Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Surveillance
3. Diagnosis
4. Staging
5. Curative-Intent Therapies
6. Locoregional Therapies
7. Systemic Therapies
8. Neoadjuvant Therapies
9. Adjuvant Therapies
10. Imaging Response Criteria
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Chiang, C.J.; Yang, Y.W.; You, S.L.; Lai, M.S.; Chen, C.J. Thirty-year outcomes of the national hepatitis B immunization program in Taiwan. JAMA 2013, 310, 276701. [Google Scholar] [CrossRef]
- Peto, T.J.; Mendy, M.E.; Lowe, Y.; Webb, E.L.; Whittle, H.C.; Hall, A.J. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia Hepatitis Intervention Study (1986–90) and in the nationwide immunisation program. BMC Infect. Dis. 2014, 14, 7. [Google Scholar] [CrossRef]
- The Gambia Hepatitis Intervention Study. The Gambia Hepatitis Study Group. Cancer Res. 1987, 47, 5782–5787. [Google Scholar]
- Sanyal, A.J.; Yoon, S.K.; Lencioni, R. The Etiology of Hepatocellular Carcinoma and Consequences for Treatment. Oncologist 2010, 15, 14–22. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Alazawi, W.; Cunningham, M.; Dearden, J.; Foster, G.R. Systematic review: Outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment. Pharmacol. Ther. 2010, 32, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Flores, J.E.; Thompson, A.J.; Ryan, M.; Howell, J. The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines 2022, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. Towards Ending Viral Hepatitis; World Health Organization: Geneva, Switzerland, 2016.
- Nishida, N. Metabolic disease as a risk of hepatocellular carcinoma. Clin. Mol. Hepatol. 2020, 27, 302. [Google Scholar] [CrossRef]
- Paik, J.M.; Golabi, P.; Younossi, Y.; Zobair, M. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of NAFLD. Hepatology 2020, 72, 31173. [Google Scholar] [CrossRef] [PubMed]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73, 31288. [Google Scholar] [CrossRef] [PubMed]
- Lew, E.A.; Garfinkel, L. Variations in mortality by weight among 750,000 men and women. J. Chronic Dis. 1979, 32, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Wolk, A.; Gridley, G.; Svensson, M.; Nyren, O.; McLaughlin, J.K.; Fraumeni, J.F.; Adami, H.O. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001, 12, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Møller, H.; Mellemgaard, A.; Lindvig, K.; Olsen, J.H. Obesity and cancer risk: A danish record-linkage study. Eur. J. Cancer 1994, 30, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Lau, E.S.H.; Wu, H.; Wu, H.; Yang, A.; Fan, B.; Shi, M.; Tam, C.H.T.; Chow, E.; Kong, A.P.S.; et al. Risk Associations of Glycemic Burden and Obesity with Liver Cancer—A 10-Year Analysis of 15,280 Patients with Type 2 Diabetes. Hepatol. Commun. 2022, 6, 1891. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Fontana, R.J.; Fu, S.; Conjeevaram, H.S.; Su, G.L.; Lok, A.S. Alcohol, tobacco and obesity are synergistic risk factors for hepatocellular carcinoma. J. Hepatol. 2005, 42, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Govindarajan, S.; Arakawa, K.; Yu, M.C. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer 2004, 101, 20427. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.L.; Limm, W.M.; Severino, R.; Wong, L.M. Improved survival with screening for hepatocellular carcinoma. Liver Transplant. 2000, 6, 4875. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Mittal, S.; Yerokun, O.A.; Chul, A.; Marrero, J.A.; Yopp, A.C.; Parikh, N.D.; Scaglione, S.J. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival among Patients with Cirrhosis in the US. Am. J. Med. 2017, 130, 1099–1106.e1. [Google Scholar] [CrossRef]
- De Lope, C.R.; Tremosini, S.; Forner, A.; Reig, M.; Bruix, J. Management of HCC. J. Hepatol. 2012, 56, S75–S87. [Google Scholar] [CrossRef]
- Habib, A.; Desai, K.; Hickey, R.; Thornburg, B.; Lewandowski, R.; Salem, R. Locoregional Therapy of Hepatocellular Carcinoma. Clin. Liver Dis. 2015, 19, 401–420. [Google Scholar] [CrossRef]
- Yang, J.D.; Heimbach, J.K. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ 2020, 371, m3544. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Singal, A.G. Surveillance for Hepatocellular Carcinoma: Current Best Practice and Future Direction. Gastroenterology 2019, 157, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 29913. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Covey, A.M. Hepatocellular carcinoma: Updates to screening and diagnosis. JNCCN J. Natl. Compr. Cancer Netw. 2018, 2018, 52. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed]
