Simple Summary
Lasting impacts and symptoms of cancer treatment can affect survivors’ daily lives. We explored how symptoms and symptom clusters can impact the ability to work among 561 cancer survivors previously diagnosed with breast, colorectal, and haematological malignancies, who were on average 58 years old and 1.5 years since diagnosis. We found almost 35% of survivors reported limitations in their ability to work. We identified three main symptom clusters: pain, emotional, and cognitive symptoms. Survivors experiencing these symptom clusters were approximately 14–17% more likely to report limitations in their work ability. Older survivors and those with more advanced cancer stages were also more likely to experience limitations in their work ability. In conclusion, a significant number of cancer survivors struggle to work due to their symptoms after treatment. Out results suggest that by better understanding and managing these symptoms, survivors can have improved opportunities to participate in work after their cancer treatment.
Abstract
Background: Cancer survivors often experience a range of symptoms after treatment which can impact their quality of life. Symptoms may cluster or co-occur. We aimed to investigate how symptoms and symptom clusters impact the ability to work among cancer survivors. Methods: We used symptom severity data and ability to work data routinely collected from cancer survivors attending a survivorship clinic after primary treatment with curative intent. We defined symptom clusters using single linkage and a threshold on the rescaled distances of <10. We then conducted a logistic regression to examine how symptoms and symptom clusters were related to the ability to work. Results: We analysed data from 561 cancer survivors, mean age 58 years and 1.5 years post diagnosis, with mixed diagnoses including breast (40.5%), colorectal (32.3%), and haematological cancers (15.3%). Limitations to work ability were reported by 34.9% of participants. Survivors experiencing pain, emotional, and cognitive symptom clusters were 14–17% more likely to report limitations in their ability to work. Older survivors and those with a higher stage disease were more likely to report limitations in their ability to work. Conclusion: A better understanding and management of symptom severity and symptom clusters may help the sizable proportion of cancer survivors experiencing symptoms to participate in work after treatment.
1. Introduction
Many people surviving cancer experience a range of symptoms and complications during and/or following their cancer treatment that may persist long-term and lead to poorer health outcomes. This can include compromised physical functioning and psychological well-being, and the development of chronic illnesses, all of which can impact an individual’s quality of life and survival [1,2,3,4,5].
Our own research in 385 early-stage cancer survivors attending their initial visit at the Sydney Cancer Survivorship Centre Clinic found that approximately 12 months after diagnosis, 56% of survivors reported five or more symptoms of at least moderate severity (>=4/10) [3]. The most commonly reported symptoms were fatigue (45%), difficulty sleeping (37%), pain (34%), sore hands or feet (30%), numbness or pins and needles (30%), difficulties with thinking skills (concentration and memory [27.5%]), and mood disturbance (anxiety [31%], depression [23%], and stress [45%]), with fear of cancer recurrence rated by a clinical psychologist based on their consultation as 62% [3]. A second study comparing the rates and severity of symptoms at the initial visit to their follow up visit, 12 months later, found some improvement in pain, fatigue, and energy, but more than 20% of survivors still had moderately severe fatigue, pain, or sleep disturbance, and 26%, particularly women, continued to report five or more symptoms of at least moderate severity [6]. Others have reported similar symptoms but at even higher rates five years after adjuvant treatment in Asian and Australian cancer survivors: fatigue (67%), loss of strength (62%), pain (62%), sleep disturbance (60%), and weight changes (58%) [7].
Similar to our finding of high rates of survivors with multiple symptoms, previous research, which has been mainly conducted in breast cancer survivors, has documented the co-occurrence of symptoms, also known as symptom clusters, in the years after treatment, particularly for sleep, fatigue, and mood disturbance [8]. Symptom clusters have been defined as consisting of two or more symptoms that are related to each other and are considered to be relatively stable over time [9,10]. Other symptom clusters among survivors include ongoing pain and symptoms associated with peripheral neuropathy, cognitive impacts, and reduced physical activity [11,12,13]. Primary cancer site, disease stage, or antitumor treatment have previously been found to predict the occurrence of symptom clusters [14], but these associations with demographic and clinical variables are not consistently found [15]. For example in a secondary analysis of 282 breast cancer patients receiving chemotherapy or radiotherapy, Kim et al. [15] found no association of treatment modality (chemotherapy vs. radiotherapy), age, employment status, disease stage, and comorbid condition.
