PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities
Abstract
Simple Summary
Abstract
1. Introduction
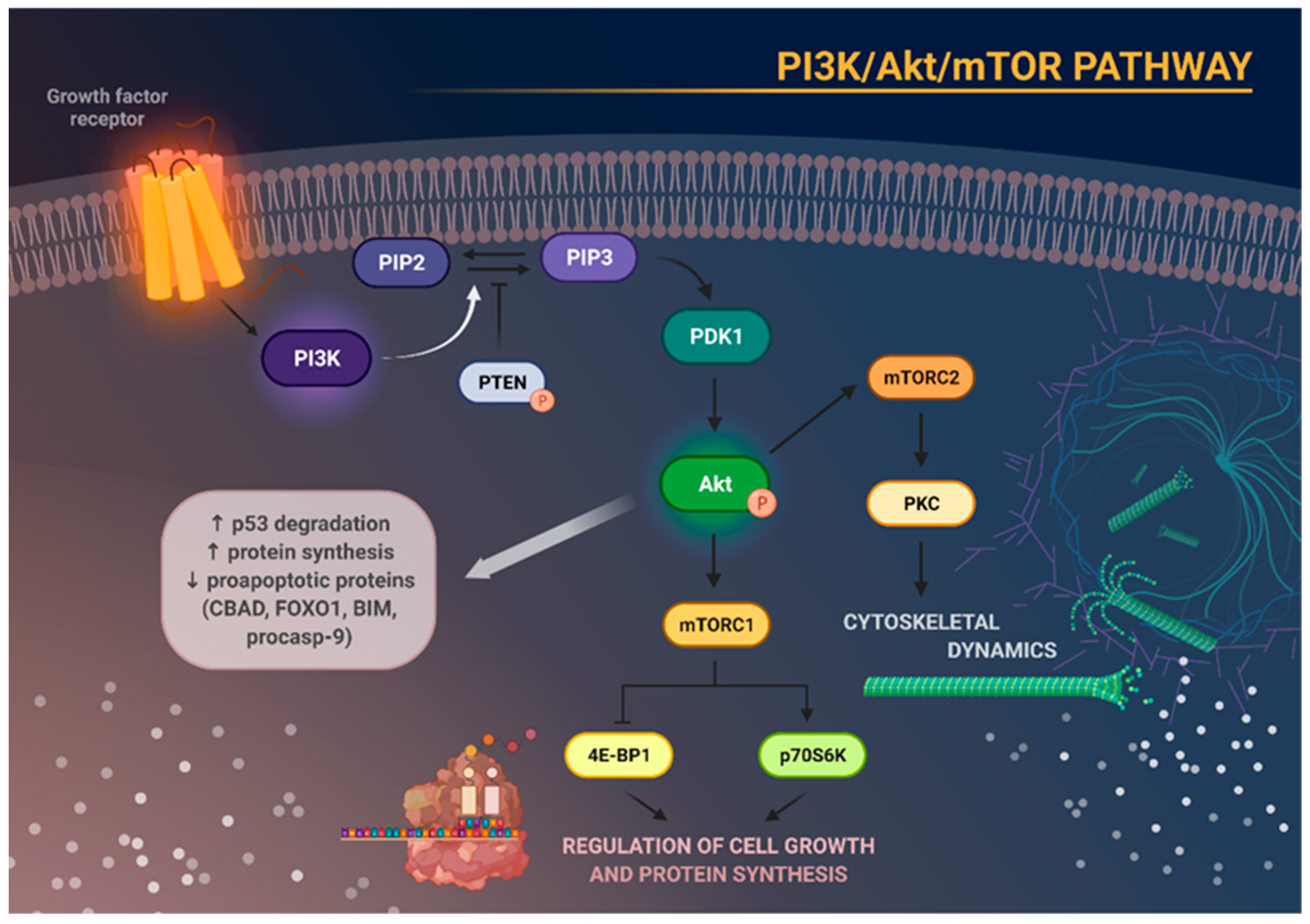
2. The PI3K/Akt/mTOR Signaling Pathway
3. The Role of PI3K/Akt/mTOR Pathway in Cancer Cells
3.1. Glucose Metabolism
3.2. Lipid Synthesis
3.3. Nucleotide Synthesis
3.4. Protein Synthesis and Degradation
4. Application of PI3K/Akt/mTOR Pathway Inhibitors in Blood Malignancies
4.1. PI3K Inhibitors
4.2. mTOR Inhibitors
4.3. Dual PI3K/mTOR Inhibitors
4.4. Akt Inhibitors
| Target | Compound Name | Type | Current Status | Number of Evaluable Patients in Trial | Results |
|---|---|---|---|---|---|
| Idelalisib | PI3K inhibitor | FDA-approved | 220 | FDA-approved Idelalisib in patients with relapsed CLL based on a significant PFS benefit. In relapsed follicular lymphoma (FL) and relapsed SLL, Idelalisib was approved under the accelerated approval program based on the tumor objective response rate data [114]. | |
| Duvelisib | PI3K inhibitor | FDA-approved | 319 | Approved by the FDA for relapsed CLL/SLL and FL. Patients with CLL/SLL treated with Duvelisib had a median PFS of 16.4 months and an overall response rate of 78%. FL patients treated with the drug had an overall response rate of 42% [115]. | |
| PI3K | ZSTK474 | PI3K inhibitor | Preclinical | In vitro research | AML [78] and CML cell proliferation decrease. Combination of ZSTK474 and Imatinib indicated synergistic effect on both cell lines [82]. |
| Buparlisib | PI3K inhibitor | CT Phase I | 14 | Safe, well tolerated, modest efficacy in advanced AML and ALL [72]. | |
| CT Phase II | 12 | Safe, well tolerated, promising results in relapse or refractory CLL—6/12 achieved PR with a median duration of response of 15.5 months [71]. | |||
| Umbralisib | PI3K inhibitor | CT Phase I | 22 | Safe, well tolerated, promising results in CLL—8/22 achieved CR and 14/22 achieved PR [116]. | |
| 90 | Safe, well tolerated, promising results in relapsed or refractory CLL. 85% of patients with relapsed or refractory CLL achieved an objective response. 8 assessable patients with high-risk cytogenetic features CLL 6 had a response, of whom 2 had a PR [77]. | ||||
| CT Phase I/IB | 44 | Safe, well tolerated, promising results in relapsed or refractory CLL. The ORR was 90% [75]. | |||
| CT Phase II | 51 | Safe, well tolerated, promising results in CLL. The ORR was 44%—19/48 PR and 2/48 CR [74]. | |||
| 28 | Safe, well tolerated, encouraging response in CLL—52% of patients achieved undetectable minimal residual disease [117]. | ||||
| RMC-4627 | mTOR inhibitor | Preclinical | In vitro research | In in vitro cell line models of Ph+ B-ALL, RMC-4627 suppressed cell cycle progression, reduced survival, and enhanced Dasatinib cytotoxicity [96]. | |
| mTOR | Everolimus | mTOR inhibitor | CT Phase I | 22 | Safe, well tolerated, promising results in childhood ALL with favorable rates, second PR (86%) and low-end reinduction minimal residual disease (68%) [88]. |
| Data unpublished | Data unpublished (NCT03328104, NCT03740334, NCT01154439, NCT00819546, NCT00636922). | ||||
| CT Phase Ib | 28 | Safe, well tolerated, promising results in AML—68% of patients achieved CR [118]. | |||
| CT Phase I/II | 24 | Safe, well tolerated, not sufficiently efficacious to recommend further development of the regimen in relapse or refractory CLL—33% of patients achieved PR but noone achieved CR [119]. | |||
| 24 | Safe, well tolerated, moderately effective in relapsed ALL, and promising response in T-ALL—ORR was 33%, response was noted in 5 of 10 heavily pretreated T-ALL patients [89]. | ||||
| 27 | Safe, well tolerated, no therapeutic effect in AML [120]. | ||||
| Data unpublished | Data unpublished (NCT00093639). | ||||
| CT Phase II | 22 | Safe, well tolerated, modest efficacy in relapse or refractory CLL—4 of 22 patients with CLL achieved a PR [121]. | |||
| Sirolimus | mTOR inhibitor | CT Phase I | 51 | Safe, well tolerated, promising results in AML—the ORR was 47% [92]. | |
| 12 | Combination of Decitabine and Sirolimus was safe and well tolerated. The primary focus of this Phase I study was not on measuring efficacy. However, it is worth noting that after one cycle, most patients showed stability or a positive response in their disease status [122]. | ||||
| Data unpublished | Data unpublished (NCT00068302, NCT00874562). | ||||
| CT Phase II | 5 | Poorly tolerated, no therapeutic effect in refractory or relapsed ALL (NCT01162551). | |||
| Data unpublished | Data unpublished (NCT00235560). | ||||
| CT Phase II | 26 | Safe, well tolerated, survival rates appear comparable to other salvage regimens in AML. The CR was 33%; median overall survival was 7.7 months in newly diagnosed elderly AML patients and 6.6 months in relapsed/refractory AML patients [123]. | |||
| Data unpublished | Data unpublished (NCT00776373). | ||||
| Temsirolimus | mTOR inhibitor | CT Phase I | 16 | Safe, well tolerated, promising results in relapsed or refractory childhood ALL—sixteen patients were included in the study, achieving an ORR of 47% and a CR of 27% [124]. | |
