Cholesterol Metabolism in Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
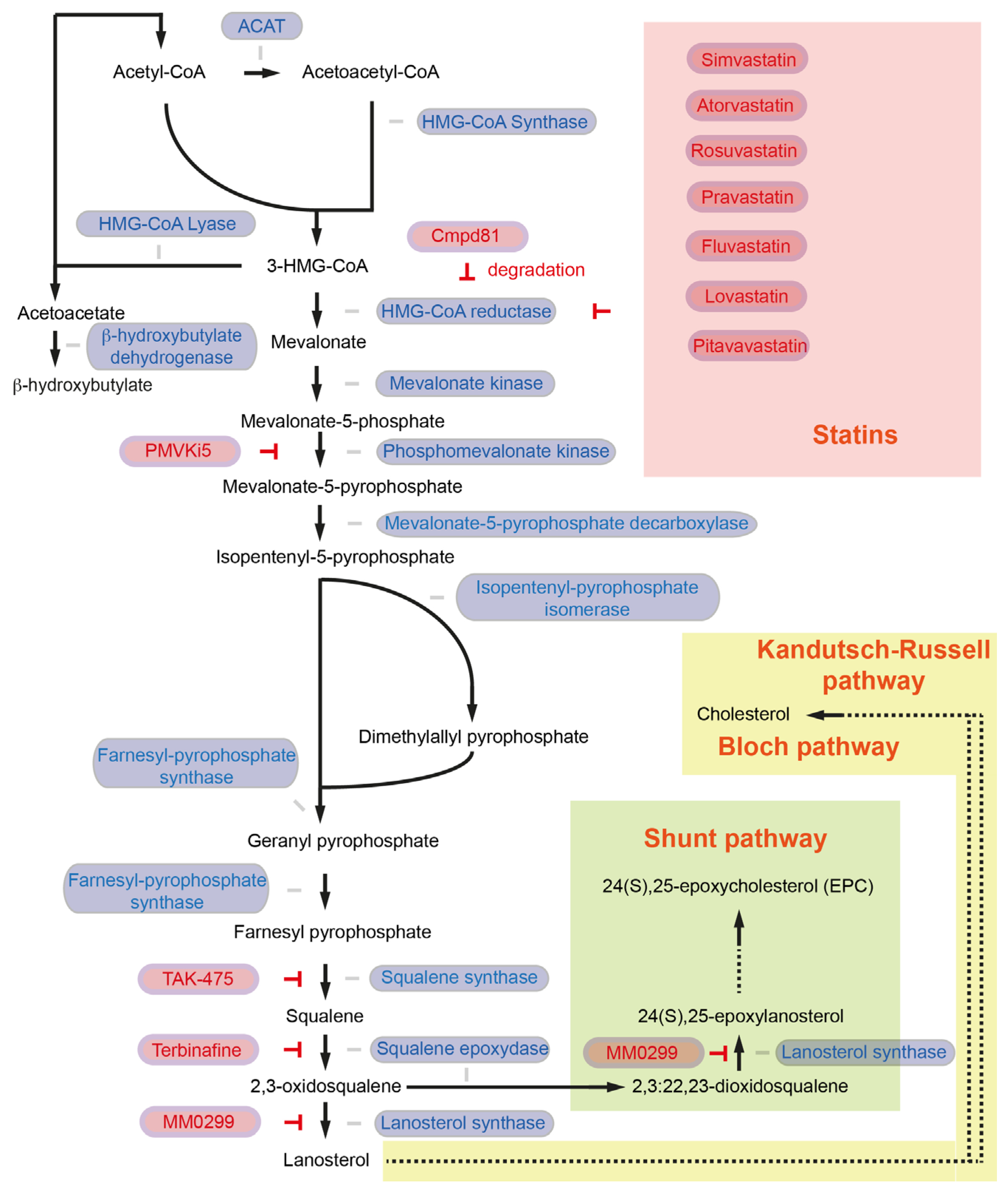
2. The Role of the Mevalonate Pathway and De Novo Cholesterol Synthesis in Pancreatic Cancer
| Intervention/Treatment | Target | Reference |
|---|---|---|
| Simvastatin | HMGCR | [25,27,55] |
| Atorvastatin | HMGCR | [26,27] |
| Rosuvastatin | HMGCR | [27] |
| Pravastatin | HMGCR | [27] |
| Fluvastatin | HMGCR | [27] |
| Lovastatin | HMGCR | [27] |
| Pitavastatin | HMGCR | [27] |
| Cmpd81 | HMGCR (degradation) | [33] |
| PMVKi5 | PMVK | [36] |
| TAK-475 | FDFT1 | [43] |
| terbinafine | SQLE | [46,48,50,51] |
| MM0299 | LSS | [54] |
| Avasimibe | SOAT1 | [56,57] |
| Surface anchor-engineered T cells with liposomal avasibime | SOAT1 | [58] |
| CP-113,818 | SOAT1 | [57] |
| K-604 | SOAT1 | [57] |
| Nilotinib | SOAT1 | [59] |
| Alirocumab | PCSK9 | [60] |
| Evolocumab | PCSK9 | [60] |
| R-IMPP | PCSK9 | [55] |
| PF-06446846 | PCSK9 | [55] |
3. Cholesterol Modification, Lipoproteins, Uptake, and Transport in Pancreatic Cancer
4. Targeting Cholesterol Metabolism and Clinical Trials
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- International Cancer Research Association. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 1 August 2023).
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sunami, Y.; Rebelo, A.; Kleeff, J. Lipid Metabolism and Lipid Droplets in Pancreatic Cancer and Stellate Cells. Cancers 2017, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Pirhonen, J.; Szkalisity, Á.; Hagström, J.; Kim, Y.; Migh, E.; Kovács, M.; Hölttä, M.; Peränen, J.; Seppänen, H.; Haglund, C.; et al. Lipid Metabolic Reprogramming Extends beyond Histologic Tumor Demarcations in Operable Human Pancreatic Cancer. Cancer Res. 2022, 82, 3932–3949. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry 2016, 55, 5483–5506. [Google Scholar] [CrossRef] [PubMed]
- Mayengbam, S.S.; Singh, A.; Pillai, A.D.; Bhat, M.K. Influence of cholesterol on cancer progression and therapy. Transl. Oncol. 2021, 14, 101043. [Google Scholar] [CrossRef]
- Juarez, D.; Fruman, D.A. Targeting the Mevalonate Pathway in Cancer. Trends Cancer 2021, 7, 525–540. [Google Scholar] [CrossRef]
- Carrer, A.; Trefely, S.; Zhao, S.; Campbell, S.L.; Norgard, R.J.; Schultz, K.C.; Sidoli, S.; Parris, J.L.D.; Affronti, H.C.; Sivanand, S.; et al. Acetyl-CoA Metabolism Supports Multistep Pancreatic Tumorigenesis. Cancer Discov. 2019, 9, 416–435. [Google Scholar] [CrossRef]
- Storz, P. Acinar cell plasticity and development of pancreatic ductal adenocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 296–304. [Google Scholar] [CrossRef]