The reasons for symptom clusters include a common aetiology or biological pathway, such as an inflammatory process and increased cytokines, immune processes, activation of the sympathetic nervous system, and activation of the hypothalamic-pituitary–adrenal axis [10,16]. However, attribution of a clear and single causal pathway is challenging due to variable symptom cluster inclusions across papers (e.g., fatigue, pain, and depression versus fatigue, pain, depression, and sleep disturbance) [10].
Reasons for not participating in work at pre-diagnosis levels are multifactorial [17,18]. Symptom burden can impact an individual’s ability to resume daily activities including returning to work or the ability to achieve expected outcomes at work (for example, completing tasks on time or to a level of previous proficiency). In addition to providing an income, working or returning to work is often regarded as an important milestone for cancer survivors as it is extrinsically linked to identity, self-esteem, and a sense of returning to normalcy for those of working age [17,19]. However, up to 40% of cancer survivors either do not return to work or do not go back to their pre-diagnosis level of employment [20]. A recent systematic review of 68 studies in cancer survivors who had completed primary cancer treatment found a trend for higher symptom burden to be associated with poorer work outcomes [21]. Fatigue, depression, and cognitive symptoms were found to be related to poorer work performance, and issues with body image and oral dysfunction were associated with a higher risk of unemployment [21].
Cognitive symptoms (also referred to as cognitive impairment throughout the literature) are likely to contribute to work outcomes due to the requirements to utilise cognitive skills at work (e.g., pay attention, complete tasks within an allotted time, remember to do tasks, plan, organise, prioritise and review tasks) [17,22,23]. In a sample of 68 breast cancer survivors who had returned to work, Von Ah et al. found perceived cognitive impairment was associated with the ability to work, while clinical and demographic factors were not. Similar results indicating a role for perceived cognitive impairment impacting markers of work productivity (e.g., time management) and engagement were also found [18,24].
Self-report of cognitive symptoms have consistently been associated with emotional symptoms of depression and anxiety, as well as fatigue [25,26]. For example, our analysis of population level data (N = 10,337, with 691 (6.7%) with a history of cancer) found moderate to high correlation between anxiety, depression and concentration difficulties (correlations ranged r = 0.52 for anxiety and concentration difficulties, to r = 0.81 for anxiety and depression), and approximately 9% of the population reported experiencing either concentration difficulties and depression or concentration difficulties and anxiety [27]. However, few studies have considered the co-occurrence of cognitive symptoms, such as attention and memory difficulties, in addition to other commonly co-occurring symptoms, such as depression, anxiety, and fatigue, and its consequent impact on the ability to work. Among a sample of 379 cancer survivors, Ehrenstein et al. [26] found self-reported cognitive symptom (memory and executive function) trajectories were related to work, fatigue, and depression. They found more cognitive symptoms which were stable over time to be associated with older age, longer time after diagnosis before returning to work, more quantitative work demands, and higher levels of depressive symptoms at baseline. However, they did not investigate the co-occurrence of cognitive symptoms, fatigue, and depression, and their compounding impact on work outcomes. Considering symptom clusters that include cognitive difficulties, their impact on the ability to work is critical, given (i) the likelihood of multiple symptoms being experienced due to cancer treatment well into survivorship and (ii) the compounding negative impact of multiple symptoms on quality of life and the ability to participate in work. Therefore, to address this gap in the literature, this study aimed to (i) identify symptom clusters based on severity of symptoms, and (ii) evaluate how these symptoms, including self-reported cognitive symptoms, were related to work ability among a sample of cancer survivors.