| 15 | Temsirolimus in combination with UK R3 chemotherapy can induce responses in children with ALL—this regimen induced remission in seven of fifteen patients with relapse ALL. However, this intensive regimen is associated with unacceptable toxicity [94,125]. | ||||
| Data unpublished | Data unpublished (NCT00101088). | ||||
| CT Phase II | 53 | Acceptable safety profile, promising results in AML—in 53 evaluable patients, the ORR was 21%. Median disease-free survival was 3–5 months, and median overall survival was 4 months [95]. | |||
| 89 | Safe, well tolerated, underperforming results in CLL—one of 15 patients with CLL had PR [126]. | ||||
| Data unpublished | Data unpublished (NCT00084916, NCT00086840). | ||||
| Dual PI3K/mTOR | Gedatolisib | Dual PI3K/mTOR inhibitor | CT Phase II | 10 | Safe, well tolerated, no clinical benefit in relapse or refractor AML—no objective response was detected for any of the 10 patients [127]. |
| BEZ235 | Dual PI3K/mTOR inhibitor | CT Phase I | 24 | Safe, well tolerated, clinical benefit for small subset of patients with ALL, with no benefit in patients with AML. CR observed in 3 of 24 patients, all of them ALL (3/11) [102]. | |
| Akt | GSK2141795 | Akt inhibitor | CT Phase II | 24 | Safe, well tolerated, no clinical benefit in AML with RAS mutations—no patient obtained CR. The study was closed early due to lack of clinical activity [113]. |
5. Summary and Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heron, M. Deaths: Leading Causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 79488. [Google Scholar]
- Karagianni, P.; Giannouli, S.; Voulgarelis, M. From the (Epi)Genome to Metabolism and Vice Versa; Examples from Hematologic Malignancy. Int. J. Mol. Sci. 2021, 22, 6321. [Google Scholar] [CrossRef] [PubMed]
- Tietsche de Moraes Hungria, V.; Chiattone, C.; Pavlovsky, M.; Abenoza, L.M.; Agreda, G.P.; Armenta, J.; Arrais, C.; Avendaño Flores, O.; Barroso, F.; Basquiera, A.L.; et al. Epidemiology of Hematologic Malignancies in Real-World Settings: Findings From the Hemato-Oncology Latin America Observational Registry Study. J. Glob. Oncol. 2019, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Damlaj, M.; El Fakih, R.; Hashmi, S.K. Evolution of Survivorship in Lymphoma, Myeloma and Leukemia: Metamorphosis of the Field into Long Term Follow-up Care. Blood Rev. 2019, 33, 63–73. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the Phosphoinositide 3-Kinase Pathway in Cancer. Nat. Rev. Drug Discov. 2009, 8, 627–644. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. MTOR Signaling Pathway and MTOR Inhibitors in Cancer: Progress and Challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, I.; Hatfield, K.J.; Grønningsæter, I.S.; Reikvam, H. The PI3K-Akt-MTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells. Int. J. Mol. Sci. 2020, 21, 2907. [Google Scholar] [CrossRef]
- Liu, H.; Hussain, Z.; Xie, Q.; Yan, X.; Zeng, C.; Zhou, G.; Cao, S. Targeting PI3K/AKT/MTOR Pathway to Enhance the Anti-Leukemia Efficacy of Venetoclax. Exp. Cell Res. 2022, 417, 113192. [Google Scholar] [CrossRef]
- Dinner, S.; Platanias, L.C. Targeting the MTOR Pathway in Leukemia. J. Cell Biochem. 2016, 117, 1745–1752. [Google Scholar] [CrossRef]
- Konopleva, M.Y. Mechanisms for Resistance in AML Insights into Molecular Pathways Mediating Resistance to Venetoclax. Best. Pract. Res. Clin. Haematol. 2021, 34, 101251. [Google Scholar] [CrossRef]
- Abbas, H.A.; Mohanty, V.; Wang, R.; Huang, Y.; Liang, S.; Wang, F.; Zhang, J.; Qiu, Y.; Hu, C.W.; Qutub, A.A.; et al. Decoupling Lineage-Associated Genes in Acute Myeloid Leukemia Reveals Inflammatory and Metabolic Signatures Associated With Outcomes. Front. Oncol. 2021, 11, 705627. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/MTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, L.M.; Yuzugullu, H.; Zhao, J.J. PI3K in Cancer: Divergent Roles of Isoforms, Modes of Activation and Therapeutic Targeting. Nat. Rev. Cancer 2015, 15, 7–24. [Google Scholar] [CrossRef]
- Tarantelli, C.; Lupia, A.; Stathis, A.; Bertoni, F. Is There a Role for Dual PI3K/MTOR Inhibitors for Patients Affected with Lymphoma? Int. J. Mol. Sci. 2020, 21, 1060. [Google Scholar] [CrossRef] [PubMed]
- Okkenhaug, K.; Vanhaesebroeck, B. PI3K in Lymphocyte Development, Differentiation and Activation. Nat. Rev. Immunol. 2003, 3, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Tassi, I.; Cella, M.; Gilfillan, S.; Turnbull, I.; Diacovo, T.G.; Penninger, J.M.; Colonna, M. P110γ and P110δ Phosphoinositide 3-Kinase Signaling Pathways Synergize to Control Development and Functions of Murine NK Cells. Immunity 2007, 27, 214–227. [Google Scholar] [CrossRef] [PubMed]
- Kok, K.; Nock, G.E.; Verrall, E.A.G.; Mitchell, M.P.; Hommes, D.W.; Peppelenbosch, M.P.; Vanhaesebroeck, B. Regulation of P110δ PI 3-Kinase Gene Expression. PLoS ONE 2009, 4, e5145. [Google Scholar] [CrossRef] [PubMed]