- Frenette, C.T.; Isaacson, A.J.; Bargellini, I.; Saab, S.; Singal, A.G. A Practical Guideline for Hepatocellular Carcinoma Screening in Patients at Risk. Mayo Clin. Proc. Innov. Qual. Outcomes 2019, 3, 302–310. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waliee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718. [Google Scholar] [CrossRef]
- Singal, A.G.; Conjeevaram, H.S.; Volk, M.L.; Fu, S.; Fontana, R.J.; Askari, F.; Su, G.L.; Lok, A.S.; Marrero, J.A. Effectiveness of hepatocellular carcinoma surveillance in patients with cirrhosis. Cancer Epidemiol. Biomark. Prev. 2012, 21, 793–799. [Google Scholar] [CrossRef]
- Saitta, C.; Pollicino, T.; Raimondo, G. Obesity and liver cancer. Ann. Hepatol. 2019, 18, 810–815. [Google Scholar] [CrossRef]
- Ramai, D.; Singh, J.; Lester, J.; Kahn, S.R.; Chandan, S.; Tartaglia, N.; Ambrosi, A.; Serviddio, G.; Facciorusso, A. Systematic review with meta-analysis: Bariatric surgery reduces the incidence of hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2021, 53, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.T.; Kum, H.C.; Park, S.; Ohsfeldt, R.L.; Shen, Y.; Parikh, N.D.; Singal, A.G. Hepatocellular Carcinoma Screening Is Associated with Increased Survival of Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2019, 17, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Beal, E.W.; Owen, M.; McNamara, M.; McAlearney, A.S.; Tsung, A. Patient-, Provider-, and System-Level Barriers to Surveillance for Hepatocellular Carcinoma in High-Risk Patients in the USA: A Scoping Review. J. Gastrointest. Cancer 2022, 54, 332–356. [Google Scholar] [CrossRef]
- Parikh, N.D.; Tayob, N.; Al-Jarrah, T.; Kramer, J.; Melcher, J.; Smith, D.; Marquardt, P.; Liu, H.; Tang, R.; Kanw, F.; et al. Barriers to Surveillance for Hepatocellular Carcinoma in a Multicenter Cohort. JAMA Netw. Open 2022, 5, 23504. [Google Scholar] [CrossRef] [PubMed]
- Dalton-Fitzgerald, E.; Tiro, J.; Kandunoori, P.; Halm, E.A.; Yopp, A.; Singal, A.G. Practice patterns and attitudes of primary care providers and barriers to surveillance of hepatocellular carcinoma in patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2015, 13, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.Y.; Chen, J.; Xia, C.C.; Cao, L.K.; Duan, T.; Song, B. Noninvasive imaging of hepatocellular carcinoma: From diagnosis to prognosis. World J. Gastroenterol. 2018, 24, 2348. [Google Scholar] [CrossRef] [PubMed]
- Farvardin, S.; Patel, J.; Khambaty, M.; Yerokun, O.A.; Huram, M.; Tiro, J.A.; Yopp, A.C.; Parikh, N.D.; Marrero, J.A.; Singal, A.G. Patient-reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology 2017, 65, 28770. [Google Scholar] [CrossRef]
- Singal, A.G.; Tiro, J.A.; Murphy, C.C.; Blackwell, J.M.; Kramer, J.R.; Khan, A.; Liu, Y.; Zhang, S.; Pillips, J.L.; Hernaez, R. Patient-Reported Barriers Are Associated with Receipt of Hepatocellular Carcinoma Surveillance in a Multicenter Cohort of Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2021, 19, 987–995. [Google Scholar] [CrossRef]
- Goldberg, D.S.; Taddei, T.H.; Serper, M.; Mehta, R.; Dieperink, E.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Pedrosa, M.; et al. Identifying barriers to hepatocellular carcinoma surveillance in a national sample of patients with cirrhosis. Hepatology 2017, 65, 28765. [Google Scholar] [CrossRef]
- Beal, E.W.; McNamara, M.; Owen, M.; McAlearney, A.S.; Tsung, A. Interventions to Improve Surveillance for Hepatocellular Carcinoma in High-Risk Patients: A Scoping Review. J. Gastrointest. Cancer 2023, in press. [Google Scholar] [CrossRef]
- Mitchell, D.G. Management. In LI-RADS CT/MRI Manual; Chernyak, V., Sirlin, C.B., Eds.; American College of Radiology: Reston, VA, USA, 2018; Chapter 11, Sections 11.1–11.13. [Google Scholar]
- Sirlin, C.B. Diagnostic Categories. In LI-RADS CT/MRI Manual; Chernyak, V., Sirlin, C.B., Eds.; American College of Radiology: Reston, VA, USA, 2018; Sections 8.1–8.39. [Google Scholar]
- Elsayes, K.M.; Kielar, A.Z.; Chernyak, V.; Morshid, A.; Furlan, A.; Masch, W.R.; Marks, R.M.; Kamaya, A.; Do, R.K.G.; Kono, Y.; et al. LI-RADS: A conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J. Hepatocell. Carcinoma 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed]
