Radical Hysterectomy or Total Mesometrial Resection—Two Anatomical Concepts for Surgical Treatment of Cancer of the Uterine Cervix
Abstract
Simple Summary
Abstract
1. Introduction
2. The Anatomy of the Parametrium
3. The Anatomy of the Pelvic Autonomic Nerves (Inferior Hypogastric Plexus)
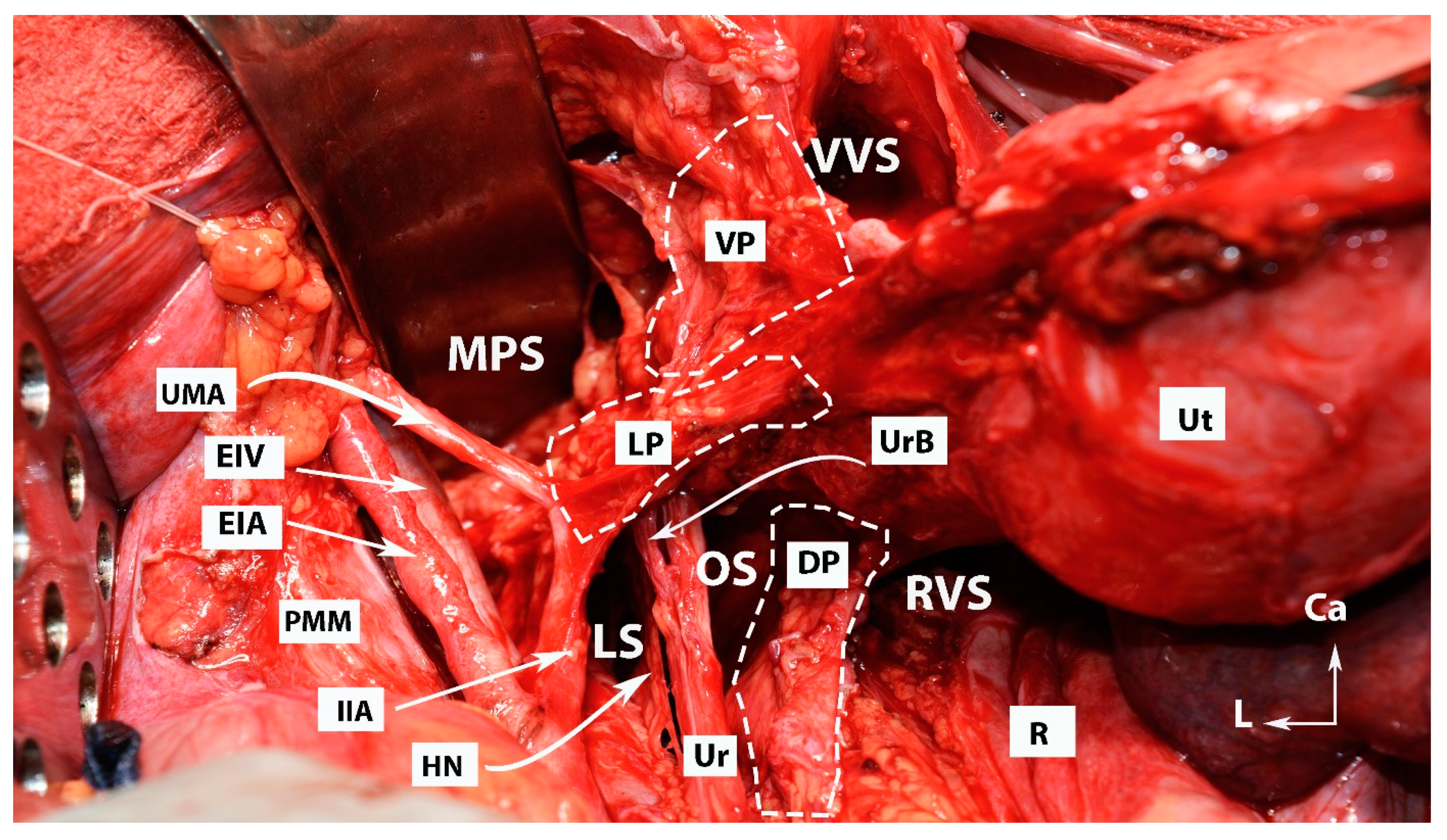
4. Avascular Spaces Nearby the Ventral Parametrium during Radical Hysterectomy
5. Discrepancy Regarding Terminology, Anatomical Description, and Surgical Technique during Radical Hysterectomy (Type C1/C2)
5.1. Dorsal Parametrium
5.1.1. Transverse
5.1.2. Longitudinal (Caudal)
5.2. Lateral Parametrium
5.2.1. Transverse
5.2.2. Longitudinal (Caudal)
5.3. Ventral Parametrium
5.3.1. Transverse
5.3.2. Longitudinal (Caudal)
6. Introduction to Ontogenetic Anatomy
6.1. Ontogenetic Anatomy and Total Mesometrial Resection
6.2. Differences between the Ontogenetic Anatomy and Traditional Anatomy for Radical Hysterectomy
7. Discussion
7.1. Differences in the Surgical Applications of Radical Hysterectomy and Total Mesometrial Resection
7.2. Lymphatic Drainage of the Cervix and Upper Vagina with Surgical Implications
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muallem, M.Z.; Jöns, T.; Seidel, N.; Sehouli, J.; Diab, Y.; Querleu, D. A Concise Paradigm on Radical Hysterectomy: The Comprehensive Anatomy of Parametrium, Paracolpium and the Pelvic Autonomic Nerve System and Its Surgical Implication. Cancers 2020, 12, 1839. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.G. A more radical method of performing hysterectomy for cancer of the uterus. Johns Hopkins Hosp. Bull. 1895, 6, 120–124. [Google Scholar]
- Wertheim, E. The extended abdominal operation for carcinoma uteri (based on 500 operative cases). Am. J. Obstet. Dis. Women Child. 1912, 66, 169–232. [Google Scholar]
- Okabayashi, H. Radical abdominal hysterectomy for cancer of the cervix uteri. Surg. Gynecol. Obstet. 1921, 33, 335–341. [Google Scholar]
- Querleu, D.; Morrow, C.P. Classification of radical hysterectomy. Lancet Oncol. 2008, 9, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y. Twenty-first century radical hysterectomy—Journey from descriptive to practical anatomy. Gynecol. Oncol. Rep. 2020, 34, 100623. [Google Scholar] [CrossRef] [PubMed]
- Santiago, I.A.; Gomes, A.P.; Heald, R.J. An ontogenetic approach to gynecologic malignancies. Insights Imaging 2016, 7, 329–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cibula, D.; Abu-Rustum, N.R.; Benedetti-Panici, P.; Köhler, C.; Raspagliesi, F.; Querleu, D.; Morrow, C.P. New classification system of radical hysterectomy: Emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol. Oncol. 2011, 122, 264–268. [Google Scholar] [CrossRef]
- Federative Committee on Anatomical Terminology (FCAT). Terminologia Anatomica: International Anatomical Terminology; Thieme: Stuttgart, Germany; New York, NY, USA, 1998. [Google Scholar]
- Selcuk, I. Correspondence on ‘Simplified anatomical nomenclature of lateral female pelvic spaces’ by Querleu et al. Int. J. Gynecol. Cancer 2022, 32, 1495. [Google Scholar] [CrossRef]
