MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
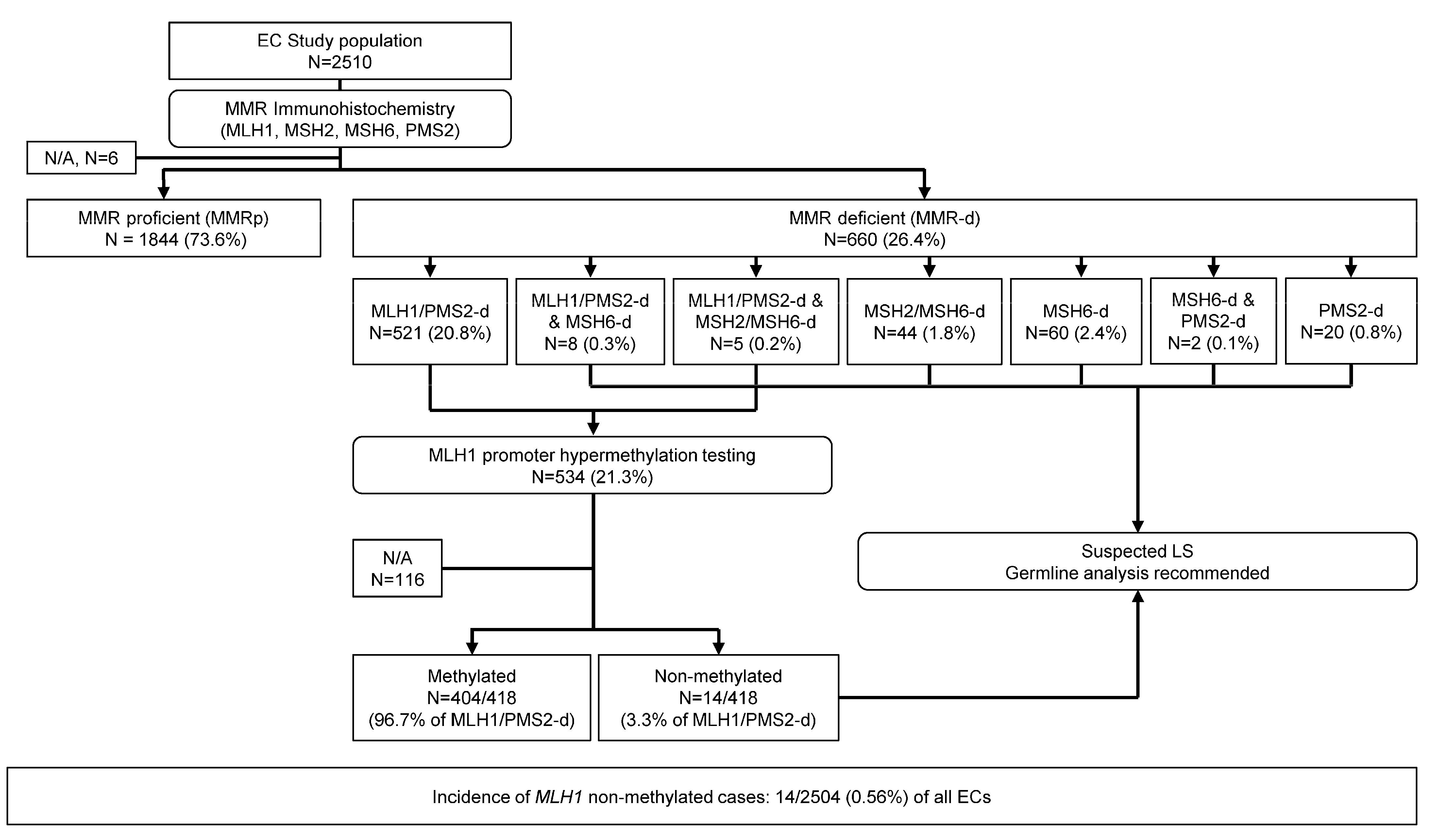
2.1. Study Population
2.2. Universal Tumor Screening Protocol
2.3. Statistical Analysis
2.4. Cost Analysis
3. Results
3.1. Cohort of Universally Screened Endometrial Carcinomas
3.2. MLH1 Promoter Hypermethylation Testing Results
3.3. Clinicopathologic Features of MLH1-Methylated and Non-Methylated Endometrial Carcinomas
3.4. Cost Analysis of Reflex MLH1 Methylation Testing
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.; Huvila, J.; Thompson, E.F.; Leung, S.; Chiu, D.; Lum, A.; McConechy, M.; Grondin, K.; Aguirre-Hernandez, R.; Salvador, S.; et al. Variation in practice in endometrial cancer and potential for improved care and equity through molecular classification. Gynecol. Oncol. 2022, 165, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.A.J.; Glaire, M.A.; Blake, D.; Cabrera-Dandy, M.; Evans, D.G.; Crosbie, E.J. The proportion of endometrial cancers associated with Lynch syndrome: A systematic review of the literature and meta-analysis. Genet. Med. 2019, 21, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Post, C.C.B.; Stelloo, E.; Smit, V.; Ruano, D.; Tops, C.M.; Vermij, L.; Rutten, T.A.; Jurgenliemk-Schulz, I.M.; Lutgens, L.; Jobsen, J.J.; et al. Prevalence and Prognosis of Lynch Syndrome and Sporadic Mismatch Repair Deficiency in Endometrial Cancer. J. Natl. Cancer Inst. 2021, 113, 1212–1220. [Google Scholar] [CrossRef] [PubMed]
- Meyer, L.A.; Broaddus, R.R.; Lu, K.H. Endometrial cancer and Lynch syndrome: Clinical and pathologic considerations. Cancer Control 2009, 16, 14–22. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research, N.; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Leon-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Consortium, R.R. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, I.; D’Oria, O.; Sgamba, L.; De Angelis, E.; Golia D’Augè, T.; Turetta, C.; Di Dio, C.; Scudo, M.; Bogani, G.; Di Donato, V.; et al. Role of Genomic and Molecular Biology in the Modulation of the Treatment of Endometrial Cancer: Narrative Review and Perspectives. Healthcare 2023, 11, 571. [Google Scholar] [CrossRef]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Stark, M.; Signore, F.; Gullo, G.; Marinelli, E. Fertility preservation in female cancer sufferers: (only) a moral obligation? Eur. J. Contracept. Reprod. Health Care 2022, 27, 335–340. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; De Jesus Acosta, A.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients With Microsatellite Instability-High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef]
- Bellone, S.; Roque, D.M.; Siegel, E.R.; Buza, N.; Hui, P.; Bonazzoli, E.; Guglielmi, A.; Zammataro, L.; Nagarkatti, N.; Zaidi, S.; et al. A phase II evaluation of pembrolizumab in recurrent microsatellite instability-high (MSI-H) endometrial cancer patients with Lynch-like versus MLH-1 methylated characteristics (NCT02899793). Ann. Oncol. 2021, 32, 1045–1046. [Google Scholar] [CrossRef]
