Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
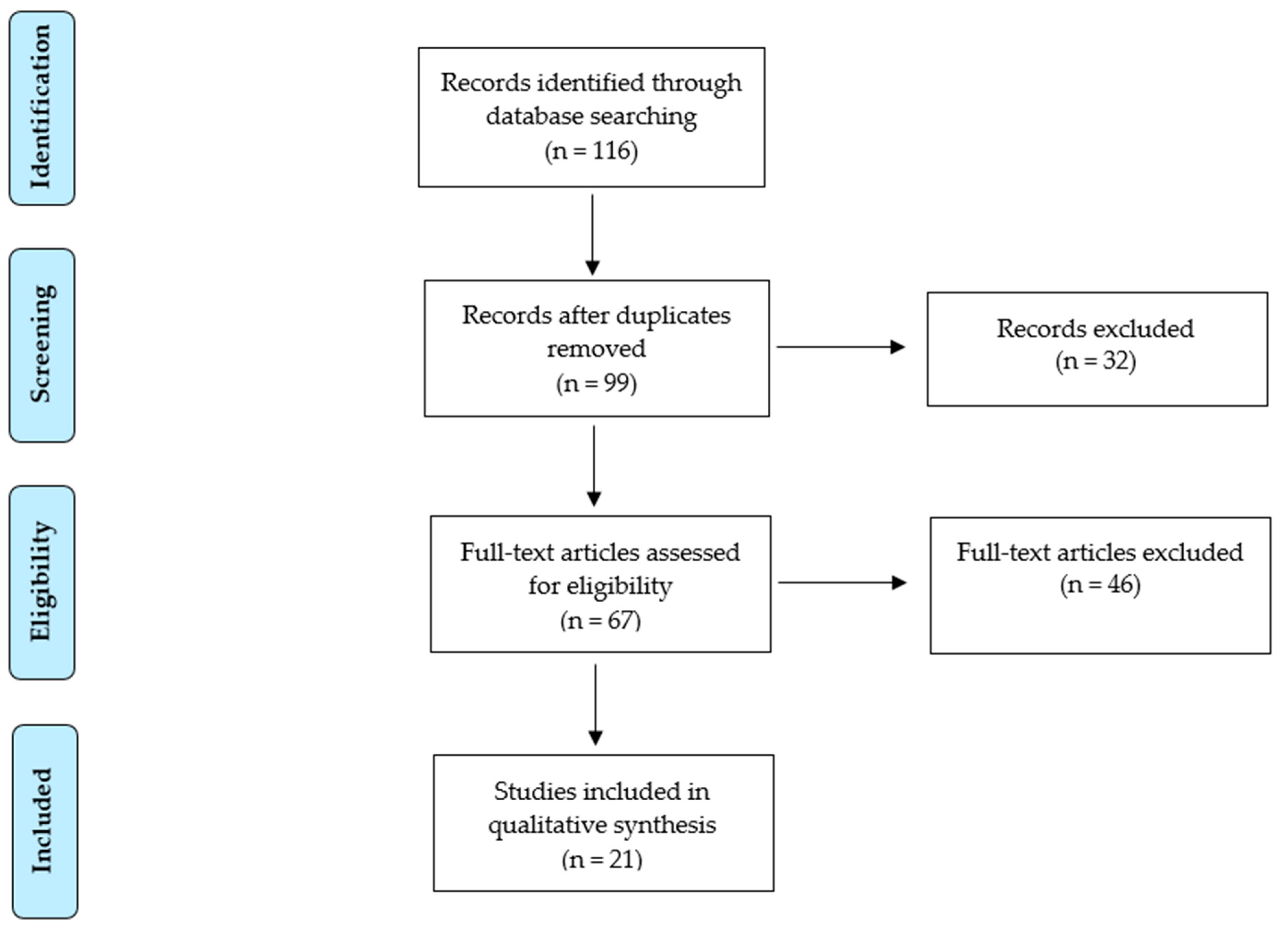
2. Materials and Methods
3. Results
4. Discussion
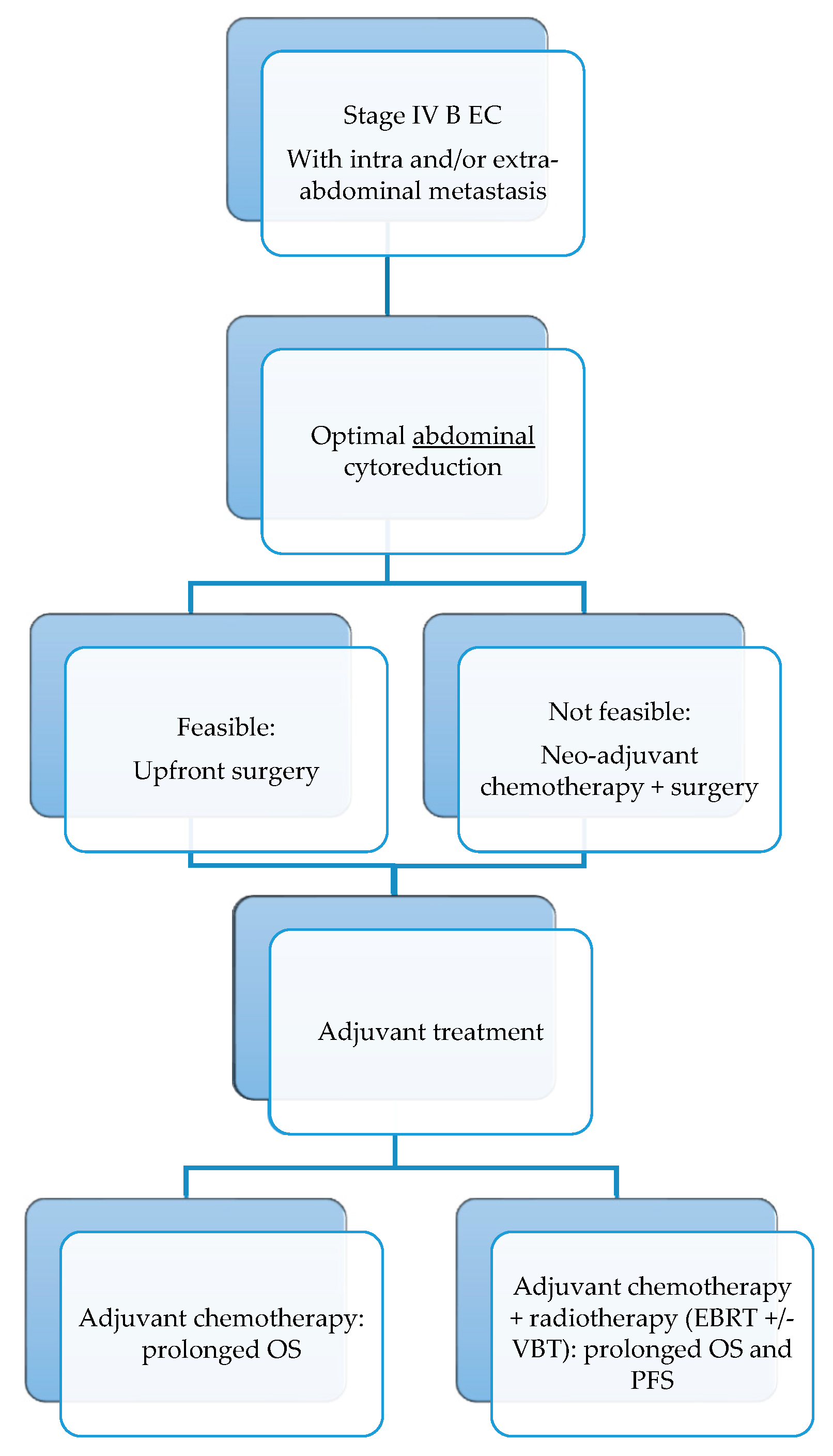
4.1. Impact of Residual Tumor at Cytoreductive Surgery
4.2. Use of Neoadjuvant Chemotherapy in Stage IVB Endometrial Cancer Patients
4.3. Adjuvant Treatment in Stage IVB Endometrial Cancer
4.4. Final Considerations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Vergote, I.; Tropé, C.G.; Amant, F.; Kristensen, G.B.; Ehlen, T.; Johnson, N.; Verheijen, R.H.M.; van der Burg, M.E.L.; Lacave, A.J.; Panici, P.B.; et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N. Engl. J. Med. 2010, 363, 943–953. [Google Scholar] [CrossRef]
- Lu, K.H.; Broaddus, R.R. Endometrial Cancer. N. Engl. J. Med. 2020, 383, 2053–2064. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Grevenkamp, F.; Karnezis, A.; Yang, W.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network; Levine, D.A. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73, Erratum in Nature 2013, 500, 242. [Google Scholar] [CrossRef]
- RAINBO Research Consortium. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Numazaki, R.; Miyagi, E.; Konnai, K.; Ikeda, M.; Yamamoto, A.; Onose, R.; Kato, H.; Okamoto, N.; Hirahara, F.; Nakayama, H. Analysis of stage IVB endometrial carcinoma patients with distant metastasis: A review of prognoses in 55 patients. Int. J. Clin. Oncol. 2009, 14, 344–350. [Google Scholar] [CrossRef]
- Creutzberg, C.L.; Lu, K.H.; Fleming, G.F. Uterine Cancer: Adjuvant Therapy and Management of Metastatic Disease. J. Clin. Oncol. 2019, 37, 2490–2500. [Google Scholar] [CrossRef]
- Huang, A.B.; Wu, J.; Chen, L.; Albright, B.B.; Previs, R.A.; Moss, H.A.; Davidson, B.A.; Havrilesky, L.J.; Melamed, A.; Wright, J.D. Neoadjuvant chemotherapy for advanced stage endometrial cancer: A systematic review. Gynecol. Oncol. Rep. 2021, 38, 100887. [Google Scholar] [CrossRef]
- Landrum, L.M.; Moore, K.N.; Myers, T.K.; Lanneau, G.S.; McMeekin, D.S.; Walker, J.L.; Gold, M.A. Stage IVB endometrial cancer: Does applying an ovarian cancer treatment paradigm result in similar outcomes? A case-control analysis. Gynecol. Oncol. 2009, 112, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Ito, K.; Yamashita, M.; Egawa-Takata, T. Complete resection after pembrolizumab treatment followed by salvage surgery in stage IVB endometrial cancer: A case report. Int. J. Surg. Case Rep. 2022, 94, 107125. [Google Scholar] [CrossRef] [PubMed]