2. Methods
2.1. Participants
Cancer survivors attending the Sydney Cancer Survivorship Centre multidisciplinary team clinic between September 2013 (when the clinic opened) and October 2020, who provided returning to work data and consented for their data to be used in research, were eligible to participate in this research study. Eligibility for attending the Sydney Cancer Survivorship Centre has been published [27] and includes an invasive cancer diagnosis, completion of primary cancer treatment (generally including chemotherapy) for early-stage cancer, or occasionally those with stage IV who have had potentially curative treatment, such as resection of metastatic disease, and no evidence of a cancer recurrence. Exclusion criteria for attending the clinic include currently undergoing primary treatment, with the exceptions that patients with breast cancer may be receiving hormonal treatment and/or targeted therapy such as trastuzumab, and patients with haematological malignancy may be on maintenance treatment. Referrals for the Sydney Cancer Survivorship Centre are accepted from medical oncologists, surgeons, and radiation oncologists [28].
2.2. Measures
Participants completed questionnaires either on paper or via online survey using the REDCap survey function prior to attending the Sydney Cancer Survivorship Centre clinic. The questionnaires included a comprehensive assessment of patient self-reported symptoms, quality of life, distress, and lifestyle factors [28]. For this analysis, we focused on self-reported symptoms and the ability to work.
Participants’ demographics, including age and sex and clinical information, including diagnosis, stage, treatment received, and time since treatment, were obtained from the patient’s medical record.
Self-reported symptoms were selected from the Patient’s Disease and Treatment Assessment (PDTA) form [29]. The PDTA asks respondents to rate the severity of 47 symptoms and aspects of wellbeing; we also added a memory question. Symptoms are rated from 0–10, with higher scores indicating greater symptom severity, and the following anchors are provided: 0 indicating no trouble and 10 indicating the worst I can imagine. For this analysis, we focused on symptoms commonly experienced during survivorship based on our included cohort of survivors [3,6] and included the following: pain, fatigue, trouble sleeping, numbness or pins and needles, diarrhoea, anxiety, depression, trouble concentrating, and problems with memory. Additionally, these symptoms were selected to ensure the coverage of the commonly occurring symptom clusters grouped into physical symptoms or psychological symptoms [14,30].
To determine whether participants were able to work, we used a single item from the Functional Assessment of Cancer Therapy–General (FACT-G): “I am able to work (include work at home)” [31]. Responses are on a 5-point Likert scale, ranging from 0 “not at all” to 4 “very much”. We dichotomised work ability as “limited ability to work” if the participant responded 0 “not at all”, 1 “a little bit”, or 2 “somewhat”; work ability was categorised as “able to work” if the participant responded 3 “quite a bit” or 4 “very much”.
2.3. Statistical Analysis
We conducted all statistical analyses using IBM SPSS Statistics version 27 (IBM Corporation, Armonk, NY, USA). We described our sample using means and standard deviations for continuous variables and counts and proportions for categorical variables. We compared participants’ work ability on demographic and clinical factors using chi-square tests for categorical variables and ANOVA for continuous variables. Previous research comparing statistical methods for identifying symptom clusters has found consistency across a principal component analysis, exploratory factor analysis, and hierarchical cluster analysis [30]. To address aim 1 and identify symptom clusters, we conducted a hierarchical cluster analysis. We defined clusters using single linkage and a threshold on the rescaled distances <10, as previously recommended [30]. We operationalised symptom cluster scores as the mean of the constituent symptoms. To address aim 2, we ran two logistic regression models with ability to work as the dependent variable. In the first model, we entered demographic variables and individual symptoms. In the second model, we entered demographic variables and any symptom clusters we had previously identified and individual symptoms. Factors were considered significantly associated with ability to work when p < 0.05.
3. Results
Ability to work data were available from 561 cancer survivors who attended the Sydney Cancer Survivorship Centre clinic. Table 1 provides the demographic characteristics of the population used for this analysis. Participants were on average 58 years old and 1.5 years post diagnosis. Most were female (69.2%), and the most common diagnosis was breast cancer (40.5%), followed by colorectal cancer (32.3%). Over a third (34.9%) of participants reported limitations in their ability to work. There was no difference between those who reported limited work ability and those able to work successfully based on clinical and demographic factors in univariable analysis. However, those with limited ability to work were more likely to report greater symptom severity than those who reported no limitation to working at the time of their initial visit (Figure 1, all p values ≤ 0.001). Fatigue did not differ significantly between those with limited work ability and those able to work (p = 0.177).