- Buchanan, D.D.; Tan, Y.Y.; Walsh, M.D.; Clendenning, M.; Metcalf, A.M.; Ferguson, K.; Arnold, S.T.; Thompson, B.A.; Lose, F.A.; Parsons, M.T.; et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J. Clin. Oncol. 2014, 32, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Batte, B.A.; Bruegl, A.S.; Daniels, M.S.; Ring, K.L.; Dempsey, K.M.; Djordjevic, B.; Luthra, R.; Fellman, B.M.; Lu, K.H.; Broaddus, R.R. Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecol. Oncol. 2014, 134, 319–325. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Favier, A.; Varinot, J.; Uzan, C.; Duval, A.; Brocheriou, I.; Canlorbe, G. The Role of Immunohistochemistry Markers in Endometrial Cancer with Mismatch Repair Deficiency: A Systematic Review. Cancers 2022, 14, 3783. [Google Scholar] [CrossRef] [PubMed]
- Goodfellow, P.J.; Billingsley, C.C.; Lankes, H.A.; Ali, S.; Cohn, D.E.; Broaddus, R.J.; Ramirez, N.; Pritchard, C.C.; Hampel, H.; Chassen, A.S.; et al. Combined Microsatellite Instability, MLH1 Methylation Analysis, and Immunohistochemistry for Lynch Syndrome Screening in Endometrial Cancers From GOG210: An NRG Oncology and Gynecologic Oncology Group Study. J. Clin. Oncol. 2015, 33, 4301–4308. [Google Scholar] [CrossRef]
- Hitchins, M.P.; Alvarez, R.; Zhou, L.; Aguirre, F.; Damaso, E.; Pineda, M.; Capella, G.; Wong, J.J.; Yuan, X.; Ryan, S.R.; et al. MLH1-methylated endometrial cancer under 60 years of age as the “sentinel” cancer in female carriers of high-risk constitutional MLH1 epimutation. Gynecol. Oncol. 2023, 171, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.; Richer, L.; Arseneau, J.; Zeng, X.; Chong, G.; Weber, E.; Foulkes, W.; Palma, L. Mismatch Repair Universal Screening of Endometrial Cancers (MUSE) in a Canadian Cohort. Curr. Oncol. 2021, 28, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Kurpiel, B.; Thomas, M.S.; Mubeen, M.; Ring, K.L.; Modesitt, S.C.; Moskaluk, C.A.; Mills, A.M. MLH1/PMS2-deficient Endometrial Carcinomas in a Universally Screened Population: MLH1 Hypermethylation and Germline Mutation Status. Int. J. Gynecol. Pathol. 2022, 41, 1–11. [Google Scholar] [CrossRef]
- Pasanen, A.; Loukovaara, M.; Kaikkonen, E.; Olkinuora, A.; Pylvanainen, K.; Alhopuro, P.; Peltomaki, P.; Mecklin, J.P.; Butzow, R. Testing for Lynch Syndrome in Endometrial Carcinoma: From Universal to Age-Selective MLH1 Methylation Analysis. Cancers 2022, 14, 1348. [Google Scholar] [CrossRef]
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. In Improving Diagnosis in Health Care; Balogh, E.P., Miller, B.T., Ball, J.R., Eds.; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouelian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results from the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Vale, N. Dostarlimab: A Review. Biomolecules 2022, 12, 1031. [Google Scholar] [CrossRef]
- Mills, A.M.; Longacre, T.A. Lynch Syndrome Screening in the Gynecologic Tract: Current State of the Art. Am. J. Surg. Pathol. 2016, 40, e35–e44. [Google Scholar] [CrossRef]