- Marmorstein, R.; Zhou, M.M. Writers and readers of histone acetylation: Structure, mechanism, and inhibition. Cold Spring Harb. Perspect. Biol. 2014, 6, a018762. [Google Scholar] [CrossRef]
- Shi, J.; Vakoc, C.R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 2014, 54, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Lin, R.; Xia, S.; Chen, D.; Elf, S.E.; Liu, S.; Pan, Y.; Xu, H.; Qian, Z.; Wang, M.; et al. Tetrameric Acetyl-CoA Acetyltransferase 1 Is Important for Tumor Growth. Mol. Cell 2016, 64, 859–874. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, A. The recent insights into the function of ACAT1: A possible anti-cancer therapeutic target. Life Sci. 2019, 232, 116592. [Google Scholar] [CrossRef]
- Souchek, J.J.; Baine, M.J.; Lin, C.; Rachagani, S.; Gupta, S.; Kaur, S.; Lester, K.; Zheng, D.; Chen, S.; Smith, L.; et al. Unbiased analysis of pancreatic cancer radiation resistance reveals cholesterol biosynthesis as a novel target for radiosensitisation. Br. J. Cancer 2014, 111, 1139–1149. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Cao, Y.; Zhao, L. Pan-cancer analysis reveals the oncogenic role of 3-hydroxy-3-methylglutaryl-CoA synthase 1. Cancer Rep. 2022, 5, e1562. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.H.; Huang, T.T.; Chen, J.L.; Chu, L.W.; Ping, Y.H.; Hsu, K.W.; Huang, K.H.; Fang, W.L.; Lee, H.C.; Chen, C.F.; et al. Mevalonate Pathway Enzyme HMGCS1 Contributes to Gastric Cancer Progression. Cancers 2020, 12, 1088. [Google Scholar] [CrossRef]
- Walsh, C.A.; Akrap, N.; Garre, E.; Magnusson, Y.; Harrison, H.; Andersson, D.; Jonasson, E.; Rafnsdottir, S.; Choudhry, H.; Buffa, F.; et al. The mevalonate precursor enzyme HMGCS1 is a novel marker and key mediator of cancer stem cell enrichment in luminal and basal models of breast cancer. PLoS ONE 2020, 15, e0236187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, J.; Du, J.; Jiang, X.; Xu, X.; Liu, Y.; He, Q.; Liang, H.; Fang, P.; Zhan, H.; et al. HMGCS1 drives drug-resistance in acute myeloid leukemia through endoplasmic reticulum-UPR-mitochondria axis. Biomed. Pharmacother. 2021, 137, 111378. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, M.D.; Yudkovitz, J.B.; Lo, C.Y.; Chen, J.S.; Alberts, A.W.; Hunt, V.M.; Chang, M.N.; Yang, S.S.; Thompson, K.L.; Chiang, Y.C.; et al. Inhibition of hydroxymethylglutaryl-coenzyme A synthase by L-659,699. Proc. Natl. Acad. Sci. USA 1987, 84, 7488–7492. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wang, Z.; Yang, S.; Li, H.; Zhao, L. Hymeglusin Enhances the Pro-Apoptotic Effects of Venetoclax in Acute Myeloid Leukemia. Front. Oncol. 2022, 12, 864430. [Google Scholar] [CrossRef]