- Moura Cunha, G.; Chernyak, V.; Fowler, K.J.; Sirlin, C.B. Up-to-Date Role of CT/MRI LI-RADS in Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2021, 8, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) version 2018: Imaging of hepatocellular carcinoma in at-risk patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- D’Avola, D.; Granito, A.; de la Torre-Aláez, M.; Piscaglia, F. The importance of liver functional reserve in the non-surgical treatment of hepatocellular carcinoma. J. Hepatol. 2022, 76, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Pons, F.; Varela, M.; Llovet, J.M. Staging systems in hepatocellular carcinoma. HPB 2005, 7, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Izumi, R.; Shimizu, K.; Il, T.; Yagi, M.O.; Matsui, O.; Nonomura, A.; Miyazaki, I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology 1994, 106, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Bruix, J.; Fuster, J.; Catells, A.; Garcia-Valdecasas, J.C.; Grande, L.; Franca, A.; Bru, C.; Navasa, M.; del Ayuso, M.; et al. Liver transplantation for small hepatocellular carcinoma: The tumor- node-metastasis classification does not have prognostic power. Hepatology 1998, 27, 1572–1577. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fabrega, J.; Burrel, M.; Garcia-Criado, A.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef]
- Leung, T.W.T.; Tang, A.M.Y.; Zee, B.; Lau, W.Y.; Lai, P.B.S.; Leung, K.L.; Lau, J.T.F.; Yu, S.C.H.; Johnson, P.J. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: A study based on 926 patients. Cancer 2002, 94, 10384. [Google Scholar] [CrossRef]
- The Cancer of the Liver Italian Program Investigators. Prospective validation of the CLIP score: A new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology 2000, 31, 5628. [Google Scholar] [CrossRef]
- Hansmann, J.; Ray, C.E. Overview of Staging Systems for Hepatocellular Carcinoma and Implications for Interventional Radiology. Semin. Interv. Radiol. 2017, 34, 1602757. [Google Scholar] [CrossRef] [PubMed]
- Lucatelli, P.; Guiu, B. 2022 Update of BCLC Treatment Algorithm of HCC: What’s New for Interventional Radiologists? Cardiovasc. Interv. Radiol. 2022, 45, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Brown, Z.J.; Tsilimigras, D.I.; Ruff, S.M.; Mohseni, A.; Kamel, I.R.; Cloyd, J.M.; Pawlik, T.M. Management of Hepatocellular Carcinoma. JAMA Surg. 2023, 158, 410. [Google Scholar] [CrossRef] [PubMed]
- Adhoute, X.; Penaranda, G.; Raoul, J.L.; Treut, P.L.; Bollon, E.; Hardwigsen, J.; Castellani, P.; Herve, P.; Bourliere, M. Usefulness of staging systems and prognostic scores for hepatocellular carcinoma treatments. World J. Hepatol. 2016, 8, 703. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.; Bhangui, P.; Yao, F.Y.; Mazzeferro, V.; Christian, T.; Akamatsu, N.; Durand, F.; Ijzermans, J.; Polak, W.; Zheng, S.; et al. Liver Transplantation for Hepatocellular Carcinoma. Working Group Report from the ILTS Transplant Oncology Consensus Conference. Transplantation 2020, 104, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Chieh Kow, A.W. Transplantation versus liver resection in patients with hepatocellular carcinoma. Transl. Gastroenterol. Hepatol. 2019, 4, 6. [Google Scholar] [CrossRef]
- Sakon, M. Clinical Significance of Hepatic Resection in Hepatocellular Carcinoma. Arch. Surg. 2000, 135, 1456. [Google Scholar] [CrossRef]
- Wang, W.; Guo, Y.; Zhong, J.; Wang, Q.; Wang, X.; Wei, H.; Li, J.; Xiu, P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci. Rep. 2021, 11, 2415. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Marrero, J.A.; Rudolph, L.; Reddy, K.R. Diagnosis and Treatment of Hepatocellular Carcinoma. Gastroenterology 2008, 134, 1752–1763. [Google Scholar] [CrossRef]
- O’Leary, C.; Mahler, M.; Soulen, M.C. Curative-Intent Therapies in Localized Hepatocellular Carcinoma. Curr. Treat. Options Oncol. 2020, 21, 31. [Google Scholar] [CrossRef] [PubMed]
- Moris, D.; Tsilimigras, D.I.; Kostakis, I.D.; Ntanasis-Stathopoulos, I.; Shah, K.N.; Felekouras, E.; Pawlik, T.M. Anatomic versus non-anatomic resection for hepatocellular carcinoma: A systematic review and meta-analysis. Eur. J. Surg. Oncol. 2018, 44, 927–938. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Kokudo, N.; Imamura, H.; Matsuyama, Y.; Aoki, T.; Minagawa, M.; Keiji, S.; Sugawara, Y.; Takayama, T.; Makuuchi, M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann. Surg. 2005, 242, 37401. [Google Scholar] [CrossRef]