Table 1.
Participant demographics and symptoms for the whole sample and whether participants were able to work or were limited in their ability to work g.
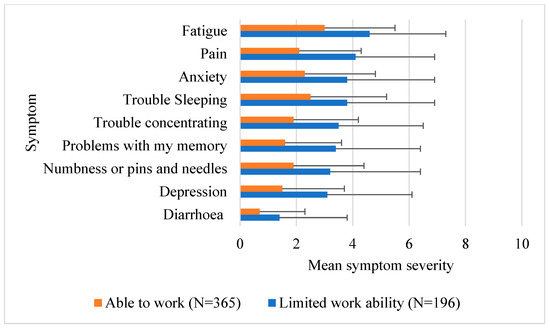
Figure 1.
Mean symptom severity rating. Higher scores out of 10 indicate greater severity.
3.1. Symptom Clusters
We determined clusters at a rescaled distance of <10 (see Figure 2). At the lower threshold, clusters were identified as an emotional cluster (anxiety and depression), a cognitive cluster (attention and memory), or a combined emotional–cognitive symptom cluster.
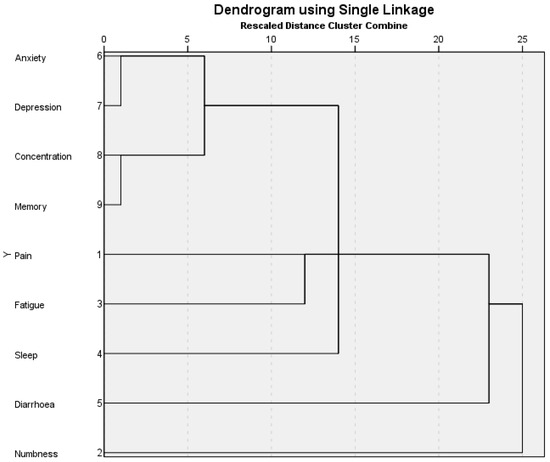
Figure 2.
Dendrogram showing symptom clustering using single linkage as previously described [29].
3.2. Factors Associated with Returning to Work
In our first logistic regression model, we entered demographic and clinical variables into the model with individual symptoms to determine the factors associated with the ability to work after cancer (Table 2). The overall model fit was significant and accounted for 33.3% of the variance in self-reported work ability (χ2(17) = 133.25, p < 0.001). Of the demographic and clinical variables entered, we found older participants were less likely to be able to work (OR: 0.964, 95%CI: 0.944–0.985). Participants with Stage III and resected Stage IV disease were less likely to be able to work compared to those with Stage I disease (OR 0.385, 95%CI: 0.168–0.883). Symptoms of pain, fatigue, depression, and problems with memory were significantly associated with the ability to work, with those reporting more severe symptoms less likely to be able to work.

Table 2.
Logistic regression with work ability as the dependent variable and clinical and demographic variables and symptoms as independent variables.
In our second logistic regression model (Table 3), we entered demographic and clinical variables into the model with the above identified symptom clusters and any remaining symptoms not included in a cluster to determine the factors associated with the ability to work after cancer. Again, the overall model fit was significant and accounted for 31.6% of the variance in self-reported ability to work (χ2(17) = 130.04, p < 0.001). Similar to the results of the first logistic regression model, older participants and those with Stage III and resected/treated Stage IV disease were less likely to be able to work. Participants reporting more severe pain, higher cognitive symptom cluster scores (i.e., more severe memory and attention symptoms on average), and those with higher emotion symptom cluster scores (i.e., more severe anxiety and depression symptoms on average) were less likely to be able to work.

Table 3.
Logistic regression with work ability as the dependent variable and clinical and demographic variables and symptom clusters identified using a rescaled distance of <10 as independent variables.