- Gouirand, V.; Gicquel, T.; Lien, E.C.; Jaune-Pons, E.; Da Costa, Q.; Finetti, P.; Metay, E.; Duluc, C.; Mayers, J.R.; Audebert, S.; et al. Ketogenic HMG-CoA lyase and its product β-hydroxybutyrate promote pancreatic cancer progression. EMBO J. 2022, 41, e110466. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Bidaut, G.; Ouaissi, M.; Servais, S.; Gouirand, V.; Olivares, O.; Lac, S.; Borge, L.; Roques, J.; Gayet, O.; et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl. Acad. Sci. USA 2015, 112, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Gunda, V.; Genaro-Mattos, T.C.; Kaushal, J.B.; Chirravuri-Venkata, R.; Natarajan, G.; Mallya, K.; Grandgenett, P.M.; Mirnics, K.; Batra, S.K.; Korade, Z.; et al. Ubiquitous Aberration in Cholesterol Metabolism across Pancreatic Ductal Adenocarcinoma. Metabolites 2022, 12, 47. [Google Scholar] [CrossRef]
- Fendrich, V.; Sparn, M.; Lauth, M.; Knoop, R.; Plassmeier, L.; Bartsch, D.K.; Waldmann, J. Simvastatin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Pancreatology 2013, 13, 502–507. [Google Scholar] [CrossRef]
- Liao, J.; Chung, Y.T.; Yang, A.L.; Zhang, M.; Li, H.; Zhang, W.; Yan, L.; Yang, G.Y. Atorvastatin inhibits pancreatic carcinogenesis and increases survival in LSL-KrasG12D-LSL-Trp53R172H-Pdx1-Cre mice. Mol. Carcinog. 2013, 52, 739–750. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zhang, Y.; Liang, M.; Sun, C.; Qu, G.; Shi, T.; Min, M.; Wu, Y.; Sun, Y. Statin Use and Risk of Pancreatic Cancer: An Updated Meta-analysis of 26 Studies. Pancreas 2019, 48, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mistafa, O.; Stenius, U. Statins inhibit Akt/PKB signaling via P2X7 receptor in pancreatic cancer cells. Biochem. Pharmacol. 2009, 78, 1115–1126. [Google Scholar] [CrossRef]
- Mohammed, A.; Qian, L.; Janakiram, N.B.; Lightfoot, S.; Steele, V.E.; Rao, C.V. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int. J. Cancer 2012, 131, 1951–1962. [Google Scholar] [CrossRef]
- Uemura, N.; Hayashi, H.; Liu, Z.; Matsumura, K.; Ogata, Y.; Yasuda, N.; Sato, H.; Shiraishi, Y.; Miyata, T.; Nakagawa, S.; et al. Statins exert anti-growth effects by suppressing YAP/TAZ expressions via JNK signal activation and eliminate the immune suppression by downregulating PD-L1 expression in pancreatic cancer. Am. J. Cancer Res. 2023, 13, 2041–2054. [Google Scholar]
- Dorsch, M.; Kowalczyk, M.; Planque, M.; Heilmann, G.; Urban, S.; Dujardin, P.; Forster, J.; Ueffing, K.; Nothdurft, S.; Oeck, S.; et al. Statins affect cancer cell plasticity with distinct consequences for tumor progression and metastasis. Cell Rep. 2021, 37, 110056. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Li, H.; Tang, J.J.; Wang, J.; Luo, J.; Liu, B.; Wang, J.K.; Shi, X.J.; Cui, H.W.; Tang, J.; et al. Discovery of a Potent HMG-CoA Reductase Degrader That Eliminates Statin-Induced Reductase Accumulation and Lowers Cholesterol. Nat. Commun. 2018, 9, 5138. [Google Scholar] [CrossRef] [PubMed]
