of Eukaryotic Initiation Factor 2 Alpha (EIF2α) Protein. Mol. Cancer Res. 2019, 17, 918–928. [Google Scholar] [CrossRef]
- Tsaytler, P.; Harding, H.P.; Ron, D.; Bertolotti, A. Selective Inhibition of a Regulatory Subunit of Protein Phosphatase 1 Restores Proteostasis. Science 2011, 332, 91–94. [Google Scholar] [CrossRef]
- Hamamura, K.; Minami, K.; Tanjung, N.; Wan, Q.; Koizumi, M.; Matsuura, N.; Na, S.; Yokota, H. Attenuation of Malignant Phenotypes of Breast Cancer Cells through EIF2α-Mediated Downregulation of Rac1 Signaling. Int. J. Oncol. 2014, 44, 1980–1988. [Google Scholar] [CrossRef]
- Koizumi, M.; Tanjung, N.G.; Chen, A.; Dynlacht, J.R.; Garrett, J.; Yoshioka, Y.; Ogawa, K.; Teshima, T.; Yokota, H. Administration of Salubrinal Enhances Radiation-Induced Cell Death of SW1353 Chondrosarcoma Cells. Anticancer Res. 2012, 32, 3667–3673. [Google Scholar]
- Jeon, Y.-J.; Kim, J.H.; Shin, J.-I.; Jeong, M.; Cho, J.; Lee, K. Salubrinal-Mediated Upregulation of EIF2α Phosphorylation Increases Doxorubicin Sensitivity in MCF-7/ADR Cells. Mol. Cells 2016, 39, 129–135. [Google Scholar] [CrossRef]
- de Bakker, T.; Journe, F.; Descamps, G.; Saussez, S.; Dragan, T.; Ghanem, G.; Krayem, M.; Van Gestel, D. Restoring P53 Function in Head and Neck Squamous Cell Carcinoma to Improve Treatments. Front. Oncol. 2022, 11, 799993. [Google Scholar] [CrossRef]
- Lee, D.; Hokinson, D.; Park, S.; Elvira, R.; Kusuma, F.; Lee, J.-M.; Yun, M.; Lee, S.-G.; Han, J. ER Stress Induces Cell Cycle Arrest at the G2/M Phase Through EIF2α Phosphorylation and GADD45α. Int. J. Mol. Sci. 2019, 20, 6309. [Google Scholar] [CrossRef]
- Brewer, J.W.; Hendershot, L.M.; Sherr, C.J.; Diehl, J.A. Mammalian Unfolded Protein Response Inhibits Cyclin D1 Translation and Cell-Cycle Progression. Proc. Natl. Acad. Sci. USA 1999, 96, 8505–8510. [Google Scholar] [CrossRef]
- Ding, Z.; Liu, Y.; Rubio, V.; He, J.; Minze, L.J.; Shi, Z.-Z. OLA1, a Translational Regulator of P21, Maintains Optimal Cell Proliferation Necessary for Developmental Progression. Mol. Cell Biol. 2016, 36, 2568–2582. [Google Scholar] [CrossRef]
- Darini, C.; Ghaddar, N.; Chabot, C.; Assaker, G.; Sabri, S.; Wang, S.; Krishnamoorthy, J.; Buchanan, M.; Aguilar-Mahecha, A.; Abdulkarim, B.; et al. An Integrated Stress Response via PKR Suppresses HER2+ Cancers and Improves Trastuzumab Therapy. Nat. Commun. 2019, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Berton, T.R.; Mitchell, D.L.; Guo, R.; Johnson, D.G. Regulation of Epidermal Apoptosis and DNA Repair by E2F1 in Response to Ultraviolet B Radiation. Oncogene 2005, 24, 2449–2460. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.F.; Barnes, L.M.; Dahler, A.L.; Smith, L.; Serewko-Auret, M.M.; Popa, C.; Abdul-Jabbar, I.; Saunders, N.A. E2F Modulates Keratinocyte Squamous Differentiation: IMPLICATIONS FOR E2F INHIBITION IN SQUAMOUS CELL CARCINOMA*. J. Biol. Chem. 2003, 278, 28516–28522. [Google Scholar] [CrossRef]
- Rosenfeldt, M.T.; Bell, L.A.; Long, J.S.; O’Prey, J.; Nixon, C.; Roberts, F.; Dufès, C.; Ryan, K.M. E2F1 Drives Chemotherapeutic Drug Resistance via ABCG2. Oncogene 2014, 33, 4164–4172. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.-H.; Wei, W.-Y.; Cao, W.-L.; Zhang, X.-S.; Xie, Y.-B.; Xiao, Q. Overexpression of E2F1 in Human Gastric Carcinoma Is Involved in Anti-Cancer Drug Resistance. BMC Cancer 2014, 14, 904. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Grandis, J.R. Emerging Drugs for Head and Neck Cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.J.; van Venrooij, W.J.; van de Putte, L.B.A. Apoptosis and Autoimmunity. Cell Death Differ. 2000, 7, 131–133. [Google Scholar] [CrossRef]