- Jiao, S.; Li, G.; Zhang, D.; Xu, Y.; Liu, J.; Li, G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int. J. Surg. 2020, 80, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Xourafas, D.; Pawlik, T.M.; Cloyd, J.M. Early Morbidity and Mortality after Minimally Invasive Liver Resection for Hepatocellular Carcinoma: A Propensity-Score Matched Comparison with Open Resection. J. Gastrointest. Surg. 2019, 23, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Gujjuri, R.R.; Hilal, M.A.; Manas, D.M.; White, S.A. Does minimally invasive liver resection improve long-term survival compared to open resection for hepatocellular carcinoma? A systematic review and meta-analysis. Scand. J. Surg. 2022, 111, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ogiso, S. Laparoscopic liver resection: Toward a truly minimally invasive approach. World J. Gastrointest. Endosc. 2015, 7, 159. [Google Scholar] [CrossRef]
- Ziogas, I.A.; Giannis, D.; Esagian, S.M.; Enconomopoulos, K.; Tohme, S.; Geller, D. Laparoscopic versus robotic major hepatectomy: A systematic review and meta-analysis. Surg. Endosc. 2021, 35, 524–535. [Google Scholar] [CrossRef]
- Lai, E.C.H.; Tang, C.N. Long-term survival analysis of robotic versus conventional laparoscopic hepatectomy for hepatocellular Carcinoma: A comparative study. Surg. Laparosc. Endosc. Percutan Tech. 2016, 26, 254. [Google Scholar] [CrossRef]
- Zeng, Q.; Wang, J. Global scientific production of robotic liver resection from 2003 to 2022: A bibliometric analysis. Laparosc. Endosc. Robot. Surg. 2023, 6, 16–23. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transplant. 2011, 17, 22365. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Rai, R. Liver Transplantatation- an Overview. Indian J. Surg. 2013, 75, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Assalino, M.; Terraz, S.; Grat, M.; Lai, Q.; Vachharajani, N.; Gringeri, E.; Bongini, M.A.; Kulik, L.; Tabrizian, P.; Agopian, V.; et al. Liver transplantation for hepatocellular carcinoma after successful treatment of macrovascular invasion—A multi-center retrospective cohort study. Transpl. Int. 2020, 33, 13586. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver transplantation for hepatocellular carcinoma: Expansion of the tumor size limits does not adversely impact survival. Hepatology 2001, 33, 24563. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.; Roberts, J. Liver Transplantation for Hepatocellular Carcinoma: Validation of the UCSF-Expanded Criteria Based on Preoperative Imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Grazi, G.L.; Piscaglia, F.; Trevisani, F.; Cescon, M.; Ercolani, G.; Vivarelli, M.; Golfieri, R.; D’Errico Grigioni, A.; Panzini, I.; et al. Liver transplantation for hepatocellular carcinoma: Results of down-staging in patients initially outside the Milan selection criteria. Am. J. Transplant. 2008, 8, 2547–2557. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, A.N.; Snaith, A.; Petrinic, T.; Friend, P.J.; Burls, A.; Silva, M.A. Systematic review of outcome of downstaging hepatocellular cancer before liver transplantation in patients outside the Milan criteria. Br. J. Surg. 2011, 98, 7561. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, J.; Li, H.; Chen, X.; Zeng, Y. Living or deceased organ donors in liver transplantation for hepatocellular carcinoma: A systematic review and meta-analysis. HPB 2019, 21, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Limkemann, A.J.P.; Abreu, P.; Sapisochin, G. How far can we go with hepatocellular carcinoma in living donor liver transplantation? Curr. Opin. Organ Transplant. 2019, 24, 644–650. [Google Scholar] [CrossRef]
- Goldaracena, N.; Gorgen, A.; Doyle, A.; Hansen, B.E.; Tomiyama, K.; Zhang, W.; Ghanekar, A.; Lilly, L.; Cattral, M.; Galvin, Z.; et al. Live donor liver transplantation for patients with hepatocellular carcinoma offers increased survival vs. deceased donation. J. Hepatol. 2019, 70, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.; Gurakar, M.; Ting, P.S.; Alsughayer, A.M.; Luu, H.; Zaffar, D.; Alqahtani, S.; Bonder, A.; Gurakar, A.; Saberi, B. Long-Term Outcomes of Living Donor Versus Deceased Donor Liver Transplant for Hepatocellular Carcinoma in the United States. Exp. Clin. Transplant. 2022, 20, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Kamath, P.S.; Kim, W.R. The Model for End-stage Liver Disease (MELD). Hepatology 2007, 45, 21563. [Google Scholar] [CrossRef] [PubMed]
- Organ Procurement and Transplantation Network. Median MELD at Transplant around Liver Donor Hospitals and Median PELD at Transplant within the Nation. Available online: https://optn.transplant.hrsa.gov/media/ukqisspz/median-meld-at-transplant-and-median-peld-at-transplant-09212023.pdf (accessed on 28 August 2023).