- Koch, P.A.; Dornan, G.L.; Hessenberger, M.; Haucke, V. The Molecular Mechanisms Mediating Class II PI 3-kinase Function in Cell Physiology. FEBS J. 2021, 288, 7025–7042. [Google Scholar] [CrossRef]
- Alzahrani, A.S. PI3K/Akt/MTOR Inhibitors in Cancer: At the Bench and Bedside. Semin. Cancer Biol. 2019, 59, 125–132. [Google Scholar] [CrossRef]
- Iida, M.; Harari, P.M.; Wheeler, D.L.; Toulany, M. Targeting AKT/PKB to Improve Treatment Outcomes for Solid Tumors. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2020, 819–820, 111690. [Google Scholar] [CrossRef]
- Song, M.; Bode, A.M.; Dong, Z.; Lee, M.-H. AKT as a Therapeutic Target for Cancer. Cancer Res. 2019, 79, 1019–1031. [Google Scholar] [CrossRef]
- Shariati, M.; Meric-Bernstam, F. Targeting AKT for Cancer Therapy. Expert Opin. Investig. Drugs 2019, 28, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Pinker, B.; Barciszewska, A.-M. MTOR Signaling and Potential Therapeutic Targeting in Meningioma. Int. J. Mol. Sci. 2022, 23, 1978. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar-Uniyal, M.; Gillick, J.L.; Neil, J.; Tobias, M.; Thwing, Z.E.; Murali, R. Distinct Signaling Mechanisms of MTORC1 and MTORC2 in Glioblastoma Multiforme: A Tale of Two Complexes. Adv. Biol. Regul. 2015, 57, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Barzegar Behrooz, A.; Talaie, Z.; Jusheghani, F.; Łos, M.J.; Klonisch, T.; Ghavami, S. Wnt and PI3K/Akt/MTOR Survival Pathways as Therapeutic Targets in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 1353. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Dai, Q.; Su, X.; Fu, J.; Feng, X.; Peng, J. Role of PI3K/AKT Pathway in Cancer: The Framework of Malignant Behavior. Mol. Biol. Rep. 2020, 47, 4587–4629. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef] [PubMed]
- Kashani, B.; Zandi, Z.; Kaveh, V.; Pourbagheri-Sigaroodi, A.; Ghaffari, S.H.; Bashash, D. Small Molecules with Huge Impacts: The Role of MiRNA-Regulated PI3K Pathway in Human Malignancies. Mol. Biol. Rep. 2021, 48, 8045–8059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kwok-Shing Ng, P.; Kucherlapati, M.; Chen, F.; Liu, Y.; Tsang, Y.H.; de Velasco, G.; Jeong, K.J.; Akbani, R.; Hadjipanayis, A.; et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/MTOR Pathway Alterations. Cancer Cell 2017, 31, 820–832.e3. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, J.; Zhong, Y.; Geng, R.; Ji, Y.; Guan, Q.; Hong, C.; Wei, Y.; Min, N.; Qi, A.; et al. PIK3CA Mutation Confers Resistance to Chemotherapy in Triple-Negative Breast Cancer by Inhibiting Apoptosis and Activating the PI3K/AKT/MTOR Signaling Pathway. Ann. Transl. Med. 2021, 9, 410. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K–AKT Network at the Interface of Oncogenic Signalling and Cancer Metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Waldhart, A.N.; Dykstra, H.; Peck, A.S.; Boguslawski, E.A.; Madaj, Z.B.; Wen, J.; Veldkamp, K.; Hollowell, M.; Zheng, B.; Cantley, L.C.; et al. Phosphorylation of TXNIP by AKT Mediates Acute Influx of Glucose in Response to Insulin. Cell Rep. 2017, 19, 2005–2013. [Google Scholar] [CrossRef]
- Roberts, D.J.; Tan-Sah, V.P.; Smith, J.M.; Miyamoto, S. Akt Phosphorylates HK-II at Thr-473 and Increases Mitochondrial HK-II Association to Protect Cardiomyocytes. J. Biol. Chem. 2013, 288, 23798–23806. [Google Scholar] [CrossRef] [PubMed]
- Hue, L.; Rider, M.H. Role of Fructose 2,6-Bisphosphate in the Control of Glycolysis in Mammalian Tissues. Biochem. J. 1987, 245, 313–324. [Google Scholar] [CrossRef]
- Betz, C.; Stracka, D.; Prescianotto-Baschong, C.; Frieden, M.; Demaurex, N.; Hall, M.N. MTOR Complex 2-Akt Signaling at Mitochondria-Associated Endoplasmic Reticulum Membranes (MAM) Regulates Mitochondrial Physiology. Proc. Natl. Acad. Sci. USA 2013, 110, 12526–12534. [Google Scholar] [CrossRef]
- Chiara, F.; Castellaro, D.; Marin, O.; Petronilli, V.; Brusilow, W.S.; Juhaszova, M.; Sollott, S.J.; Forte, M.; Bernardi, P.; Rasola, A. Hexokinase II Detachment from Mitochondria Triggers Apoptosis through the Permeability Transition Pore Independent of Voltage-Dependent Anion Channels. PLoS ONE 2008, 3, e1852. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, K.; Shi, L.; Xiang, F.; Tao, K.; Wang, G. Prognostic Significance of the Metabolic Marker Hexokinase-2 in Various Solid Tumors: A Meta-Analysis. PLoS ONE 2016, 11, e0166230. [Google Scholar] [CrossRef]
- Bouchard, C.; Marquardt, J.; Brás, A.; Medema, R.H.; Eilers, M. Myc-Induced Proliferation and Transformation Require Akt-Mediated Phosphorylation of FoxO Proteins. EMBO J. 2004, 23, 2830–2840. [Google Scholar] [CrossRef]
- Krencz, I.; Sztankovics, D.; Danko, T.; Sebestyen, A.; Khoor, A. Progression and Metastasis of Small Cell Lung Carcinoma: The Role of the PI3K/Akt/MTOR Pathway and Metabolic Alterations. Cancer Metastasis Rev. 2021, 40, 1141–1157. [Google Scholar] [CrossRef]