4. Discussion
In our sample of 561 cancer survivors with mixed diagnoses who were attending a large survivorship multidisciplinary team clinic in Sydney, Australia, around 35% of survivors reported limitations in their ability to work. Reported symptom severity differed between those reporting limitations in their ability to work and those not reporting limitations in their ability to work after cancer. The factors that were significantly associated with increased odds of being able to work included younger age, early-stage disease, and less severe pain, emotional, and cognitive symptoms.
Our results are consistent with those of other studies showing impacts on work engagement and participation among cancer survivors [17,18,21,24,32]. Previous research has documented an associated impact on financial well-being, with survivors not able to work reporting reduced earnings [14,22,32]. The flow-on effect to overall quality of life is likely complex and age-dependent. Cancer survivors may reduce work hours or start retirement earlier than otherwise planned due to positive (i.e., cancer has caused a refocus on personal values such as spending more time with loved ones) and negative (i.e., the lasting symptoms of cancer treatment prohibit work and impact overall quality of life) impacts of cancer [17]. Our results do not tease apart these bidirectional impacts and future research is warranted. However, the average age of our sample was below the Australian retirement age (>65 years of age), and our results may reflect changed work plans, further highlighting the importance of assessing for lasting symptom impacts after cancer treatment is finished.
Similar to previous research, we found emotional or psychological symptoms of anxiety and depression clustered together, and cognitive symptoms of attention and difficulties with memory were clustered together [30,33]. Our results are important in highlighting that the ability to work after cancer is impacted by multiple symptoms, in addition to survivor age and disease stage. While these symptoms are self-reported, the presence of these symptoms was associated with returning to work. These results may reflect impacts on the capacity to fulfil work duties as highlighted by others [34]. In a sample of 1562 people with mixed diagnoses of advanced cancer, both on and off treatment, four symptom clusters were reliably identified using different statistical methods and included tense–worry–irritable–depressed (emotional cluster), fatigue–pain, nausea–vomiting, and concentration–memory (cognitive cluster) [30]. This study also found the emotional cluster was the strongest predictor of overall quality of life, in contrast to other identified clusters [30]. As cognitive symptoms, depression, and fatigue may all be relatively stable over time [26], this further highlights the importance of addressing psychosocial aspects in symptom cluster management [30] to help improve work outcomes for cancer survivors.
5. Implications for Cancer Survivors and Clinical Practice
We found older survivors and those with a higher stage of disease were at particular risk of not achieving their work goals and therefore may also be at a potentially increased risk of financial toxicity. Considering symptom presence (particularly pain and cognitive and emotional symptoms), severity, and any potential clustering or compounding effects during survivorship clinical encounters, particularly for older survivors and those with a higher stage disease, may be particularly helpful to support survivors in achieving their work-related goals. Although treating a symptom in isolation may miss the full impact on overall quality of life during survivorship, conversely, focusing on reducing the severity of one symptom may reduce or prevent escalation of the other symptoms [35]. As such, it is recommended that clinicians screen for the presence of multiple symptoms and determine if a sentinel symptom exists within a symptom cluster [10], as this may be amenable to an evidence-based intervention.
A review of previous intervention research for symptom clusters has found interventions often targeted one symptom only [10], though some studies have reported improvements in symptoms, in addition to the primary symptom targeted [36]. In a pilot randomised controlled trial with 86 patients with advanced lung, prostate, colorectal, or gynaecologic cancers receiving treatment, a two week audio-recorded and delivered training program of twelve relaxation, imagery, or distraction exercises reduced the severity of the pain, fatigue, and sleep disturbance symptom cluster at two weeks post intervention, compared to a waitlist control group [35]. However, no impact was observed on symptom interference with daily life [35].
6. Strengths and Limitations
Previous research has largely focused on breast cancer survivors, and a strength of our study is the inclusion of a large sample of survivors with mixed cancer diagnoses and stages; however, there are several limitations worth noting. We used a single item measure of the ability to work and the experience of symptoms. While these questions are used in current clinical encounters and serve as a reasonable screen of symptoms [37] and the ability to work, research studies employing a more comprehensive assessment of the presence of multiple symptoms and their severity, in addition to validated measures of the ability to work, are warranted [37]. To address this gap clinically, at the Sydney Cancer Survivorship Centre clinic, we have recently started collecting more information on patients’ work status, changes in their working plans since their cancer diagnosis, and their ability to work after cancer.