- Senguttuvan, R.N.; Wei, C.; Raoof, M.; Dellinger, T.H.; Wang, E.W. Complete Pathologic Response to PARP Inhibitor Olaparib in a Patient with Stage IVB Recurrent Endometrioid Endometrial Adenocarcinoma. J. Clin. Med. 2023, 12, 3839. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.; Oxman, A.D.; Akl, E.A.; Kunz, R.; Vist, G.; Brozek, J.; Norris, S.; Falck-Ytter, Y.; Glasziou, P.; DeBeer, H.; et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011, 64, 383–394. [Google Scholar] [CrossRef]
- Bristow, R.E.; Zerbe, M.J.; Rosenshein, N.B.; Grumbine, F.C.; Montz, F. Stage IVB endometrial carcinoma: The role of cytoreductive surgery and determinants of survival. Gynecol. Oncol. 2000, 78, 85–91. [Google Scholar] [CrossRef]
- Ayhan, A.; Taskiran, C.; Celik, C.; Yuce, K.; Kucukali, T. The influence of cytoreductive surgery on survival and morbidity in stage IVB endometrial cancer. Int. J. Gynecol. Cancer 2002, 12, 448–453. [Google Scholar] [CrossRef]
- Haight, P.J.; Riedinger, C.J.; Backes, F.J.; O’Malley, D.M.; Cosgrove, C.M. The right time for change: A report on the heterogeneity of IVB endometrial cancer and improved risk-stratification provided by new 2023 FIGO staging criteria. Gynecol. Oncol. 2023, 175, 32–40. [Google Scholar] [CrossRef]
- Lee, L.J.; DeMaria, R.; Berkowitz, R.; Matulonis, U.; Viswanathan, A.N. Clinical predictors of long-term survival for stage IVB uterine papillary serous carcinoma confined to the abdomen. Gynecol. Oncol. 2014, 132, 65–69. [Google Scholar] [CrossRef]
- Eto, T.; Saito, T.; Kasamatsu, T.; Nakanishi, T.; Yokota, H.; Satoh, T.; Nogawa, T.; Yoshikawa, H.; Kamura, T.; Konishi, I. Clinicopathological prognostic factors and the role of cytoreduction in surgical stage IVb endometrial cancer: A retrospective multi-institutional analysis of 248 patients in Japan. Gynecol. Oncol. 2012, 127, 338–344. [Google Scholar] [CrossRef]
- Ueda, Y.; Enomoto, T.; Miyatake, T.; Egawa-Takata, T.; Ugaki, H.; Yoshino, K.; Fujita, M.; Kimura, T. Endometrial carcinoma with extra-abdominal metastasis: Improved prognosis following cytoreductive surgery. Ann. Surg. Oncol. 2010, 17, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, S.; Hook, J.; Nankivell, M.; Jayson, G.C.; Kitchener, H.; Lopes, T.; Luesley, D.; Perren, T.; Bannoo, S.; Mascarenhas, M.; et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet 2015, 386, 249–257. [Google Scholar] [CrossRef]