- Berwick, D.C.; Hers, I.; Heesom, K.J.; Moule, S.K.; Tavareá, J.M. The Identification of ATP-Citrate Lyase as a Protein Kinase B (Akt) Substrate in Primary Adipocytes. J. Biol. Chem. 2002, 277, 33895–33900. [Google Scholar] [CrossRef] [PubMed]
- Potapova, I.A.; El-Maghrabi, M.R.; Doronin, S.V.; Benjamin, W.B. Phosphorylation of Recombinant Human ATP:Citrate Lyase by CAMP-Dependent Protein Kinase Abolishes Homotropic Allosteric Regulation of the Enzyme by Citrate and Increases the Enzyme Activity. Allosteric Activation of ATP:Citrate Lyase by Phosphorylated Sugars. Biochemistry 2000, 39, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.V.; Carrer, A.; Shah, S.; Snyder, N.W.; Wei, S.; Venneti, S.; Worth, A.J.; Yuan, Z.-F.; Lim, H.-W.; Liu, S.; et al. Akt-Dependent Metabolic Reprogramming Regulates Tumor Cell Histone Acetylation. Cell Metab. 2014, 20, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Porstmann, T.; Griffiths, B.; Chung, Y.-L.; Delpuech, O.; Griffiths, J.R.; Downward, J.; Schulze, A. PKB/Akt Induces Transcription of Enzymes Involved in Cholesterol and Fatty Acid Biosynthesis via Activation of SREBP. Oncogene 2005, 24, 6465–6481. [Google Scholar] [CrossRef]
- Buttrick, G.J.; Wakefield, J.G. PI3-K and GSK-3: Akt-Ing Together with Microtubules. Cell Cycle 2008, 7, 2621–2625. [Google Scholar] [CrossRef]
- Kim, K.H.; Song, M.J.; Yoo, E.J.; Choe, S.S.; Park, S.D.; Kim, J.B. Regulatory Role of Glycogen Synthase Kinase 3 for Transcriptional Activity of ADD1/SREBP1c. J. Biol. Chem. 2004, 279, 51999–52006. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.N.; Fan, T.W.-M. Regulation of Mammalian Nucleotide Metabolism and Biosynthesis. Nucleic Acids Res. 2015, 43, 2466–2485. [Google Scholar] [CrossRef]
- Villa, E.; Ali, E.; Sahu, U.; Ben-Sahra, I. Cancer Cells Tune the Signaling Pathways to Empower de Novo Synthesis of Nucleotides. Cancers 2019, 11, 688. [Google Scholar] [CrossRef]
- Saha, A.; Connelly, S.; Jiang, J.; Zhuang, S.; Amador, D.T.; Phan, T.; Pilz, R.B.; Boss, G.R. Akt Phosphorylation and Regulation of Transketolase Is a Nodal Point for Amino Acid Control of Purine Synthesis. Mol. Cell 2014, 55, 264–276. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a Metabolic Gene Regulatory Network Downstream of MTOR Complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Sarfraz, I.; Rasul, A.; Hussain, G.; Shah, M.A.; Zahoor, A.F.; Asrar, M.; Selamoglu, Z.; Ji, X.; Adem, Ş.; Sarker, S.D. 6-Phosphogluconate Dehydrogenase Fuels Multiple Aspects of Cancer Cells: From Cancer Initiation to Metastasis and Chemoresistance. BioFactors 2020, 46, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Li, F.; Handler, J.; Huang, C.R.L.; Xiang, Y.; Neretti, N.; Sedivy, J.M.; Zeller, K.I.; Dang, C.V. Global Regulation of Nucleotide Biosynthetic Genes by C-Myc. PLoS ONE 2008, 3, e2722. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Howell, J.J.; Asara, J.M.; Manning, B.D. Stimulation of de Novo Pyrimidine Synthesis by Growth Signaling Through MTOR and S6K1. Science 2013, 339, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sahra, I.; Hoxhaj, G.; Ricoult, S.J.H.; Asara, J.M.; Manning, B.D. MTORC1 Induces Purine Synthesis through Control of the Mitochondrial Tetrahydrofolate Cycle. Science 2016, 351, 728–733. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and Downstream of MTOR. Genes. Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.M.; Blenis, J. Molecular Mechanisms of MTOR-Mediated Translational Control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Cirone, M. Cancer Cells Dysregulate PI3K/AKT/MTOR Pathway Activation to Ensure Their Survival and Proliferation: Mimicking Them Is a Smart Strategy of Gammaherpesviruses. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and MTOR Regulate Autophagy through Direct Phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Napolitano, G.; Esposito, A.; Choi, H.; Matarese, M.; Benedetti, V.; Di Malta, C.; Monfregola, J.; Medina, D.L.; Lippincott-Schwartz, J.; Ballabio, A. MTOR-Dependent Phosphorylation Controls TFEB Nuclear Export. Nat. Commun. 2018, 9, 3312. [Google Scholar] [CrossRef]
- Jung, C.H.; Jun, C.B.; Ro, S.-H.; Kim, Y.-M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.-H. ULK-Atg13-FIP200 Complexes Mediate MTOR Signaling to the Autophagy Machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Poillet-Perez, L.; Despouy, G.; Delage-Mourroux, R.; Boyer-Guittaut, M. Interplay between ROS and Autophagy in Cancer Cells, from Tumor Initiation to Cancer Therapy. Redox Biol. 2015, 4, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, B.; Gygi, S.P.; Goldberg, A.L. MTOR Inhibition Activates Overall Protein Degradation by the Ubiquitin Proteasome System as Well as by Autophagy. Proc. Natl. Acad. Sci. USA 2015, 112, 15790–15797. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Choi, W.H.; Lee, M.J. Effects of MTORC1 Inhibition on Proteasome Activity and Levels. BMB Rep. 2022, 55, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Patel, H.; Alanazi, S.; Kilroy, M.K.; Garrett, J.T. PI3K Inhibitors in Cancer: Clinical Implications and Adverse Effects. Int. J. Mol. Sci. 2021, 22, 3464. [Google Scholar] [CrossRef]