7. Conclusions
We found that almost 35% of cancer survivors attending a survivorship clinic reported limitations in their ability to work and that this was associated with age, stage of disease, and the presence of multiple symptoms including pain and emotional and cognitive symptoms. A better understanding of symptom severity and clusters, and reducing symptom burden after cancer treatment, may support survivors to better engage in work. Future research on work engagement and the ability of cancer survivors to work should seek to further explore and address the whole person context, including symptom burden and benefits and barriers to work after cancer, to better understand and support cancer survivors in achieving their work goals.
Author Contributions
Conceptualization, J.E.F. and J.L.V.; data curation, S.Y.T. and K.K.-A.; formal analysis, J.E.F.; methodology, J.E.F. and J.L.V.; resources, S.Y.T. and K.K.-A.; writing—original draft, J.E.F. and J.L.V.; writing—review and editing, J.E.F., S.Y.T., K.K.-A., H.M.D., and J.L.V. All authors have read and agreed to the published version of the manuscript.
Funding
Joanna E. Fardell is a Maridulu Budyari Gumal (SPHERE) Cancer CAG Senior Research Fellow and is supported by a Cancer Institute NSW Research Capacity Building Grant (2021/CBG003). Janette L. Vardy is supported by a National Health Medical Research Council Investigator Grant [APP1176221].
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Sydney Local Health District—Concord General Repatriation Hospital Human Research Ethic Committee—(HREC/14/CRGH/23, approved 6 May 2014).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available on reasonable request from the author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eakin, E.G.; Youlden, D.R.; Baade, P.D.; Lawler, S.P.; Reeves, M.M.; Heyworth, J.S.; Fritschi, L. Health status of long-term cancer survivors: Results from an Australian population-based sample. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Turner, J.; Kerin-Ayres, K.; Butler, S.; Deguchi, C.; Khatri, S.; Mo, C.; Warby, A.; Cunningham, I.; Malalasekera, A.; et al. Health concerns of cancer survivors after primary anti-cancer treatment. Support. Care Cancer 2019, 27, 3739–3747. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; Norredam, M.; Landrum, M.B.; Huskamp, H.A.; Meara, E. Physical and mental health status of older long-term cancer survivors. J. Am. Geriatr. Soc. 2005, 53, 2145–2152. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Fritschi, L.; Eakin, E.G. Non-cancer mortality among people diagnosed with cancer (Australia). Cancer Causes Control 2006, 17, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Liew, A.; Turner, J.; Kerin-Ayres, K.; Butler, S.; Deguchi, C.; Khatri, S.; Wildbore, C.; Mo, C.; Hamayun, M.; et al. What happens to cancer survivors attending a structured cancer survivorship clinic? Symptoms, quality of life and lifestyle changes over the first year at the Sydney Cancer Survivorship Centre clinic. Support. Care Cancer 2021, 29, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Yates, P.; Li, Q.; So, W.K.; Pongthavornkamol, K.; Pittayapan, P.; Komatsu, H.; Thandar, M.; Yi, M.; Chacko, S.T.; et al. Mapping unmet supportive care needs, quality-of-life perceptions and current symptoms in cancer survivors across the Asia-Pacific region: Results from the International STEP Study. Ann. Oncol. 2019, 30, 493. [Google Scholar] [CrossRef] [PubMed]
- Carson, E.-K.; Vardy, J.L.; Dhillon, H.M.; Brown, C.; Kiely, B.E. Relationship between sleep disturbance, symptoms, and alcohol use in breast cancer survivors attending Sydney Cancer Survivorship Clinic. Support. Care Cancer 2021, 29, 6233–6242. [Google Scholar] [CrossRef]