- Boucher, Y.; Kamekura, M.; Doolittle, W.F. Origins and evolution of isoprenoid lipid biosynthesis in archaea. Mol. Microbiol. 2004, 52, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Clizbe, D.B.; Owens, M.L.; Masuda, K.R.; Shackelford, J.E.; Krisans, S.K. IDI2, a second isopentenyl diphosphate isomerase in mammals. J. Biol. Chem. 2007, 282, 6668–6676. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, X.; Zhou, X.; Tang, Y.; Lu, M.; Zhao, J.; Tian, C.; Wu, M.; Liu, Y.; Prochownik, E.V.; et al. Phosphomevalonate Kinase Controls β-Catenin Signaling via the Metabolite 5-Diphosphomevalonate. Adv. Sci. 2023, 10, e2204909. [Google Scholar] [CrossRef]
- Seshacharyulu, P.; Rachagani, S.; Muniyan, S.; Siddiqui, J.A.; Cruz, E.; Sharma, S.; Krishnan, R.; Killips, B.J.; Sheinin, Y.; Lele, S.M.; et al. FDPS cooperates with PTEN loss to promote prostate cancer progression through modulation of small GTPases/AKT axis. Oncogene 2019, 38, 5265–5280. [Google Scholar] [CrossRef]
- Schmid, R.M. HMG-CoA reductase inhibitors for the treatment of pancreatic cancer. Gastroenterology 2002, 122, 565–567. [Google Scholar] [CrossRef]
- Van de Donk, N.W.; Kamphuis, M.M.; van Kessel, B.; Lokhorst, H.M.; Bloem, A.C. Inhibition of protein geranylgeranylation induces apoptosis in myeloma plasma cells by reducing Mcl-1 protein levels. Blood 2003, 102, 3354–3362. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The Critical Driver and Therapeutic Target for Pancreatic Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Kemp, S.B.; Cheng, N.; Markosyan, N.; Sor, R.; Kim, I.K.; Hallin, J.; Shoush, J.; Quinones, L.; Brown, N.V.; Bassett, J.B.; et al. Efficacy of a Small-Molecule Inhibitor of KrasG12D in Immunocompetent Models of Pancreatic Cancer. Cancer Discov. 2023, 13, 298–311. [Google Scholar] [CrossRef]
- Kazi, A.; Xiang, S.; Yang, H.; Chen, L.; Kennedy, P.; Ayaz, M.; Fletcher, S.; Cummings, C.; Lawrence, H.R.; Beato, F.; et al. Dual Farnesyl and Geranylgeranyl Transferase Inhibitor Thwarts Mutant KRAS-Driven Patient-Derived Pancreatic Tumors. Clin. Cancer Res. 2019, 25, 5984–5996. [Google Scholar] [CrossRef]
- Biancur, D.E.; Kapner, K.S.; Yamamoto, K.; Banh, R.S.; Neggers, J.E.; Sohn, A.S.W.; Wu, W.; Manguso, R.T.; Brown, A.; Root, D.E.; et al. Functional Genomics Identifies Metabolic Vulnerabilities in Pancreatic Cancer. Cell Metab. 2021, 33, 199–210.e8. [Google Scholar] [CrossRef]