- Puła, B.; Giza, A.; Długosz-Danecka, M.; Rybka, J.; Subocz, E.; Waszczuk-Gajda, A.; Piotrowska, M.; Rej, M.; Jamroziak, K.; Jurczak, W. Ocena Profilu Korzyści i Ryzyka Leczenia Idelalizybem u Chorych Na Przewlekłą Białaczkę Limfocytową i Chłoniaki Nie-Hodgkina. Hematologia 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Davids, M.S.; Fisher, D.C.; Tyekucheva, S.; McDonough, M.; Hanna, J.; Lee, B.; Francoeur, K.; Montegaard, J.; Odejide, O.; Armand, P.; et al. A Phase 1b/2 Study of Duvelisib in Combination with FCR (DFCR) for Frontline Therapy for Younger CLL Patients. Leukemia 2021, 35, 1064–1072. [Google Scholar] [CrossRef]
- Flinn, I.W.; O’Brien, S.; Kahl, B.; Patel, M.; Oki, Y.; Foss, F.F.; Porcu, P.; Jones, J.; Burger, J.A.; Jain, N.; et al. Duvelisib, a Novel Oral Dual Inhibitor of PI3K-δ,γ, Is Clinically Active in Advanced Hematologic Malignancies. Blood 2018, 131, 877–887. [Google Scholar] [CrossRef]
- Flinn, I.W.; Cherry, M.A.; Maris, M.B.; Matous, J.V.; Berdeja, J.G.; Patel, M. Combination Trial of Duvelisib (IPI-145) with Rituximab or Bendamustine/Rituximab in Patients with Non-Hodgkin Lymphoma or Chronic Lymphocytic Leukemia. Am. J. Hematol. 2019, 94, 1325–1334. [Google Scholar] [CrossRef]
- Assouline, S.; Amrein, L.; Aloyz, R.; Banerji, V.; Caplan, S.; Owen, C.; Hasegawa, W.; Robinson, S.; Shivakumar, S.; Prica, A.; et al. IND.216: A Phase II Study of Buparlisib and Associated Biomarkers, Raptor and P70S6K, in Patients with Relapsed and Refractory Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2020, 61, 1653–1659. [Google Scholar] [CrossRef] [PubMed]
- Ragon, B.K.; Kantarjian, H.; Jabbour, E.; Ravandi, F.; Cortes, J.; Borthakur, G.; DeBose, L.; Zeng, Z.; Schneider, H.; Pemmaraju, N.; et al. Buparlisib, a PI3K Inhibitor, Demonstrates Acceptable Tolerability and Preliminary Activity in a Phase I Trial of Patients with Advanced Leukemias. Am. J. Hematol. 2017, 92, 7–11. [Google Scholar] [CrossRef]
- Amrein, L.; Shawi, M.; Grenier, J.; Aloyz, R.; Panasci, L. The Phosphatidylinositol-3 Kinase I Inhibitor BKM120 Induces Cell Death in B-Chronic Lymphocytic Leukemia Cells In Vitro. Int. J. Cancer 2013, 133, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Ghosh, N.; Schuster, S.J.; Lamanna, N.; Pagel, J.M.; Flinn, I.W.; Barrientos, J.C.; Rai, K.R.; Reeves, J.A.; Cheson, B.D.; et al. Phase 2 Study of the Safety and Efficacy of Umbralisib in Patients with CLL Who Are Intolerant to BTK or PI3Kδ Inhibitor Therapy. Blood 2021, 137, 2817–2826. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Kim, H.T.; Nicotra, A.; Savell, A.; Francoeur, K.; Hellman, J.M.; Bazemore, J.; Miskin, H.P.; Sportelli, P.; Stampleman, L.; et al. Umbralisib in Combination with Ibrutinib in Patients with Relapsed or Refractory Chronic Lymphocytic Leukaemia or Mantle Cell Lymphoma: A Multicentre Phase 1–1b Study. Lancet Haematol. 2019, 6, e38–e47. [Google Scholar] [CrossRef]
- Davids, M.S.; O’Connor, O.A.; Jurczak, W.; Samaniego, F.; Fenske, T.S.; Zinzani, P.L.; Patel, M.R.; Ghosh, N.; Cheson, B.D.; Derenzini, E.; et al. Integrated Safety Analysis of Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor, in Relapsed/Refractory Lymphoid Malignancies. Blood Adv. 2021, 5, 5332–5343. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.A.; Flinn, I.W.; Patel, M.R.; Fenske, T.S.; Deng, C.; Brander, D.M.; Gutierrez, M.; Essell, J.H.; Kuhn, J.G.; Miskin, H.P.; et al. Umbralisib, a Novel PI3Kδ and Casein Kinase-1ε Inhibitor, in Relapsed or Refractory Chronic Lymphocytic Leukaemia and Lymphoma: An Open-Label, Phase 1, Dose-Escalation, First-in-Human Study. Lancet Oncol. 2018, 19, 486–496. [Google Scholar] [CrossRef]
- Stengel, S.; Petrie, K.R.; Sbirkov, Y.; Stanko, C.; Ghazvini Zadegan, F.; Gil, V.; Skopek, R.; Kamiński, P.; Szymański, Ł.; Brioli, A.; et al. Suppression of MYC by PI3K/AKT/MTOR Pathway Inhibition in Combination with All-Trans Retinoic Acid Treatment for Therapeutic Gain in Acute Myeloid Leukaemia. Br. J. Haematol. 2022, 198, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Campolo, M.; Paterniti, I. Antioxidants and Anti-Inflammatory Effects in Neurodegenerative Diseases (NDs). Antioxidants 2022, 11, 1172. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Ultimo, S.; Martelli, A.M.; Zauli, G.; Milani, D.; McCubrey, J.A.; Capitani, S.; Neri, L.M. Synergistic Effects of Selective Inhibitors Targeting the PI3K/AKT/MTOR Pathway or NUP214-ABL1 Fusion Protein in Human Acute Lymphoblastic Leukemia. Oncotarget 2016, 7, 79842–79853. [Google Scholar] [CrossRef] [PubMed]