- Fujii, S.; Takakura, K.; Matsumura, N.; Higuchi, T.; Yura, S.; Mandai, M.; Baba, T.; Yoshioka, S. Anatomic identification and functional outcomes of the nerve sparing Okabayashi radical hysterectomy. Gynecol. Oncol. 2007, 107, 4–13. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Armbrust, R.; Neymeyer, J.; Miranda, A.; Muallem, J. Nerve Sparing Radical Hysterectomy: Short-Term Oncologic, Surgical, and Functional Outcomes. Cancers 2020, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Sekiyama, K.; Fujii, S.; Mandai, M. Anatomical location of the surgically identifiable bladder branch of the inferior hypogastric plexus for nerve-sparing radical hysterectomy. Gynecol. Oncol. Rep. 2023, 46, 101152. [Google Scholar] [CrossRef]
- Muallem, M.Z.; Diab, Y.; Sehouli, J.; Fujii, S. Nerve-sparing radical hysterectomy: Steps to standardize surgical technique. Int. J. Gynecol. Cancer 2019, 29, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Querleu, D.; Bizzarri, N.; Fanfani, F.; Fagotti, A.; Scambia, G. Simplified anatomical nomenclature of lateral female pelvic spaces. Int. J. Gynecol. Cancer 2022, 32, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Kostov, S.; Slavchev, S.; Dzhenkov, D.; Mitev, D.; Yordanov, A. Avascular Spaces of the Female Pelvis-Clinical Applications in Obstetrics and Gynecology. J. Clin. Med. 2020, 9, 1460. [Google Scholar] [CrossRef]
- Fujii, S.; Sekiyama, K. Precise Neurovascular Anatomy for Radical Hysterectomy; Springer Nature PTe., Ltd.: Singapore, 2020; pp. 20–53. [Google Scholar]
- Zapardiel, I.; Ceccaroni, M.; Minig, L.; Halaska, M.J.; Fujii, S. Avascular spaces in radical hysterectomy. Int. J. Gynecol. Cancer 2023, 33, 285–292. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Ni, T.; Huang, Y.; Wang, L.; Yuan, J.; Fan, Q.; Li, Y.; Wang, Y. A practical method of using the anatomical space of the vesicouterine ligament for laparoscopic radical hysterectomy: A retrospective cohort study. J. Int. Med. Res. 2020, 48, 300060520926857. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Asamoto, A.; Hoshiba, T.; Nishimoto, H.; Nishikawa, Y.; Nakajima, T. Radical hysterectomy: An anatomic evaluation of parametrial dissection. Gynecol. Oncol. 2000, 77, 155–163. [Google Scholar] [CrossRef]
- Fritsch, H.; Hötzinger, H. Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin. Anat. 1995, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Haylen, B.T.; Tse, K.; Farnsworth, A. Surgical anatomy of the uterosacral ligament. Int. Urogynecol. J. 2010, 21, 1123–1128. [Google Scholar] [CrossRef]
- Umek, W.H.; Morgan, D.M.; Ashton-Miller, J.A.; DeLancey, J.O. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet. Gynecol. 2004, 103, 447–451. [Google Scholar] [CrossRef]
- Ceccaroni, M.; Clarizia, R.; Roviglione, G.; Ruffo, G. Neuro-anatomy of the posterior parametrium and surgical considerations for a nerve-sparing approach in radical pelvic surgery. Surg. Endosc. 2013, 27, 4386–4394. [Google Scholar] [CrossRef]
- Ramanah, R.; Berger, M.B.; Parratte, B.M.; DeLancey, J.O.L. Anatomy and histology of apical support: A literature review concerning cardinal and uterosacral ligaments. Int. Urogynecol. J. 2012, 23, 1483–1494. [Google Scholar] [CrossRef]
- Ercoli, A.; Delmas, V.; Fanfani, F.; Gadonneix, P.; Ceccaroni, M.; Fagotti, A.; Mancuso, S.; Scambia, G. Terminologia Anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: A dissection-based comparative study. Am. J. Obstet. Gynecol. 2005, 193, 1565–1573. [Google Scholar] [CrossRef]