- Jiang, H.; Tang, E.; Chen, Y.; Liu, H.; Zhao, Y.; Lin, M.; He, L. Squalene synthase predicts poor prognosis in stage I–III colon adenocarcinoma and synergizes squalene epoxidase to promote tumor progression. Cancer Sci. 2022, 113, 971–985. [Google Scholar] [CrossRef] [PubMed]
- Bai, R.; Rebelo, A.; Kleeff, J.; Sunami, Y. Identification of prognostic lipid droplet-associated genes in pancreatic cancer patients via bioinformatics analysis. Lipids Health Dis. 2021, 20, 58. [Google Scholar] [CrossRef]
- Wang, S.; Dong, L.; Ma, L.; Yang, S.; Zheng, Y.; Zhang, J.; Wu, C.; Zhao, Y.; Hou, Y.; Li, H.; et al. SQLE facilitates the pancreatic cancer progression via the lncRNA-TTN-AS1/miR-133b/SQLE axis. J. Cell. Mol. Med. 2022, 26, 3636–3647. [Google Scholar] [CrossRef]
- Xu, R.; Song, J.; Ruze, R.; Chen, Y.; Yin, X.; Wang, C.; Zhao, Y. SQLE promotes pancreatic cancer growth by attenuating ER stress and activating lipid rafts-regulated Src/PI3K/Akt signaling pathway. Cell Death Dis. 2023, 14, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Huang, Y.; Zhang, Y.; Li, X.; Chen, K.; Long, Y.; Li, F.; Ma, X. SQLE inhibition suppresses the development of pancreatic ductal adenocarcinoma and enhances its sensitivity to chemotherapeutic agents in vitro. Mol. Biol. Rep. 2022, 49, 6613–6621. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, L.; Li, W.; Yang, F.; Zhang, Z.; Liu, Z.; Du, W. p53 transcriptionally regulates SQLE to repress cholesterol synthesis and tumor growth. EMBO Rep. 2021, 22, e52537. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wong, C.C.; Fu, L.; Chen, H.; Zhao, L.; Li, C.; Zhou, Y.; Zhang, Y.; Xu, W.; Yang, Y.; et al. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci. Transl. Med. 2018, 10, eaap9840. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Y.; Liu, D.; Wong, C.C.; Coker, O.O.; Zhang, X.; Liu, C.; Zhou, Y.; Liu, Y.; Kang, W.; et al. Squalene epoxidase drives cancer cell proliferation and promotes gut dysbiosis to accelerate colorectal carcinogenesis. Gut 2022, 71, 2253–2265. [Google Scholar] [CrossRef]
- Jun, S.Y.; Brown, A.J.; Chua, N.K.; Yoon, J.Y.; Lee, J.J.; Yang, J.O.; Jang, I.; Jeon, S.J.; Choi, T.I.; Kim, C.H.; et al. Reduction of Squalene Epoxidase by Cholesterol Accumulation Accelerates Colorectal Cancer Progression and Metastasis. Gastroenterology 2021, 160, 1194–1207.e28. [Google Scholar] [CrossRef]