- Evangelisti, C.; Cappellini, A.; Oliveira, M.; Fragoso, R.; Barata, J.T.; Bertaina, A.; Locatelli, F.; Simioni, C.; Neri, L.M.; Chiarini, F.; et al. Phosphatidylinositol 3-Kinase Inhibition Potentiates Glucocorticoid Response in B-Cell Acute Lymphoblastic Leukemia. J. Cell Physiol. 2018, 233, 1796–1811. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, Y.; Chen, X.; Zhao, W.; Zhong, Y.; Wang, R.; Jin, M.; Qiu, Y.; Kong, D. In Vitro Antileukemia Activity of ZSTK474 on K562 and Multidrug Resistant K562/A02 Cells. Int. J. Biol. Sci. 2016, 12, 631–638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ultimo, S.; Simioni, C.; Martelli, A.M.; Zauli, G.; Evangelisti, C.; Celeghini, C.; McCubrey, J.A.; Marisi, G.; Ulivi, P.; Capitani, S.; et al. PI3K Isoform Inhibition Associated with Anti Bcr-Abl Drugs Shows in Vitro Increased Anti-Leukemic Activity in Philadelphia Chromosome-Positive B-Acute Lymphoblastic Leukemia Cell Lines. Oncotarget 2017, 8, 23213–23227. [Google Scholar] [CrossRef] [PubMed]
- Mao, B.; Zhang, Q.; Ma, L.; Zhao, D.-S.; Zhao, P.; Yan, P. Overview of Research into MTOR Inhibitors. Molecules 2022, 27, 5295. [Google Scholar] [CrossRef] [PubMed]
- Buono, R.; Alhaddad, M.; Fruman, D.A. Novel Pharmacological and Dietary Approaches to Target MTOR in B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2023, 13, 1162694. [Google Scholar] [CrossRef]
- Hasskarl, J. Everolimus. In Small Molecules in Oncology; Recent Results in Cancer Research; Springer: Cham, Switzerland, 2018; Volume 211, pp. 101–123. [Google Scholar]
- Burnett, A.K.; Das Gupta, E.; Knapper, S.; Khwaja, A.; Sweeney, M.; Kjeldsen, L.; Hawkins, T.; Betteridge, S.E.; Cahalin, P.; Clark, R.E.; et al. Addition of the Mammalian Target of Rapamycin Inhibitor, Everolimus, to Consolidation Therapy in Acute Myeloid Leukemia: Experience from the UK NCRI AML17 Trial. Haematologica 2018, 103, 1654–1661. [Google Scholar] [CrossRef]
- Place, A.E.; Pikman, Y.; Stevenson, K.E.; Harris, M.H.; Pauly, M.; Sulis, M.-L.; Hijiya, N.; Gore, L.; Cooper, T.M.; Loh, M.L.; et al. Phase I Trial of the MTOR Inhibitor Everolimus in Combination with Multi-Agent Chemotherapy in Relapsed Childhood Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2018, 65, e27062. [Google Scholar] [CrossRef]
- Daver, N.; Boumber, Y.; Kantarjian, H.; Ravandi, F.; Cortes, J.; Rytting, M.E.; Kawedia, J.D.; Basnett, J.; Culotta, K.S.; Zeng, Z.; et al. A Phase I/II Study of the MTOR Inhibitor Everolimus in Combination with HyperCVAD Chemotherapy in Patients with Relapsed/Refractory Acute Lymphoblastic Leukemia. Clin. Cancer Res. 2015, 21, 2704–2714. [Google Scholar] [CrossRef] [PubMed]
- Selvarani, R.; Mohammed, S.; Richardson, A. Effect of Rapamycin on Aging and Age-Related Diseases—Past and Future. Geroscience 2021, 43, 1135–1158. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.S.; Vautier, M.; Allenbach, Y.; Zahr, N.; Benveniste, O.; Funck-Brentano, C.; Salem, J.-E. Sirolimus and MTOR Inhibitors: A Review of Side Effects and Specific Management in Solid Organ Transplantation. Drug Saf. 2019, 42, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.T.; Mick, R.; Jeschke, G.R.; Carabasi, M.; Filicko-O’Hara, J.; Flomenberg, N.; Frey, N.V.; Hexner, E.O.; Luger, S.M.; Loren, A.W.; et al. Sirolimus Enhances Remission Induction in Patients with High Risk Acute Myeloid Leukemia and MTORC1 Target Inhibition. Investig. New Drugs 2018, 36, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Litzow, M.R.; Wang, X.V.; Carroll, M.P.; Karp, J.E.; Ketterling, R.P.; Zhang, Y.; Kaufmann, S.H.; Lazarus, H.M.; Luger, S.M.; Paietta, E.M.; et al. A Randomized Trial of Three Novel Regimens for Recurrent Acute Myeloid Leukemia Demonstrates the Continuing Challenge of Treating This Difficult Disease. Am. J. Hematol. 2019, 94, 111–117. [Google Scholar] [CrossRef]
- Rheingold, S.R.; Tasian, S.K.; Whitlock, J.A.; Teachey, D.T.; Borowitz, M.J.; Liu, X.; Minard, C.G.; Fox, E.; Weigel, B.J.; Blaney, S.M. A Phase 1 Trial of Temsirolimus and Intensive Re-Induction Chemotherapy for 2nd or Greater Relapse of Acute Lymphoblastic Leukaemia: A Children’s Oncology Group Study (ADVL1114). Br. J. Haematol. 2017, 177, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Stasi, R.; Martelli, A.M.; Venditti, A.; Meloni, G.; Pane, F.; Martinelli, G.; Lunghi, M.; Pagano, L.; Cilloni, D.; et al. Temsirolimus, an MTOR Inhibitor, in Combination with Lower-Dose Clofarabine as Salvage Therapy for Older Patients with Acute Myeloid Leukaemia: Results of a Phase II GIMEMA Study (AML-1107). Br. J. Haematol. 2012, 156, 205–212. [Google Scholar] [CrossRef]
- Lee, B.J.; Mallya, S.; Dinglasan, N.; Fung, A.; Nguyen, T.; Herzog, L.; Thao, J.; Lorenzana, E.G.; Wildes, D.; Singh, M.; et al. Efficacy of a Novel Bi-Steric MTORC1 Inhibitor in Models of B-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2021, 11, 673213. [Google Scholar] [CrossRef]
- Burnett, G.L.; Yang, Y.C.; Aggen, J.B.; Pitzen, J.; Gliedt, M.K.; Semko, C.M.; Marquez, A.; Evans, J.W.; Wang, G.; Won, W.S.; et al. Discovery of RMC-5552, a Selective Bi-Steric Inhibitor of MTORC1, for the Treatment of MTORC1-Activated Tumors. J. Med. Chem. 2023, 66, 149–169. [Google Scholar] [CrossRef]