- Kiyomatsu, T.; Ishihara, S.; Murono, K.; Otani, K.; Yasuda, K.; Nishikawa, T.; Tanaka, T.; Hata, K.; Kawai, K.; Nozawa, H.; et al. Anatomy of the middle rectal artery: A review of the historical literature. Surg. Today 2017, 47, 14–19. [Google Scholar] [CrossRef]
- Cosma, S.; Ferraioli, D.; Mitidieri, M.; Ceccaroni, M.; Zola, P.; Micheletti, L.; Benedetto, C. A simplified fascial model of pelvic anatomical surgery: Going beyond parametrium-centered surgical anatomy. Anat. Sci. Int. 2021, 96, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Murakami, G.; Yabuki, Y. Does the cardinal ligament of the uterus contain a nerve that should be preserved in radical hysterectomy? Anat. Sci. Int. 2002, 77, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y. Two lateral parametria in radical hysterectomy: History and outcome. J. Obstet. Gynaecol. Res. 2023, 49, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Lührs, O.; Ekdahl, L.; Geppert, B.; Lönnerfors, C.; Persson, J. Resection of the upper paracervical lymphovascular tissue should be an integral part of a pelvic sentinel lymph node algorithm in early stage cervical cancer. Gynecol. Oncol. 2021, 163, 289–293. [Google Scholar] [CrossRef]
- Sakuragi, N.; Kaneuchi, M. Nerve-Sparing Radical Hysterectomy Using the Okabayashi-Kobayashi Method. Surg. J. 2021, 7 (Suppl. 2), S48–S56. [Google Scholar] [CrossRef]
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175. [Google Scholar]
- Sinnatamby, C.S. Last’s Anatomy, International Edition: Regional and Applied; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Höckel, M. Cancer field surgery for locoregional tumor control of cervical carcinoma. J. Cancer Metastasis Treat. 2021, 7, 64. [Google Scholar] [CrossRef]
- Höckel, M.; Horn, L.-C.; Manthey, N.; Braumann, U.-D.; Wolf, U.; Teichmann, G.; Frauenschläger, K.; Dornhöfer, N.; Einenkel, J. Resection of the embryologically defined uterovaginal (Müllerian) compartment and pelvic control in patients with cervical cancer: A prospective analysis. Lancet Oncol. 2009, 10, 683–692. [Google Scholar] [CrossRef]
- Gerrelli, D.; Lisgo, S.; Copp, A.J.; Lindsay, S. Enabling research with human embryonic and fetal tissue resources. Development 2015, 142, 3073–3076. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J. The ‘Holy Plane’ of rectal surgery. J. R. Soc. Med. 1988, 81, 503–508. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, C.F.; Wei, D.; Wang, C.; Meng, W.J. Anatomy of the lateral ligaments of the rectum: A controversial point of view. World J. Gastroenterol. 2010, 16, 5411–5415. [Google Scholar] [CrossRef]
- Zarzecki, M.P.; Ostrowski, P.; Wałęga, P.; Iwanaga, J.; Walocha, J.A. The middle anorectal artery: A systematic review and meta-analysis of 880 patients/1905 pelvic sides. Clin. Anat. 2022, 35, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Höckel, M. Principles and practice of surgical treatment for cervical cancer: It’s time for a change. Women’s Health 2009, 5, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Ahmed, D. Embryology, Kidney, Bladder, and Ureter. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Touboul, C.; Fauconnier, A.; Zareski, E.; Bouhanna, P.; Daraï, E. The lateral infraureteral parametrium: Myth or reality? Am. J. Obstet. Gynecol. 2008, 199, 242.e1–242.e6. [Google Scholar] [CrossRef]