- Kim, H.-J.; McGuire, D.B.; Tulman, L.; Barsevick, A.M. Symptom Clusters: Concept Analysis and Clinical Implications for Cancer Nursing. Cancer Nurs. 2005, 28, 270–282. [Google Scholar] [CrossRef]
- Miaskowski, C.; Barsevick, A.; Berger, A.; Casagrande, R.; Grady, P.A.; Jacobsen, P.; Kutner, J.; Patrick, D.; Zimmerman, L.; Xiao, C.; et al. Advancing Symptom Science Through Symptom Cluster Research: Expert Panel Proceedings and Recommendations. J. Natl. Cancer Inst. 2017, 109, djw253. [Google Scholar] [CrossRef]
- Hardy-Leger, I.; Charles, C.; Lange, M.; Joly, F.; Roux, P.; Capel, A.; Petrucci, J.; Rigal, O.; Le Fel, J.; Vanlemmens, L.; et al. Differentiation of groups of patients with cognitive complaints at breast cancer diagnosis: Results from a sub-study of the French CANTO cohort. Psycho-Oncology 2021, 30, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Klaver, K.M.; Schagen, S.B.; Kieffer, J.M.; van der Beek, A.J.; Duijts, S.F.A. Trajectories of Cognitive Symptoms in Sick-Listed Cancer Survivors. Cancers 2021, 13, 2444. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Thompson, W.; Ancoli-Israel, S.; Liu, L.; Palmer, B.; Natarajan, L. Cognition, quality-of-life, and symptom clusters in breast cancer: Using Bayesian networks to elucidate complex relationships. Psycho-Oncology 2018, 27, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Kirkova, J.; Aktas, A.; Walsh, D.; Davis, M.P. Cancer Symptom Clusters: Clinical and Research Methodology. J. Palliat. Med. 2011, 14, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; Barsevick, A.M.; Tulman, L.; McDermott, P.A. Treatment-Related Symptom Clusters in Breast Cancer: A Secondary Analysis. J. Pain Symptom Manag. 2008, 36, 468–479. [Google Scholar] [CrossRef] [PubMed]
- So, W.K.; Law, B.M.; Ng, M.S.; He, X.; Chan, D.N.; Chan, C.W.; McCarthy, A.L. Symptom clusters experienced by breast cancer patients at various treatment stages: A systematic review. Cancer Med. 2021, 10, 2531–2565. [Google Scholar] [CrossRef] [PubMed]
- Butow, P.; Laidsaar-Powell, R.; Konings, S.; Lim, C.Y.S.; Koczwara, B. Return to work after a cancer diagnosis: A meta-review of reviews and a meta-synthesis of recent qualitative studies. J. Cancer Surviv. 2020, 14, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; Storey, S.; Crouch, A. Relationship between self-reported cognitive function and work-related outcomes in breast cancer survivors. J. Cancer Surviv. Res. Pract. 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Porro, B.; Durand, M.J.; Petit, A.; Bertin, M.; Roquelaure, Y. Return to work of breast cancer survivors: Toward an integrative and transactional conceptual model. J. Cancer Surviv. 2022, 16, 590–603. [Google Scholar] [CrossRef]
- Mehnert, A. Employment and work-related issues in cancer survivors. Crit. Rev. Oncol. Hematol. 2011, 77, 109–130. [Google Scholar] [CrossRef]
- Tan, C.J.; Yip, S.Y.C.; Chan, R.J.; Chew, L.; Chan, A. Investigating how cancer-related symptoms influence work outcomes among cancer survivors: A systematic review. J. Cancer Surviv. Res. Pract. 2022, 16, 1065–1078. [Google Scholar] [CrossRef] [PubMed]
- Boykoff, N.; Moieni, M.; Subramanian, S.K. Confronting chemobrain: An in-depth look at survivors’ reports of impact on work, social networks, and health care response. J. Cancer Surviv. Res. Pract. 