- Fagotti, A.; Ferrandina, M.G.; Vizzielli, G.; Pasciuto, T.; Fanfani, F.; Gallotta, V.; Margariti, P.A.; Chiantera, V.; Costantini, B.; Alletti, S.G.; et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int. J. Gynecol. Cancer 2020, 30, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Reuss, A.; Du Bois, A.; Harter, P.; Fotopoulou, C.; Sehouli, J.; Aletti, G.; Guyon, F.; Greggi, S.; Mosgaard, B.J.; Reinthaller, A.; et al. TRUST: Trial of Radical Upfront Surgical Therapy in advanced ovarian cancer (ENGOT ov33/AGO-OVAR OP7). Int. J. Gynecol. Cancer 2019, 29, 1327–1331. [Google Scholar] [CrossRef]
- Memarzadeh, S.; Holschneider, C.H.; Bristow, R.E.; Jones, N.L.; Fu, Y.S.; Karlan, B.Y.; Berek, J.S.; Farias-Eisner, R. FIGO stage III and IV uterine papillary serous carcinoma: Impact of residual disease on survival. Int. J. Gynecol. Cancer 2002, 12, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Saito, T.; Aoki, D.; Watanabe, Y.; Ushijima, K.; Takano, M.; Sugiyama, T.; Yaegashi, N.; Takehara, K. JGOG2046: A feasibility study of neoadjuvant chemotherapy followed by debulking surgery for clinically diagnosed FIGO stage IVb endometrial cancer. Int. J. Clin. Oncol. 2023, 28, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, I.; Van Calster, B.; Capoen, A.; Leunen, K.; Berteloot, P.; Neven, P.; Moerman, P.; Vergote, I.; Amant, F. Neoadjuvant chemotherapy followed by interval debulking surgery in patients with serous endometrial cancer with transperitoneal spread (stage IV): A new preferred treatment? Br. J. Cancer 2009, 101, 244–249. [Google Scholar] [CrossRef]
- Eto, T.; Saito, T.; Shimokawa, M.; Hatae, M.; Takeshima, N.; Kobayashi, H.; Kasamatsu, T.; Yoshikawa, H.; Kamura, T.; Konishi, I. Status of treatment for the overall population of patients with stage IVb endometrial cancer, and evaluation of the role of preoperative chemotherapy: A retrospective multi-institutional study of 426 patients in Japan. Gynecol. Oncol. 2013, 131, 574–580. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Yang, S. Survival benefit of surgical treatment for patients with stage IVB endometrial cancer: A propensity score-matched SEER database analysis. J. Obstet. Gynaecol. 2023, 43, 2204937. [Google Scholar] [CrossRef]