- Ercoli, A.; Delmas, V.; Iannone, V.; Fagotti, A.; Fanfani, F.; Corrado, G.; Ferrandina, G.; Scambia, G. The lymphatic drainage of the uterine cervix in adult fresh cadavers: Anatomy and surgical implications. Eur. J. Surg. Oncol. 2010, 36, 298–303. [Google Scholar] [CrossRef]
- Kraima, A.C.; Derks, M.; Smit, N.N.; Van Munsteren, J.C.; Van der Velden, J.; Kenter, G.G.; DeRuiter, M.C. Lymphatic drainage pathways from the cervix uteri: Implications for radical hysterectomy? Gynecol. Oncol. 2014, 132, 107–113. [Google Scholar] [CrossRef]
- Buderath, P.; Stukan, M.; Ruhwedel, W.; Strutas, D.; Feisel-Schwickardi, G.; Wimberger, P.; Kimmig, R. Total mesometrial resection (TMMR) for cervical cancer FIGO IB-IIA: First results from the multicentric TMMR register study. J. Gynecol. Oncol. 2022, 33, e9. [Google Scholar] [CrossRef]
- Höckel, M.; Wolf, B.; Schmidt, K.; Mende, M.; Aktas, B.; Kimmig, R.; Dornhöfer, N.; Horn, L.-C. Surgical resection based on ontogenetic cancer field theory for cervical cancer: Mature results from a single-centre, prospective, observational, cohort study. Lancet Oncol. 2019, 20, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Kubitschke, H.; Wolf, B.; Morawetz, E.; Horn, L.-C.; Aktas, B.; Behn, U.; Höckel, M.; Käs, J. Roadmap to Local Tumour Growth: Insights from Cervical Cancer. Sci. Rep. 2019, 9, 12768. [Google Scholar] [CrossRef]
- Plentl, A.A.; Friedman, E.A. Clinical significance of cervical lymphatics. In Lymphatic System of the Female Genitalia: The Morphologic Basis of Oncologic Diagnosis and Therapy; W. B. Saunders: Philadelphia, PA, USA, 1971; pp. 85–115. [Google Scholar]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R. 2017 Update on the Querleu-Morrow Classification of Radical Hysterectomy. Ann. Surg. Oncol. 2017, 24, 3406–3412. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- van de Lande, J.; von Mensdorff-Pouilly, S.; Lettinga, R.G.; Piek, J.M.; Verheijen, R.H. Open versus laparoscopic pelvic lymph node dissection in early stage cervical cancer: No difference in surgical or disease outcome. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2012, 22, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Pecorino, B.; D’Agate, M.G.; Scibilia, G.; Scollo, P.; Giannini, A.; Di Donna, M.C.; Chiantera, V.; Laganà, A.S. Evaluation of Surgical Outcomes of Abdominal Radical Hysterectomy and Total Laparoscopic Radical Hysterectomy for Cervical Cancer: A Retrospective Analysis of Data Collected before the LACC Trial. Int. J. Environ. Res. Public Health 2022, 19, 13176. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Raspollini, M.R.; Planchamp, F.; Centeno, C.; Chargari, C.; Felix, A.; Fischerová, D.; Jahnn-Kuch, D.; Joly, F.; Kohler, C.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with cervical cancer—Update 2023. Int. J. Gynecol. Cancer 2023, 33, 649–666. [Google Scholar] [CrossRef]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Arechvo, A.; Persson, J. A study on uterine lymphatic anatomy for standardization of pelvic sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2017, 145, 256–261. [Google Scholar] [CrossRef]