- Ceresa, C.D.L.; Nasralla, D.; Coussios, C.C.; Friend, P.J. The case for normothermic machine perfusion in liver transplantation. Liver Transplant. 2018, 24, 25000. [Google Scholar] [CrossRef] [PubMed]
- Goldaracena, N.; Cullen, J.M.; Kim, D.S.; Ekser, B.; Halazun, K.J. Expanding the donor pool for liver transplantation with marginal donors. Int. J. Surg. 2020, 82, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Merion, R.M.; Goodrich, N.P.; Feng, S. How can we define expanded criteria for liver donors? J. Hepatol. 2006, 45, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Ohara, S.; Lizaola-Mayo, B.; Macdonough, E.; Morgan, P.; Das, D.; Egbert, L.; Brooks, A.; Mathur, A.K.; Aqel, B.; Reddy, K.S.; et al. Reassessing Geographic, Logistical, and Cold Ischemia Cutoffs in Liver Transplantation. Prog. Transplant. 2023, 33, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef]
- Bailey, C.W.; Sydnor, M.K. Current State of Tumor Ablation Therapies. Dig. Dis. Sci. 2019, 64, 951–958. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, Y.; He, G.; Yu, M.; Zheng, M.; Liu, L.; Zhou, X. Effects of radiofrequency ablation versus other ablating techniques on hepatocellular carcinomas: A systematic review and meta-analysis. World J. Surg. Oncol. 2017, 15, 126. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Suwa, K.; Seki, T.; Aoi, K.; Yamashina, M.; Murata, M.; Yamashiki, N.; Nishio, A.; Shimatani, M.; Naganuma, M. Efficacy of microwave ablation versus radiofrequency ablation for hepatocellular carcinoma: A propensity score analysis. Abdom. Radiol. 2021, 46, 3790–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Rhim, H. Thermal ablation for hepatocellular carcinoma: What’s new in 2019. Chin. Clin. Oncol. 2019, 8, 27367. [Google Scholar] [CrossRef]
- Couillard, A.B.; Knott, E.A.; Zlevor, A.M.; Mezrich, J.D.; Cristescu, M.M.; Agarwal, P.; Ziemlewicz, T.J.; Longhurst, C.; Lubner, M.G.; Hinshaw, J.L.; et al. Microwave Ablation as Bridging to Liver Transplant for Patients with Hepatocellular Carcinoma: A Single-Center Retrospective Analysis. J. Vasc. Interv. Radiol. 2022, 33, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Bauschke, A.; Altendorf-Hofmann, A.; Ardelt, M.; Kissler, H.; Tautenhahn, H.M.; Settmacher, U. Impact of successful local ablative bridging therapy prior to liver transplantation on long-term survival in patients with hepatocellular carcinoma in cirrhosis. J. Cancer Res. Clin. Oncol. 2020, 146, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.W.; Raman, S.S.; Asvadi, N.H.; Siripongsakun, S.; Hicks, R.M.; Chen, J.; Worakitsitisatorn, A.; McWilliams, J.; Tong, M.J.; Finn, R.S.; et al. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: A 10-year intention-to-treat analysis. Hepatology 2017, 65, 29098. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Zhao, F.; Xu, Y.; Wang, C.; Liu, K.; Liu, B.; Zheng, H.; Wei, Y.; Wang, X.; et al. Microwave ablation versus radiofrequency ablation as bridge therapy in potentially transplantable patients with single HCC ≤ 3 cm: A propensity score-matched study. Eur. J. Radiol. 2023, 164, 110860. [Google Scholar] [CrossRef]
- Gbolahan, O.B.; Schacht, M.A.; Beckley, E.W.; LaRoche, T.P.; O’Neil, B.H.; Pyko, M. Locoregional and systemic therapy for hepatocellular carcinoma. J. Gastrointest. Oncol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Torimura, T.; Iwamoto, H. Optimizing the management of intermediate-stage hepatocellular carcinoma: Current trends and prospects. Clin. Mol. Hepatol. 2021, 27, 204. [Google Scholar] [CrossRef]
- Raoul, J.L.; Sangro, B.; Forner, A.; Mazzaferro, V.; Piscaglia, F.; Bolondi, L.; Lencioni, R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: Available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat. Rev. 2011, 37, 212–220. [Google Scholar] [CrossRef]
- Guiu, B.; Garin, E.; Allimant, C.; Edeline, J.; Salem, R. TARE in Hepatocellular Carcinoma: From the Right to the Left of BCLC. Cardiovasc. Interv. Radiol. 2022, 45, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, J.F.H.; Salem, R.; Carr, B.I.; Soulen, M.C.; Thurston, K.G.; Goin, K.A.; Van Buskirk, M.; Roberts, C.A.; Goin, J.E. Yttrium-90 microspheres for the treatment of hepatocellular carcinoma. Gastroenterology 2004, 127, S194–S205. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, R.J.; Kulik, L.M.; Riaz, A.; Senthilnathan, S.; Mulcahy, M.F.; Ryu, R.K.; Ibrahim, S.M.; Sato, K.T.; Baker, T.; Miller, F.H. A Comparative Analysis of Transarterial Downstaging for Hepatocellular Carcinoma: Chemoembolization Versus Radioembolization. Am. J. Transplant. 