- Kanno, M.; Yunokawa, M.; Kurihara, N.; Aoki, Y.; Omi, M.; Tanigawa, T.; Kanao, H. Efficacy of intra-abdominal cytoreductive surgery in advanced endometrial cancer with distant metastasis. J. Gynecol. Oncol. 2023, 34, e77. [Google Scholar] [CrossRef]
- Tobias, C.J.; Chen, L.; Melamed, A.; Clair, C.S.; Khoury-Collado, F.; Tergas, A.I.; Hou, J.Y.; Hur, C.; Ananth, C.V.; Neugut, A.I.; et al. Association of Neoadjuvant Chemotherapy with Overall Survival in Women with Metastatic Endometrial Cancer. JAMA Netw. Open 2020, 3, e2028612. [Google Scholar] [CrossRef]
- Jooya, N.D.; Ciccone, M.A.; Brunette, L.L.; Pham, H.Q.; Yessaian, A.A.; Muderspach, L.I.; Roman, L.D.; Matsuo, K. Population-level uptake of neoadjuvant chemotherapy for stage IVB endometrial cancer. Gynecol. Oncol. 2022, 165, 428–436. [Google Scholar] [CrossRef]
- Bogani, G.; Ditto, A.; Maggiore, U.L.R.; Scaffa, C.; Mosca, L.; Chiappa, V.; Martinelli, F.; Lorusso, D.; Raspagliesi, F. Neoadjuvant chemotherapy followed by interval debulking surgery for unresectable stage IVB Serous endometrial cancer. Tumori J. 2019, 105, 92–97. [Google Scholar] [CrossRef]
- Wilkinson-Ryan, I.; Frolova, A.I.; Liu, J.; Massad, L.S.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Hagemann, A.R. Neoadjuvant chemotherapy versus primary cytoreductive surgery for stage IV uterine serous carcinoma. Int. J. Gynecol. Cancer 2015, 25, 63–68. [Google Scholar] [CrossRef]
- Unsal, M.; Kilic, C.; Cakir, C.; Kilic, F.; Ersak, B.; Karakas, S.; Tokgozoglu, N.; Varli, B.; Oktar, O.; Comert, G.K.; et al. Neoadjuvant chemotherapy in patients with stage IVB uterine serous carcinoma: A Turkish multicentric study. J. Obstet. Gynaecol. 2023, 43, 2151355. [Google Scholar] [CrossRef]
- van den Heerik, A.S.V.M.; Horeweg, N.; de Boer, S.M.; Bosse, T.; Creutzberg, C.L. Adjuvant therapy for endometrial cancer in the era of molecular classification: Radiotherapy, chemoradiation and novel targets for therapy. Int. J. Gynecol. Cancer 2021, 31, 594–604. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; Colombo, A.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 295–309. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit from Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef]