- Bonneau, C.; Cortez, A.; Lis, R.; Mirshahi, M.; Fauconnier, A.; Ballester, M.; Daraï, E.; Touboul, C. Lymphatic and nerve distribution throughout the parametrium. Gynecol. Oncol. 2013, 131, 708–713. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Maneschi, F.; D’Andrea, G.; Cutillo, G.; Rabitti, C.; Congiu, M.; Coronetta, F.; Capelli, A. Early cervical carcinoma: The natural history of lymph node involvement redefined on the basis of thorough parametrectomy and giant section study. Cancer 2000, 88, 2267–2274. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Cutillo, G.; D’Andrea, G.; Rabitti, C.; Coronetta, F.; Capelli, A.; Mancuso, S. Lymphatic spread of cervical cancer: An anatomical and pathological study based on 225 radical hysterectomies with systematic pelvic and aortic lymphadenectomy. Gynecol. Oncol. 1996, 62, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Balaya, V.; Mathevet, P.; Magaud, L.; Bonsang-Kitzis, H.; Delomenie, M.; Macias, R.M.; Ngô, C.; Bats, A.; Lécuru, F. Predictive factors of unexpected lymphatic drainage pathways in early-stage cervical cancer. Gynecol. Oncol. 2019, 154, 102–109. [Google Scholar] [CrossRef] [PubMed]












| Differences in Terminology and Conception of Anatomy | Radical Hysterectomy (C1/C2) | Total Mesometrial Resection |
|---|---|---|
| Avascular pelvic spaces | Exist | The concept of avascular spaces is not applicable for TMMR, despite some of the spaces developed during procedure (Latzko, Okabayashi, presacral) |
| Uterus ligaments | Exist | Only round and ovarian ligaments |
| Lateral parametrium | ||
| Cranial part | Parauterine tissue | Vascular mesometrium Distal mesoureter Mesobladder |
| Caudal part | Paracervix | Mesobladder IHP Mesocolpos |
| Ventral parametrium | ||
| Cranial part | Vesicouterine ligament | Mesobladder Vesicovaginal venous plexus |
| Caudal part | Vesicovaginal ligament | Mesobladder |
| Dorsal parametrium | ||
| Cranial part | Sacrouterine ligament | Ligamentous mesometrium—upper part |
| Caudal part | Rectovaginal ligament | Ligamentous mesometrium—lower part |
| Lateral ligament of the rectum | Exist | Does not exist |
| Middle rectal artery | Exist | Does not exist |
| Mesoureter | In the same axis and part of the hypogastric nerve | Not part of the hypogastric nerve. The nerve is located between the mesoureter and ligamentous mesometria |
| Autonomic Nerve | Surgical Step | Functional Outcome |
|---|---|---|
| Lumbar splanchnic nerves | Lumbo-aortic lymph node dissection | Obstipation, anorectal dysfunction, sexual dysfunction (lubrication and orgasm disorders) |
| Superior hypogastric plexus | Lumbo-aortic lymph node dissection | Obstipation, anorectal dysfunction, sexual dysfunction (lubrication and orgasm disorders) |
| Hypogastric nerve | Rectouterine (uterosacral) ligament resection | Obstipation, flatus, anorectal dysfunction, lack of bladder-urine sensation |
| Pelvic splanchnic nerves | Paracervix resection | Lack of bladder-urine sensation and urinary retention, impaired voluntarily voiding, obstipation-anorectal dysfunction |