2009, 9, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Lobo, L.; Yakoub, D.; Picado, O.; Ripat, C.; Pendola, F.; Sharma, R.; ElTawil, R.; Kwon, D.; Venkat, S.; Portelance, L.; et al. Unresectable Hepatocellular Carcinoma: Radioembolization Versus Chemoembolization: A Systematic Review and Meta-analysis. Cardiovasc. Interv. Radiol. 2016, 39, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J.; Kulik, L.; Wang, E.; Riaz, A.; Rya, R.K.; Sato, K.T.; Gupta, R.; Nikolaidis, P.; Miller, F.H.; et al. Radioembolization Results in Longer Time-to-Progression and Reduced Toxicity Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2011, 140, 497–507.e2. [Google Scholar] [CrossRef] [PubMed]
- Song, M.J. Hepatic artery infusion chemotherapy for advanced hepatocellular carcinoma. World J. Gastroenterol. 2015, 21, 3843. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Fujisawa, K.; Matsumoto, T.; Hidaka, I.; Marumoto, Y.; Ishikawa, T.; et al. Treatment strategies for advanced hepatocellular carcinoma: Sorafenib vs hepatic arterial infusion chemotherapy. World J. Hepatol. 2018, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dawson, L.A. Advances in Stereotactic Body Radiation Therapy for Hepatocellular Carcinoma. Semin. Radiat. Oncol. 2017, 27, 247–255. [Google Scholar] [CrossRef]
- Lewis, S.; Dawson, L.; Barry, A.; Stanescu, T.; Mohamad, I.; Hosni, A. Stereotactic body radiation therapy for hepatocellular carcinoma: From infancy to ongoing maturity. JHEP Rep. 2022, 4, 100498. [Google Scholar] [CrossRef]
- Kim, N.; Cheng, J.; Jung, I.; Liang, J.A.; Shih, Y.L.; Huang, W.Y.; Kimura, T.; Lee, V.H.F.; Zeng, Z.C.; Zhenggan, R.; et al. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J. Hepatol. 2020, 73, 121–129. [Google Scholar] [CrossRef]
- Marisi, G.; Cucchetti, A.; Ulivi, P.; Canale, M.; Cabibbo, G.; Solaini, L.; Foschi, F.G.; De Matteis, S.; Ercolani, G.; Valgiusti, M.; et al. Ten years of sorafenib in hepatocellular carcinoma: Are there any predictive and/or prognostic markers? World J. Gastroenterol. 2018, 24, 4152. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A Review in Hepatocellular Carcinoma. Drugs 2019, 79, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Zhu, A.X. Evolution of Systemic Therapy for Hepatocellular Carcinoma. Hepatology 2021, 73, 31306. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; Qin, S.; Ikeda, M.; Galle, P.; Ducreux, M.; Zhu, A.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. IMbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, ix186–ix187. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. Fda approval summary: Atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Lau, G.; Kudo, M.; Chan, S.L.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.; Van Dao, T.; De Toni, E.N.; et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022, 1, 1–12. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Chan, S.L.; Kudo, M.; Lau, G.; Kelley, R.K.; Furuse, J.; Sukeepaisarnjaroen, W.; Kang, Y.; Dao, T.V.; De Toni, E.N.; et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (pts) with unresectable hepatocellular carcinoma (uHCC): HIMALAYA. J. Clin. Oncol. 2022, 40, 379. [Google Scholar] [CrossRef]
- Foerster, F.; Galle, P.R. The current landscape of clinical trials for systemic treatment of hcc. Cancers 2021, 13, 1962. [Google Scholar] [CrossRef]
- Kudo, M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg. Nutr. 2022, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 10064. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Miksad, R.; Cicin, I.; Chen, Y.; Klumpen, H.J.; Kim, S.; Lin, Z.Z.; Youkstetter, J.; Sen, S.; Cheng, A.-L.; et al. Outcomes based on albumin-bilirubin (ALBI) grade in the phase III CELESTIAL trial of cabozantinib versus placebo in patients with advanced hepatocellular carcinoma (HCC). Ann. Oncol. 2019, 30, ix45–ix46. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). J. Clin. Oncol. 2019, 37, 4004. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.H.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Updated efficacy and safety of KEYNOTE-224: A phase II study of pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. Eur. J. Cancer 2022, 167, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Saung, M.T.; Pelosof, L.; Casak, S.; Donoghue, M.; Lemery, S.; Yuan, M.; Rodriguez, L.; Schotland, P.; Chuk, M.; Davis, G.; et al. FDA Approval Summary: Nivolumab Plus Ipilimumab for the Treatment of Patients with Hepatocellular Carcinoma Previously Treated with Sorafenib. Oncologist 2021, 26, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Kudo, M.; Meyer, T.; Finn, R.S.; Vogel, A.; Vai, Y.; Guo, Y.; Meng, Z.; Zhang, T.; Satoh, T.; et al. LBA36 Final analysis of RATIONALE-301: Randomized, phase III study of tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Ann. Oncol. 