- Lasunción, M.A.; Martín-Sánchez, C.; Canfrán-Duque, A.; Busto, R. Post-lanosterol biosynthesis of cholesterol and cancer. Curr. Opin. Pharmacol. 2012, 12, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Wang, W.; Sternisha, A.C.; Corley, C.D.; Wang, H.L.; Wang, X.; Ortiz, F.; Lim, S.K.; Abdullah, K.G.; Parada, L.F.; et al. Selective and brain-penetrant lanosterol synthase inhibitors target glioma stem-like cells by inducing 24(S),25-epoxycholesterol production. Cell Chem. Biol. 2023, 30, 214–229.e18. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Wu, J.L.; Ji, F.; Kang, W.; Bian, X.; Chen, H.; Chan, L.S.; Luk, S.T.Y.; Tong, S.; Xu, J.; et al. The cholesterol uptake regulator PCSK9 promotes and is a therapeutic target in APC/KRAS-mutant colorectal cancer. Nat. Commun. 2022, 13, 3971. [Google Scholar] [CrossRef]
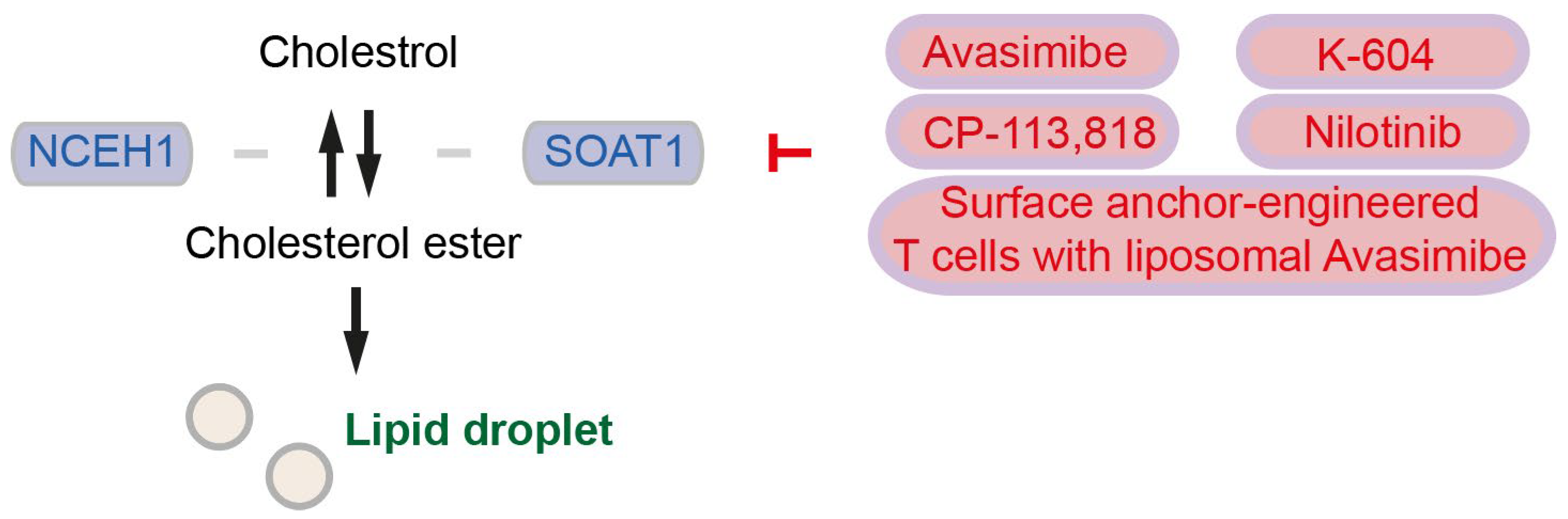
- Li, J.; Gu, D.; Lee, S.S.; Song, B.; Bandyopadhyay, S.; Chen, S.; Konieczny, S.F.; Ratliff, T.L.; Liu, X.; Xie, J.; et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene 2016, 35, 6378–6388. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Bai, Y.; Xiong, Y.; Zhang, J.; Chen, S.; Zheng, X.; Meng, X.; Li, L.; Wang, J.; Xu, C.; et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 2016, 531, 651–655. [Google Scholar] [CrossRef]
- Hao, M.; Hou, S.; Li, W.; Li, K.; Xue, L.; Hu, Q.; Zhu, L.; Chen, Y.; Sun, H.; Ju, C.; et al. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci. Transl. Med. 2020, 12, eaaz6667. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Zhang, M.; Xu, K.; Zhang, X.; Xie, Y.; Zhang, Y.; Chang, C.; Li, X.; Sun, A.; et al. High-affinity SOAT1 ligands remodeled cholesterol metabolism program to inhibit tumor growth. BMC Med. 2022, 20, 292. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Chen, H.; Zhang, T.; He, D.; Luo, Q.; Chi, J.; Hong, Z.; Liao, Y.; Zhang, S.; et al. PCSK9 Inhibition: From Current Advances to Evolving Future. Cells 2022, 11, 2972. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. Lipid droplets and cellular lipid metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef]
- Oni, T.E.; Biffi, G.; Baker, L.A.; Hao, Y.; Tonelli, C.; Somerville, T.D.D.; Deschênes, A.; Belleau, P.; Hwang, C.I.; Sánchez-Rivera, F.J.; et al. SOAT1 promotes mevalonate pathway dependency in pancreatic cancer. J. Exp. Med. 2020, 217, e20192389. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, L.; Chen, X.; Zhang, Q. NCEH1 may be a prognostic biomarker for pancreatic cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 2746–2752. [Google Scholar]
- Huang, J.K.; Lee, H.C. Emerging Evidence of Pathological Roles of Very-Low-Density Lipoprotein (VLDL). Int. J. Mol. Sci. 2022, 23, 4300. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Endotext [Internet]; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef]
- Go, G.W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar] [PubMed]
- Dowds, C.M.; Kornell, S.C.; Blumberg, R.S.; Zeissig, S. Lipid antigens in immunity. Biol. Chem. 2014, 395, 61–81. [Google Scholar] [CrossRef] [PubMed]