- Gao, H.; Li, Z.; Wang, K.; Zhang, Y.; Wang, T.; Wang, F.; Xu, Y. Design, Synthesis, and Biological Evaluation of Sulfonamide Methoxypyridine Derivatives as Novel PI3K/MTOR Dual Inhibitors. Pharmaceuticals 2023, 16, 461. [Google Scholar] [CrossRef]
- Tasian, S.K.; Teachey, D.T.; Li, Y.; Shen, F.; Harvey, R.C.; Chen, I.-M.; Ryan, T.; Vincent, T.L.; Willman, C.L.; Perl, A.E.; et al. Potent Efficacy of Combined PI3K/MTOR and JAK or ABL Inhibition in Murine Xenograft Models of Ph-like Acute Lymphoblastic Leukemia. Blood 2017, 129, 177–187. [Google Scholar] [CrossRef]
- Lindblad, O.; Cordero, E.; Puissant, A.; Macaulay, L.; Ramos, A.; Kabir, N.N.; Sun, J.; Vallon-Christersson, J.; Haraldsson, K.; Hemann, M.T.; et al. Aberrant Activation of the PI3K/MTOR Pathway Promotes Resistance to Sorafenib in AML. Oncogene 2016, 35, 5119–5131. [Google Scholar] [CrossRef]
- Gazi, M.; Moharram, S.A.; Marhäll, A.; Kazi, J.U. The Dual Specificity PI3K/MTOR Inhibitor PKI-587 Displays Efficacy against T-Cell Acute Lymphoblastic Leukemia (T-ALL). Cancer Lett. 2017, 392, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Wunderle, L.; Badura, S.; Schleyer, E.; Brüggemann, M.; Serve, H.; Schnittger, S.; Gökbuget, N.; Pfeifer, H.; Wagner, S.; et al. A Phase I Study of a Dual PI3-Kinase/MTOR Inhibitor BEZ235 in Adult Patients with Relapsed or Refractory Acute Leukemia. BMC Pharmacol. Toxicol. 2020, 21, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, X.; Ma, C.; Xu, S.; Xu, M.; Yang, J.; Wang, R.; Xue, L. Dual PI3K/MTOR Inhibitor NVP-BEZ235 Decreases the Proliferation of Doxorubicin-resistant K562 Cells. Mol. Med. Rep. 2021, 23, 301. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Jiang, L.; Lin, X.; Tseng, K.-F.; Liu, Y.; Zhang, X.; Dong, R.; Lu, Z.; Wang, X. The PI3K/MTOR Dual Inhibitor BEZ235 Suppresses Proliferation and Migration and Reverses Multidrug Resistance in Acute Myeloid Leukemia. Acta Pharmacol. Sin. 2017, 38, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Sandhöfer, N.; Metzeler, K.H.; Rothenberg, M.; Herold, T.; Tiedt, S.; Groiß, V.; Carlet, M.; Walter, G.; Hinrichsen, T.; Wachter, O.; et al. Dual PI3K/MTOR Inhibition Shows Antileukemic Activity in MLL-Rearranged Acute Myeloid Leukemia. Leukemia 2015, 29, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.P.; Reynolds, C.P.; Kang, M.H. Modulation of Glucocorticoid Resistance in Pediatric T-Cell Acute Lymphoblastic Leukemia by Increasing BIM Expression with the PI3K/MTOR Inhibitor BEZ235. Clin. Cancer Res. 2016, 22, 621–632. [Google Scholar] [CrossRef]
- Xin, P.; Xu, W.; Zhu, X.; Li, C.; Zheng, Y.; Zheng, T.; Cheng, W.; Peng, Q. Protective Autophagy or Autophagic Death: Effects of BEZ235 on Chronic Myelogenous Leukemia. Cancer Manag. Res. 2019, 11, 7933–7951. [Google Scholar] [CrossRef]
- Xin, P.; Li, C.; Zheng, Y.; Peng, Q.; Xiao, H.; Huang, Y.; Zhu, X. Efficacy of the Dual PI3K and MTOR Inhibitor NVP-BEZ235 in Combination with Imatinib Mesylate against Chronic Myelogenous Leukemia Cell Lines. Drug Des. Develop. Ther. 2017, 11, 1115–1126. [Google Scholar] [CrossRef]
- Ishikawa, C.; Senba, M.; Mori, N. Effects of NVP-BEZ235, a Dual Phosphatidylinositol 3-Kinase/Mammalian Target of Rapamycin Inhibitor, on HTLV-1-Infected T-Cell Lines. Oncol. Lett. 2018, 15, 5311–5317. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Yu, L.; Yang, L. Mechanisms of Venetoclax Resistance and Solutions. Front. Oncol. 2022, 12, 1005659. [Google Scholar] [CrossRef]
- Bose, P.; Gandhi, V.; Konopleva, M. Pathways and Mechanisms of Venetoclax Resistance. Leuk. Lymphoma 2017, 58, 2026–2039. [Google Scholar] [CrossRef]
- Dumble, M.; Crouthamel, M.-C.; Zhang, S.-Y.; Schaber, M.; Levy, D.; Robell, K.; Liu, Q.; Figueroa, D.J.; Minthorn, E.A.; Seefeld, M.A.; et al. Discovery of Novel AKT Inhibitors with Enhanced Anti-Tumor Effects in Combination with the MEK Inhibitor. PLoS ONE 2014, 9, e100880. [Google Scholar] [CrossRef] [PubMed]
- Ragon, B.K.; Odenike, O.; Baer, M.R.; Stock, W.; Borthakur, G.; Patel, K.; Han, L.; Chen, H.; Ma, H.; Joseph, L.; et al. Oral MEK 1/2 Inhibitor Trametinib in Combination with AKT Inhibitor GSK2141795 in Patients with Acute Myeloid Leukemia with RAS Mutations: A Phase II Study. Clin. Lymphoma Myeloma Leuk. 2019, 19, 431–440.e13. [Google Scholar] [CrossRef] [PubMed]
- Raedler, L.A. Zydelig (Idelalisib): First-in-Class PI3 Kinase Inhibitor Approved for the Treatment of 3 Hematologic Malignancies. Am. Health Drug Benefits 2015, 8, 157–162. [Google Scholar] [PubMed]
- Duvelisib Approved for Leukemia, Lymphoma. Cancer Discov. 2018, 8, OF4. [CrossRef]
- Nastoupil, L.J.; Lunning, M.A.; Vose, J.M.; Schreeder, M.T.; Siddiqi, T.; Flowers, C.R.; Cohen, J.B.; Burger, J.A.; Wierda, W.G.; O’Brien, S.; et al. Tolerability and Activity of Ublituximab, Umbralisib, and Ibrutinib in Patients with Chronic Lymphocytic Leukaemia and Non-Hodgkin Lymphoma: A Phase 1 Dose Escalation and Expansion Trial. Lancet Haematol. 2019, 6, e100–e109. [Google Scholar] [CrossRef]