2022, 33, S1402–S1403. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, A.; Macarulla, T.M.; Tomasella, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1805–1945. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, T.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Kelley, R.K.; Yau, T.; Cheng, A.-L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.; Sukeepaisarnjaroen, W.; Breder, V.; Verset, G.; et al. VP10-2021: Cabozantinib (C) plus atezolizumab (A) versus sorafenib (S) as first-line systemic treatment for advanced hepatocellular carcinoma (aHCC): Results from the randomized phase III COSMIC-312 trial. Ann. Oncol. 2022, 33, 114–116. [Google Scholar] [CrossRef]
- Kelley, R.K.; Rimassa, L.; Cheng, A.L.; Kaseb, A.; Qin, S.; Zhu, A.X.; Chan, S.L.; Melkadze, T.; Sukeepaisarnjaroen, W.; Breder, V.; et al. Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 995–1008. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Kudo, M.; Merle, P.; Meyer, T.; Qin, S.; Ikeda, M.; Xu, R.; Edeline, J.; Ryoo, B.-Y.; Ren, Z.; et al. LBA34 Primary results from the phase III LEAP-002 study: Lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). Ann. Oncol. 2022, 33, S1401. [Google Scholar] [CrossRef]
- Bristol-Myers Squibb. A Study of Nivolumab in Combination with Ipilimumab in Participants with Advanced Hepatocellular Carcinoma (CheckMate 9DW). Available online: https://clinicaltrials.gov/study/NCT04039607 (accessed on 11 October 2023).
- Sanduzzi Zamparelli, M.; Matilla, A.; Lledó, J.L.; Martínez, S.M.; Varela, M.; Iñarrairaegui, M.; Perelló, C.; Minguez, B.; Llarch, N.; Márquez, L.; et al. Early nivolumab addition to regorafenib in patients with hepatocellular carcinoma progressing under first-line therapy (GOING trial), interim analysis and safety profile. J. Clin. Oncol. 2022, 40, 428. [Google Scholar] [CrossRef]
- Cheng, A.-L. Regorafenib Plus Tislelizumab as First-line Systemic Therapy for Patients with Advanced Hepatocellular Carcinoma. Available online: https://www.clinicaltrials.gov/study/NCT04183088 (accessed on 11 October 2023).
- Tvardi Therapeutics Incorporated. A Study of TTI-101 as Monotherapy and in Combination in Participants with Locally Advanced or Metastatic, and Unresectable Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/study/NCT05440708 (accessed on 11 October 2023).
- Pasche, V.K. Electromagnetic Fields versus Placebo for Child-Pugh A and B Patients with Advanced Hepatocellular Carcinoma (ARTEMIS). Available online: https://clinicaltrials.gov/study/NCT04797884 (accessed on 11 October 2023).
- Xu, A. A Study of GPC3 Redirected Autologous T Cells for Advanced HCC (GPC3-CART). Available online: https://clinicaltrials.gov/study/NCT02715362 (accessed on 11 October 2023).
- Chen Zhinan Air Force Military Medical University China. A Study of CD147-targeted CAR-T by Hepatic Artery Infusions for Very Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/study/NCT03993743 (accessed on 11 October 2023).
- Lo, C.M.; Liu, C.L.; Chan, S.C.; Lam, C.M.; Poon, R.T.P.; Ng, I.O.L.; Fan, S.T.; Wong, J. A randomized, controlled trial of postoperative adjuvant interferon therapy after resection of hepatocellular carcinoma. Ann. Surg. 2007, 245, 45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, L.; Xu, Y.; Lu, X.; Zhao, H.; Yang, H.; Sang, X. Neoadjuvant therapy and immunotherapy strategies for hepatocellular carcinoma. Am. J. Cancer Res 2020, 10, 1658. [Google Scholar] [PubMed]
- Sahin, I.H.; Khalil, L.; Millett, R.; Kaseb, A. Neoadjuvant and adjuvant treatment approaches for hepatocellular carcinoma: Future outlook. Chin. Clin. Oncol. 2021, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.C.; Acharya, S.K. New Developments in the Treatment of Hepatocellular Carcinoma: The Concept of Adjuvant and Neoadjuvant Chemotherapy. J. Clin. Exp. Hepatol. 2021, 11, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Pinato, D.J.; Fessas, P.; Sapisochin, G.; Marron, T.U. Perspectives on the Neoadjuvant Use of Immunotherapy in Hepatocellular Carcinoma. Hepatology 2021, 74, 31697. [Google Scholar] [CrossRef]
- Foerster, F.; Gairing, S.J.; Ilyas, S.I.; Galle, P.R. Emerging immunotherapy for HCC: A guide for hepatologists. Hepatology 2022, 75, 32447. [Google Scholar] [CrossRef]