2009, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Duijts, S.F.; Van Der Beek, A.J.; Boelhouwer, I.G.; Schagen, S.B. Cancer-related cognitive impairment and patients’ ability to work: A current perspective. Curr. Opin. Support. Palliat. Care 2017, 11, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; Crouch, A. Relationship of perceived everyday cognitive function and work engagement in breast cancer survivors. Support. Care Cancer 2021, 29, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Pullens, M.J.J.; De Vries, J.; Roukema, J.A. Subjective cognitive dysfunction in breast cancer patients: A systematic review. Psycho-Oncology 2010, 19, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Ehrenstein, J.K.; van Zon, S.K.; Duijts, S.F.; Stewart, R.E.; Almansa, J.; Amick, I.I.I.B.C.; Schagen, S.B.; Bültmann, U. Trajectories of cognitive symptoms and associated factors in cancer survivors after return to work: An 18-month longitudinal cohort study. J. Cancer Surviv. 2022, 17, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Tan, C.; Turner, J.D.; Dhillon, H. Health status and needs of cancer survivors attending the Sydney Survivorship Centre clinics and programmes: A protocol for longitudinal evaluation of the centre’s services. BMJ Open 2017, 7, e014803. [Google Scholar] [CrossRef] [PubMed]
- Stockler, M.R.; O’Connell, R.; Nowak, A.K.; Goldstein, D.; Turner, J.; Wilcken, N.R.; Wyld, D.; Abdi, E.A.; Glasgow, A.; Beale, P.J.; et al. Effect of sertraline on symptoms and survival in patients with advanced cancer, but without major depression: A placebo-controlled double-blind randomised trial. Lancet Oncol. 2007, 8, 603–612. [Google Scholar] [CrossRef]
- Dong, S.T.; Costa, D.S.; Butow, P.N.; Lovell, M.R.; Agar, M.; Velikova, G.; Teckle, P.; Tong, A.; Tebbutt, N.C.; Clarke, S.J.; et al. Symptom Clusters in Advanced Cancer Patients: An Empirical Comparison of Statistical Methods and the Impact on Quality of Life. J. Pain Symptom Manag. 2016, 51, 88–98. [Google Scholar] [CrossRef]
- Cella, D.F.; Tulsky, D.S.; Gray, G.; Sarafian, B.; Linn, E.; Bonomi, A.; Silberman, M.; Yellen, S.B.; Winicour, P.; Brannon, J.; et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J. Clin. Oncol. 1993, 11, 570–579. [Google Scholar] [CrossRef]
- Mols, F.; Tomalin, B.; Pearce, A.; Kaambwa, B.; Koczwara, B. Financial toxicity and employment status in cancer survivors. A systematic literature review. Support. Care Cancer 2020, 28, 5693–5708. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Tomalin, B.; Kaambwa, B.; Horevoorts, N.; Duijts, S.; Mols, F.; van de Poll-Franse, L.; Koczwara, B. Financial toxicity is more than costs of care: The relationship between employment and financial toxicity in long-term cancer survivors. J. Cancer Surviv. 2019, 13, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Duijts, S.F.; Van Egmond, M.P.; Spelten, E.; Van Muijen, P.; Anema, J.R.; van der Beek, A.J. Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psycho-Oncology 2014, 23, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, K.L.; Abbott-Anderson, K.; Cherwin, C.; Roiland, R.; Serlin, R.C.; Ward, S.E. Pilot randomized controlled trial of a patient-controlled cognitive-behavioral intervention for the pain, fatigue, and sleep disturbance symptom cluster in cancer. J. Pain Symptom Manag. 2012, 44, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Kwekkeboom, K.L.; Cherwin, C.H.; Lee, J.W.; Wanta, B. Mind-body treatments for the pain-fatigue-sleep disturbance symptom cluster in persons with cancer. J. Pain Symptom Manag. 2010, 39, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Fardell, J.E.; Bray, V.; Bell, M.L.; Rabe, B.; Dhillon, H.; Vardy, J.L. Screening for cognitive symptoms among cancer patients during chemotherapy: Sensitivity and specificity of a single item self-report cognitive change score. Psychooncology 2022, 31, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Rosbjerg, R.; Hansen, D.G.; Zachariae, R.; Stapelfeldt, C.M.; Hoejris, I.; Rasmussen, M.T.; Drysdale, S.W.; Labriola, M. Validation of the Return To Work Self-Efficacy questionnaire in a population of employees undergoing treatment for cancer. Eur. J. Cancer Care 2021, 30, e13373. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).