- Zyla, R.; Graham, T.; Aronson, M.; Velsher, L.; Mrkonjic, M.; Turashvili, G. MLH1 epimutation is a rare mechanism for Lynch syndrome: A case report and review of the literature. Genes Chromosomes Cancer 2021, 60, 635–639. [Google Scholar] [CrossRef]
- Hitchins, M.P. Finding the needle in a haystack: Identification of cases of Lynch syndrome with MLH1 epimutation. Fam. Cancer 2016, 15, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Pinto, D.; Pinto, C.; Guerra, J.; Pinheiro, M.; Santos, R.; Vedeld, H.M.; Yohannes, Z.; Peixoto, A.; Santos, C.; Pinto, P.; et al. Contribution of MLH1 constitutional methylation for Lynch syndrome diagnosis in patients with tumor MLH1 downregulation. Cancer Med. 2018, 7, 433–444. [Google Scholar] [CrossRef]
- Takeda, T.; Banno, K.; Yanokura, M.; Adachi, M.; Iijima, M.; Kunitomi, H.; Nakamura, K.; Iida, M.; Nogami, Y.; Umene, K.; et al. Methylation Analysis of DNA Mismatch Repair Genes Using DNA Derived from the Peripheral Blood of Patients with Endometrial Cancer: Epimutation in Endometrial Carcinogenesis. Genes 2016, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Mrkonjic, M.; Turashvili, G. EPM2AIP1 Immunohistochemistry Can Be Used as Surrogate Testing for MLH1 Promoter Methylation in Endometrial Cancer. Am. J. Surg. Pathol. 2022, 46, 376–382. [Google Scholar] [CrossRef] [PubMed]
| MLH1 Non-Methylated n = 14 | MLH1 Methylated n = 404 | p-Value | |
|---|---|---|---|
| Age, median (range) | 57 (37–71) | 64 (39–94) | 0.018 |
| Histological type | 0.686 | ||
| Endometrioid | 12 (85.7%) | 323 (80.0%) | |
| G1 | 6 | 137 | |
| G2 | 5 | 141 | |
| G3 | 1 | 44 | |
| N/A | - | 1 | |
| Mucinous | - | 3 (0.7%) | |
| Clear cell | - | 2 (0.5%) | |
| Carcinosarcoma | - | 6 (1.5%) | |
| Mixed | - | 11 (2.7%) | |
| Serous | 1 (7.1%) | - | |
| Dedifferentiated/Undifferentiated | 1 (7.1%) | 7 (1.7%) | |
| High grade carcinoma, unclassifiable | - | 16 (4.0%) | |
| N/A | - | 36 | |
| P53 | 0.051 | ||
| Wild-type | 4 (66.7%) | 168 (94.4%) | |
| Abnormal | 2 (33.3%) | 10 (5.6%) | |
| N/A | 8 | 226 | |
| ER | 0.492 | ||
| Positive | 3 (21.4%) | 69 (85.2%) | |
| Negative | 1 (7.1%) | 12 (14.8%) | |
| N/A | 10 | 323 | |
| POLE | - | ||
| Wild-type | - | 9 (2.2%) | |
| Mutated | - | - | |
| N/A | 13 | 395 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plotkin, A.; Olkhov-Mitsel, E.; Nofech-Mozes, S. MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal. Cancers 2023, 15, 5188. https://doi.org/10.3390/cancers15215188
Plotkin A, Olkhov-Mitsel E, Nofech-Mozes S. MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal. Cancers. 2023; 15(21):5188. https://doi.org/10.3390/cancers15215188
Chicago/Turabian StylePlotkin, Anna, Ekaterina Olkhov-Mitsel, and Sharon Nofech-Mozes. 2023. "MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal" Cancers 15, no. 21: 5188. https://doi.org/10.3390/cancers15215188
APA StylePlotkin, A., Olkhov-Mitsel, E., & Nofech-Mozes, S. (2023). MLH1 Methylation Testing as an Integral Component of Universal Endometrial Cancer Screening—A Critical Appraisal. Cancers, 15(21), 5188. https://doi.org/10.3390/cancers15215188