- Roeker, L.E.; Feldman, T.A.; Soumerai, J.D.; Falco, V.; Panton, G.; Dorsey, C.; Zelenetz, A.D.; Falchi, L.; Park, J.H.; Straus, D.J.; et al. Adding Umbralisib and Ublituximab (U2) to Ibrutinib in Patients with CLL: A Phase II Study of an MRD-Driven Approach. Clin. Cancer Res. 2022, 28, 3958–3964. [Google Scholar] [CrossRef]
- Park, S.; Chapuis, N.; Saint Marcoux, F.; Recher, C.; Prebet, T.; Chevallier, P.; Cahn, J.-Y.; Leguay, T.; Bories, P.; Witz, F.; et al. A Phase Ib GOELAMS Study of the MTOR Inhibitor RAD001 in Association with Chemotherapy for AML Patients in First Relapse. Leukemia 2013, 27, 1479–1486. [Google Scholar] [CrossRef]
- Zent, C.S.; Bowen, D.A.; Conte, M.J.; LaPlant, B.R.; Call, T.G. Treatment of Relapsed/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma with Everolimus (RAD001) and Alemtuzumab: A Phase I/II Study. Leuk. Lymphoma 2016, 57, 1585–1591. [Google Scholar] [CrossRef]
- Yee, K.W.L.; Zeng, Z.; Konopleva, M.; Verstovsek, S.; Ravandi, F.; Ferrajoli, A.; Thomas, D.; Wierda, W.; Apostolidou, E.; Albitar, M.; et al. Phase I/II Study of the Mammalian Target of Rapamycin Inhibitor Everolimus (RAD001) in Patients with Relapsed or Refractory Hematologic Malignancies. Clin. Cancer Res. 2006, 12, 5165–5173. [Google Scholar] [CrossRef]
- Zent, C.S.; LaPlant, B.R.; Johnston, P.B.; Call, T.G.; Habermann, T.M.; Micallef, I.N.; Witzig, T.E. The Treatment of Recurrent/Refractory Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL) with Everolimus Results in Clinical Responses and Mobilization of CLL Cells into the Circulation. Cancer 2010, 116, 2201–2207. [Google Scholar] [CrossRef]
- Liesveld, J.L.; O’Dwyer, K.; Walker, A.; Becker, M.W.; Ifthikharuddin, J.J.; Mulford, D.; Chen, R.; Bechelli, J.; Rosell, K.; Minhajuddin, M.; et al. A Phase I Study of Decitabine and Rapamycin in Relapsed/Refractory AML. Leuk. Res. 2013, 37, 1622–1627. [Google Scholar] [CrossRef]
- Liesveld, J.L.; Baran, A.; Azadniv, M.; Misch, H.; Nedrow, K.; Becker, M.; Loh, K.P.; O’Dwyer, K.M.; Mendler, J.H. A Phase II Study of Sequential Decitabine and Rapamycin in Acute Myelogenous Leukemia. Leuk. Res. 2022, 112, 106749. [Google Scholar] [CrossRef] [PubMed]
- Rheingold, S.R.; Silverman, L.B.; Whitlock, J.A.; Sposto, R.; Schafer, E.S.; Schultz, K.R.; Hutchinson, R.J.; Gaynon, P.S.; Bateman, C.; Cooper, T.M.; et al. Temsirolimus Combined with Etoposide and Cyclophosphamide for Relapsed/Refractory Acute Lymphoblastic Leukemia: Therapeutic Advances in Childhood Leukemia Consortium (TACL 2014-001) Trial. J. Clin. Oncol. 2020, 38, 10512. [Google Scholar] [CrossRef]
- Rheingold, S.R.; Whitlock, J.; Tasian, S.K.; Teachey, D.T.; Borowitz, M.J.; Liu, X.; Ahern, C.H.; Minard, C.; Fox, E.; Weigel, B.; et al. Temsirolimus and Intensive Re-Induction Chemotherapy for 2nd or Greater Relapse of Acute Lymphoblastic Leukemia (ALL): A Children’s Oncology Group Study. J. Clin. Oncol. 2015, 33, 10029. [Google Scholar] [CrossRef]
- Smith, S.M.; van Besien, K.; Karrison, T.; Dancey, J.; McLaughlin, P.; Younes, A.; Smith, S.; Stiff, P.; Lester, E.; Modi, S.; et al. Temsirolimus Has Activity in Non–Mantle Cell Non-Hodgkin’s Lymphoma Subtypes: The University of Chicago Phase II Consortium. J. Clin. Oncol. 2010, 28, 4740–4746. [Google Scholar] [CrossRef] [PubMed]
- Vargaftig, J.; Farhat, H.; Ades, L.; Briaux, A.; Benoist, C.; Turbiez, I.; Vey, N.; Glaisner, S.; Callens, C. Phase 2 Trial of Single Agent Gedatolisib (PF-05212384), a Dual PI3K/MTOR Inhibitor, for Adverse Prognosis and Relapse/Refractory AML: Clinical and Transcriptomic Results. Blood 2018, 132, 5233. [Google Scholar] [CrossRef]
| PI3K Class | Subunits | Isoforms | Encoding Gene |
|---|---|---|---|
| IA | Catalytic subunits + regulating subunit isoform | p110α, p110β, p110δ | PIK3CA, PIK3CB, PI3KCD |
| IB | - | p110γ | PIK3CG |
| II | Only the catalytic subunit | PI3K-C2α, PI3K-C2β, PI3K-C2γ | PIK3C2A, PIK3C2B, PIK3C2G |
| III | Vps34 | - | PIK3C3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiese, W.; Barczuk, J.; Racinska, O.; Siwecka, N.; Rozpedek-Kaminska, W.; Slupianek, A.; Sierpinski, R.; Majsterek, I. PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities. Cancers 2023, 15, 5297. https://doi.org/10.3390/cancers15215297
Wiese W, Barczuk J, Racinska O, Siwecka N, Rozpedek-Kaminska W, Slupianek A, Sierpinski R, Majsterek I. PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities. Cancers. 2023; 15(21):5297. https://doi.org/10.3390/cancers15215297
Chicago/Turabian StyleWiese, Wojciech, Julia Barczuk, Olga Racinska, Natalia Siwecka, Wioletta Rozpedek-Kaminska, Artur Slupianek, Radoslaw Sierpinski, and Ireneusz Majsterek. 2023. "PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities" Cancers 15, no. 21: 5297. https://doi.org/10.3390/cancers15215297
APA StyleWiese, W., Barczuk, J., Racinska, O., Siwecka, N., Rozpedek-Kaminska, W., Slupianek, A., Sierpinski, R., & Majsterek, I. (2023). PI3K/Akt/mTOR Signaling Pathway in Blood Malignancies—New Therapeutic Possibilities. Cancers, 15(21), 5297. https://doi.org/10.3390/cancers15215297