- Kepp, O.; Semeraro, M.; Bravo-San Pedro, J.M.; Bloy, N.; Buqué, A.; Huang, X.; Zhou, H.; Senovilla, L.; Kroemer, G.; Galluzzi, L. EIF2α Phosphorylation as a Biomarker of Immunogenic Cell Death. Semin. Cancer Biol. 2015, 33, 86–92. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.-L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin Exposure Dictates the Immunogenicity of Cancer Cell Death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Bezu, L.; Gomes-de-Silva, L.C.; Dewitte, H.; Breckpot, K.; Fucikova, J.; Spisek, R.; Galluzzi, L.; Kepp, O.; Kroemer, G. Combinatorial Strategies for the Induction of Immunogenic Cell Death. Front. Immunol. 2015, 6, 187. [Google Scholar] [CrossRef]
- Fucikova, J.; Kepp, O.; Kasikova, L.; Petroni, G.; Yamazaki, T.; Liu, P.; Zhao, L.; Spisek, R.; Kroemer, G.; Galluzzi, L. Detection of Immunogenic Cell Death and Its Relevance for Cancer Therapy. Cell Death Dis. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Li, M.-H.; Ito, D.; Sanada, M.; Odani, T.; Hatori, M.; Iwase, M.; Nagumo, M. Effect of 5-Fluorouracil on G1 Phase Cell Cycle Regulation in Oral Cancer Cell Lines. Oral. Oncol. 2004, 40, 63–70. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Xu, S.-J.; Chen, W.-S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Beigi, K.; Doroodizadeh, T.; Haghnegahdar, M.; Golfeshan, F.; Ranjbar, R.; Tebyanian, H. Therapeutic Applications of Herbal/Synthetic/Bio-Drug in Oral Cancer: An Update. Eur. J. Pharmacol. 2021, 890, 173657. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, B.; Guzzi, N.; Gola, A.; Fiore, V.F.; Sendoel, A.; Nikolova, M.; Barrows, D.; Carroll, T.S.; Pasolli, H.A.; Fuchs, E. The Integrated Stress Response Remodels the Microtubule-Organizing Center to Clear Unfolded Proteins Following Proteotoxic Stress. eLife 2022, 11, e77780. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Y.; Zhou, X.; Xu, J.; Zhu, W.; Shu, Y.; Liu, P. Efficacy of Therapy with Bortezomib in Solid Tumors: A Review Based on 32 Clinical Trials. Future Oncol. 2014, 10, 1795–1807. [Google Scholar] [CrossRef] [PubMed]




| Patient Characteristics | Number of Cases (%) | |
|---|---|---|
| Sex | Male | 73 (88%) |
| Female | 10 (12%) | |
| Total | 83 (100%) | |
| Tumor localization | Larynx | 57 (69.7%) |
| Pharynx | 13 (15.7%) | |
| Tongue/tongue base | 6 (7.2%) | |
| Tonsil | 2 (2.4%) | |
| Other | 5 (6%) | |
| Metastasis | Localized disease | 47 (56.6%) |
| Nodal metastasis | 16 (19.3%) | |
| Distant metastasis | 15 (18.1%) | |
| N/D | 5 (6.0%) | |
| HPV status | Positive | 11 (13.2%) |
| Negative | 66 (79.5%) | |
| N/D | 6 (7.2%) | |
| P16 status | Positive | 20 (24.1%) |
| Negative | 56 (67.5%) | |
| N/D | 7 (8.4%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cyran, A.M.; Kleinegger, F.; Nass, N.; Naumann, M.; Haybaeck, J.; Arens, C. Inhibition of EIF2α Dephosphorylation Decreases Cell Viability and Synergizes with Standard-of-Care Chemotherapeutics in Head and Neck Squamous Cell Carcinoma. Cancers 2023, 15, 5350. https://doi.org/10.3390/cancers15225350
Cyran AM, Kleinegger F, Nass N, Naumann M, Haybaeck J, Arens C. Inhibition of EIF2α Dephosphorylation Decreases Cell Viability and Synergizes with Standard-of-Care Chemotherapeutics in Head and Neck Squamous Cell Carcinoma. Cancers. 2023; 15(22):5350. https://doi.org/10.3390/cancers15225350
Chicago/Turabian StyleCyran, Anna M., Florian Kleinegger, Norbert Nass, Michael Naumann, Johannes Haybaeck, and Christoph Arens. 2023. "Inhibition of EIF2α Dephosphorylation Decreases Cell Viability and Synergizes with Standard-of-Care Chemotherapeutics in Head and Neck Squamous Cell Carcinoma" Cancers 15, no. 22: 5350. https://doi.org/10.3390/cancers15225350
APA StyleCyran, A. M., Kleinegger, F., Nass, N., Naumann, M., Haybaeck, J., & Arens, C. (2023). Inhibition of EIF2α Dephosphorylation Decreases Cell Viability and Synergizes with Standard-of-Care Chemotherapeutics in Head and Neck Squamous Cell Carcinoma. Cancers, 15(22), 5350. https://doi.org/10.3390/cancers15225350