| Inferior hypogastric plexus | Paracervix and rectovaginal ligament resection | Lack of bladder-urine sensation and urinary retention, impaired voluntarily voiding, obstipation-anorectal dysfunction |
| Inferior hypogastric plexus rectal branches | Rectovaginal ligament resection | Obstipation, anorectal dysfunction |
| Inferior hypogastric plexus vesical fibers | Vesicovaginal ligament and paracolpium resection | Lack of bladder-urine sensation and urinary retention, impaired voluntarily voiding |
| Parametrium Tissue | Type C1 | Type C2 | TMMR | |
|---|---|---|---|---|
| Lateral parametrium | ||||
| Parauterine tissue | Excised from the origin at the level of the internal iliac artery | Excised from the origin at the level of the internal iliac artery | Excised from the origin at the level of the internal iliac artery | |
| Paracervix tissue | Partially removed, to the level of the deep uterine vein (vaginal vein) (IHP and PSN are preserved) | Totally removed, caudal to the deep uterine vein, to the level of levator ani (iliococcygeus) (IHP and PSN are sacrificed) | Paracervix is excised medial to mesoureter. Vaginal branches of IHP and vaginal veins are transected medial to the mesouterer | |
| Dorsal parametrium | ||||
| Rectouterine (uterosacral) ligament | Excised from the level of rectosacral angle (HN is preserved) | Excised from the level of rectosacral angle (HN is sacrificed) | Excised from the level of rectosacral angle (HN is preserved) | |
| Rectovaginal ligament | Partially excised—according to the resection plane of the vagina (IHP is preserved) | Excised from the level of pelvic parietal fascia (IHP is sacrificed) | Excised from the level of pelvic parietal fascia (IHP is preserved) | |
| Ventral parametrium | ||||
| Vesicouterine ligament (anterior leaf) | Excised medial to the distal ureter | Excised from the level of the bladder | Excised medial to the distal ureter | |
| Vesicovaginal ligament (vesicouterine ligament posterior leaf) | Excised above the IHP vesical fibers | Totally excised with the paracolpium to the level of pubococcygeus (IHP vesical fibers are sacrificed) | Not removed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Sorokin, P.; Rezende, B.; Yalçın, H.; Selçuk, I. Radical Hysterectomy or Total Mesometrial Resection—Two Anatomical Concepts for Surgical Treatment of Cancer of the Uterine Cervix. Cancers 2023, 15, 5295. https://doi.org/10.3390/cancers15215295
Kostov S, Sorokin P, Rezende B, Yalçın H, Selçuk I. Radical Hysterectomy or Total Mesometrial Resection—Two Anatomical Concepts for Surgical Treatment of Cancer of the Uterine Cervix. Cancers. 2023; 15(21):5295. https://doi.org/10.3390/cancers15215295
Chicago/Turabian StyleKostov, Stoyan, Pavel Sorokin, Bruno Rezende, Hakan Yalçın, and Ilker Selçuk. 2023. "Radical Hysterectomy or Total Mesometrial Resection—Two Anatomical Concepts for Surgical Treatment of Cancer of the Uterine Cervix" Cancers 15, no. 21: 5295. https://doi.org/10.3390/cancers15215295
APA StyleKostov, S., Sorokin, P., Rezende, B., Yalçın, H., & Selçuk, I. (2023). Radical Hysterectomy or Total Mesometrial Resection—Two Anatomical Concepts for Surgical Treatment of Cancer of the Uterine Cervix. Cancers, 15(21), 5295. https://doi.org/10.3390/cancers15215295