- Cirik, D.A.; Karalok, A.; Ureyen, I.; Tasci, T.; Koc, S.; Turan, T.; Tulunay, G. Stage IVB endometrial cancer confined to the abdomen: Is chemotherapy superior to radiotherapy? Eur. J. Gynaecol. Oncol. 2016, 37, 226–231. [Google Scholar] [PubMed]
- Barrington, D.A.; Fox, B.; Meade, C.; Quick, A.; Felix, A.S.; Chambers, L.M. Does the addition of radiation improve survival compared to chemotherapy alone in women with stage IV endometrial carcinoma? Analysis of the NCDB and SEER databases. Gynecol. Oncol. 2022, 165, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Albright, B.B.; Monuszko, K.A.; Kaplan, S.J.; Davidson, B.A.; Moss, H.A.; Huang, A.B.; Melamed, A.; Wright, J.D.; Havrilesky, L.J.; Previs, R.A. Primary cytoreductive surgery for advanced stage endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 225, 237.e1–237.e24. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Gordhandas, S.; Zammarrelli, W.A.; Rios-Doria, E.V.; Green, A.K.; Makker, V. Current Evidence-Based Systemic Therapy for Advanced and Recurrent Endometrial Cancer. J. Natl. Compr. Cancer Netw. 2023, 21, 217–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capozzi, V.A.; Scarpelli, E.; De Finis, A.; Rotondella, I.; Scebba, D.; Gallinelli, A.; Montrucchio, C.; Martignon, G.; Leotta, M.; Ghi, T.; et al. Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review. Cancers 2023, 15, 5123. https://doi.org/10.3390/cancers15215123
Capozzi VA, Scarpelli E, De Finis A, Rotondella I, Scebba D, Gallinelli A, Montrucchio C, Martignon G, Leotta M, Ghi T, et al. Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review. Cancers. 2023; 15(21):5123. https://doi.org/10.3390/cancers15215123
Chicago/Turabian StyleCapozzi, Vito Andrea, Elisa Scarpelli, Alessandra De Finis, Isabella Rotondella, Davide Scebba, Asya Gallinelli, Carlotta Montrucchio, Giulia Martignon, Martina Leotta, Tullio Ghi, and et al. 2023. "Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review" Cancers 15, no. 21: 5123. https://doi.org/10.3390/cancers15215123
APA StyleCapozzi, V. A., Scarpelli, E., De Finis, A., Rotondella, I., Scebba, D., Gallinelli, A., Montrucchio, C., Martignon, G., Leotta, M., Ghi, T., & Berretta, R. (2023). Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review. Cancers, 15(21), 5123. https://doi.org/10.3390/cancers15215123