- Engelen, S.E.; Ververs, F.A.; Markovska, A.; Lagerholm, B.C.; Kraaijenhof, J.M.; Yousif, L.I.; Zurke, Y.X.; Gulersonmez, C.M.; Kooijman, S.; Goddard, M.; et al. Lipoproteins act as vehicles for lipid antigen delivery and activation of invariant natural killer T cells. JCI Insight 2023, 8, e158089. [Google Scholar] [CrossRef]
- Acier, A.; Godard, M.; Gassiot, F.; Finetti, P.; Rubis, M.; Nowak, J.; Bertucci, F.; Iovanna, J.L.; Tomasini, R.; Lécorché, P.; et al. LDL receptor-peptide conjugate as in vivo tool for specific targeting of pancreatic ductal adenocarcinoma. Commun. Biol. 2021, 4, 987. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Luo, H.; Lu, Q.; Yu, S. PCSK9 promotes the progression and metastasis of colon cancer cells through regulation of EMT and PI3K/AKT signaling in tumor cells and phenotypic polarization of macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 303. [Google Scholar] [CrossRef]
- Kemp, S.B.; Carpenter, E.S.; Steele, N.G.; Donahue, K.L.; Nwosu, Z.C.; Pacheco, A.; Velez-Delgado, A.; Menjivar, R.E.; Lima, F.; The, S.; et al. Apolipoprotein E Promotes Immune Suppression in Pancreatic Cancer through NF-κB-Mediated Production of CXCL1. Cancer Res. 2021, 81, 4305–4318. [Google Scholar] [CrossRef]
- Tavazoie, M.F.; Pollack, I.; Tanqueco, R.; Ostendorf, B.N.; Reis, B.S.; Gonsalves, F.C.; Kurth, I.; Andreu-Agullo, C.; Derbyshire, M.L.; Posada, J.; et al. LXR/ApoE Activation Restricts Innate Immune Suppression in Cancer. Cell 2018, 172, 825–840.e18. [Google Scholar] [CrossRef]
- El-Refai, S.M.; Brown, J.D.; Arnold, S.M.; Black, E.P.; Leggas, M.; Talbert, J.C. Epidemiologic Analysis Along the Mevalonate Pathway Reveals Improved Cancer Survival in Patients Who Receive Statins Alone and in Combination with Bisphosphonates. JCO Clin. Cancer Inform. 2017, 1, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gabitova-Cornell, L.; Surumbayeva, A.; Peri, S.; Franco-Barraza, J.; Restifo, D.; Weitz, N.; Ogier, C.; Goldman, A.R.; Hartman, T.R.; Francescone, R.; et al. Cholesterol Pathway Inhibition Induces TGF-β Signaling to Promote Basal Differentiation in Pancreatic Cancer. Cancer Cell. 2020, 38, 567–583.e11. [Google Scholar] [CrossRef] [PubMed]
- Di Bello, E.; Zwergel, C.; Mai, A.; Valente, S. The Innovative Potential of Statins in Cancer: New Targets for New Therapies. Front. Chem. 2020, 8, 516. [Google Scholar] [CrossRef] [PubMed]
- Ricco, N.; Kron, S.J. Statins in Cancer Prevention and Therapy. Cancers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Gbelcová, H.; Rimpelová, S.; Ruml, T.; Fenclová, M.; Kosek, V.; Hajšlová, J.; Strnad, H.; Kolář, M.; Vítek, L. Variability in statin-induced changes in gene expression profiles of pancreatic cancer. Sci. Rep. 2017, 7, 44219. [Google Scholar] [CrossRef]

| Cholesterol Metabolic Gene | Role in Cancer | Inhibitor | Reference |
|---|---|---|---|
| HMGCR | Upregulated expression in pancreatic cancer mouse model and patients; HMGCR inhibitors attenuate pancreatic cancer development | Simvastatin, Atorvastatin, Rosuvastatin, Pravastatin, Fluvastatin, Lovastatin, Pitavavastatin | [23,24,25,26,27,55] |
| SOAT1 | Patients with SOAT1 expression in pancreatic cancer have significantly shorter overall survival; Inhibition of SOAT1 reduces pancreatic cancer cell proliferation | Avasimibe, K-604, CP-113,818, Nilotinib, Surface anchor-engineered T cells with liposomal Avasimibe | [56,58] |
| LDLR | High LDLR expression observed in all stages of pancreatic cancer; Associated with increased risk of pancreatic cancer recurrence | - | [23] |
| PCSK9 | High PCSK9 protein levels associated with shorter survival of colorectal cancer mice; PCSK9 inhibitors inhibit CRC growth | Alirocumab, R-IMPP, Evolocumab, PF-06446846 | [55,60] |
| APOE | High APOE expression associated with shorter survival of pancreatic cancer patients | - | [72] |
| Intervention/ Treatment | Condition or Disease | NCT Number | Stage of Clinical Trial | Recruitment Status (Recruiting, Completed, not Yet Recruiting. Last Update) | Last Update |
|---|---|---|---|---|---|
| Atorvastatin Evolocumab Ezetimibe FOLFILINOX | Metastatic pancreatic cancer | NCT04862260 | Early Phase 1 | Recruiting | 7 June 2023 |
| Simvastatin Metformin Digoxin | Advanced pancreatic cancer | NCT03889795 | Phase 1 | Recruiting | 17 November 2021 |
| Simvastatin Gemcitabine | Pancreatic cancer | NCT00944463 | Phase 2 | Completed | 17 February 2017 |
| Valproic acid Simvastatin Gemcitabine Nab paclitaxel Cisplatin Capecitabine | Untreated Metastatic Pancreatic Adenocarcinoma | NCT05821556 | Phase 2 | Recruiting | 15 June 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebelo, A.; Kleeff, J.; Sunami, Y. Cholesterol Metabolism in Pancreatic Cancer. Cancers 2023, 15, 5177. https://doi.org/10.3390/cancers15215177
Rebelo A, Kleeff J, Sunami Y. Cholesterol Metabolism in Pancreatic Cancer. Cancers. 2023; 15(21):5177. https://doi.org/10.3390/cancers15215177
Chicago/Turabian StyleRebelo, Artur, Jörg Kleeff, and Yoshiaki Sunami. 2023. "Cholesterol Metabolism in Pancreatic Cancer" Cancers 15, no. 21: 5177. https://doi.org/10.3390/cancers15215177
APA StyleRebelo, A., Kleeff, J., & Sunami, Y. (2023). Cholesterol Metabolism in Pancreatic Cancer. Cancers, 15(21), 5177. https://doi.org/10.3390/cancers15215177