- Ho, W.J.; Zhu, Q.; Durham, J.; Popovic, A.; Xavier, S.; Leatherman, J.; Mohan, A.; Mo, G.; Zhang, S.; Gross, N.; et al. Neoadjuvant cabozantinib and nivolumab convert locally advanced hepatocellular carcinoma into resectable disease with enhanced antitumor immunity. Nat. Cancer 2021, 2, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Laschtowitz, A.; Roderburg, C.; Tacke, F.; Mohr, R. Preoperative Immunotherapy in Hepatocellular Carcinoma: Current State of the Art. J. Hepatocell. Carcinoma 2023, 10, 347944. [Google Scholar] [CrossRef] [PubMed]
- Hack, S.P.; Spahn, J.; Chen, M.; Cheng, A.L.; Kaseb, A.; Kudo, M.; Lee, H.C.; Yopp, A.; Chow, P.; Qin, S. IMbrave 050: A Phase III trial of atezolizumab plus bevacizumab in high-risk hepatocellular carcinoma after curative resection or ablation. Future Oncol. 2020, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Arase, Y.; Saitoh, S.; Kobayashi, M.; Suzuki, Y.; Suzuki, F.; Tsubota, A.; Chayama, K.; Murashima, N.; Kumada, H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor—A prospective randomized study of hepatitis C virus—Related liver cancer. Hepatology 2000, 32, 9409. [Google Scholar] [CrossRef]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Exposito, M.J.; Akce, M.; Alvarez, J.L.M.; Assenat, E.; Balart, L.A.; Baron, A.D.; Decaens, T.; Heurgue-Berlot, A.; Martin, A.O.; Paik, S.W.; et al. CA209-9DX: Phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann. Oncol. 2018, 29, ix65. [Google Scholar] [CrossRef]
- Zhong, J.H.; Li, L.Q. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol. Res. 2010, 40, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Kudo, M.; Vogel, A.; Yau, T.; Zhou, J.; Kim, E.; Malhotra, U.; Siegel, A.B.; Cheng, A.-L. Abstract CT284: Phase 3 KEYNOTE-937: Adjuvant pembrolizumab versus placebo in patients with hepatocellular carcinoma and complete radiologic response after surgical resection or local ablation. Cancer Res. 2020, 80, CT284. [Google Scholar] [CrossRef]
- Boucher, E.; Corbinais, S.; Rolland, Y.; Bourguet, P.; Guyader, D.; Boudjema, K.; Meunier, B.; Raoul, J.L. Adjuvant Intra-arterial Injection of Iodine-131-Labeled Lipiodol after Resection of Hepatocellular Carcinoma. Hepatology 2003, 38, 50473. [Google Scholar] [CrossRef]
- Lau, W.Y.; Lai, E.C.H.; Leung, T.W.T.; Yu, S.C.H. Adjuvant intra-arterial iodine-131-labeled lipiodol for resectable hepatocellular carcinoma: A prospective randomized trial—Update on 5-year and 10-year survival. Ann. Surg. 2008, 247, 43–48. [Google Scholar] [CrossRef]
- Xu, L.; Chen, L.; Zhang, W. Neoadjuvant treatment strategies for hepatocellular carcinoma. World J. Gastrointest. Surg. 2021, 13, 1550. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Sun, P.; Hu, Q.G.; Song, Z.F.; Xiong, J.; Zheng, Q.C. Transarterial (chemo)embolization for curative resection of hepatocellular carcinoma: A systematic review and meta-analyses. J. Cancer Res. Clin. Oncol. 2014, 140, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Hoogstraten, B.; Staquet, M.; Winkler, A. Reporting results of cancer treatment. Cancer 1981, 47, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; Van Oosterom, A.T.; Christian, M.C.; et al. New guidelines to evaluate the response to treatment in solid tumors. J. Natl. Cancer Inst. 2000, 92, 205. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, S.; Terroir, M.; Caramella, C. Advances in oncological treatment: Limitations of RECIST 1.1 criteria. Q. J. Nucl. Med. Mol. Imaging 2018, 62, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M.; Llovet, J.M.; Beaugrand, M.; Lencioni, R.; Burroughs, A.K.; Christensen, E.; Pagliaro, L.; Colombo, M.; Rodés, J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona—2000 EASL conference. European Association for the Study of the Liver. J. Hepatol. 2001, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Sherman, M. Management of hepatocellular carcinoma. Hepatology 2005, 42, 1208–1236. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J.M. Modified recist (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 1247132. [Google Scholar] [CrossRef]
- Lin, M.; Pellerin, O.; Bhagat, N.; Rao, P.P.; Loffroy, R.; Ardon, R.; Mory, B.; Reyes, D.K.; Geschwind, J.F. Quantitative and volumetric European association for the study of the liver and response evaluation criteria in solid tumors measurements: Feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J. Vasc. Interv. Radiol. 2012, 23, 1629–1637. [Google Scholar] [CrossRef]
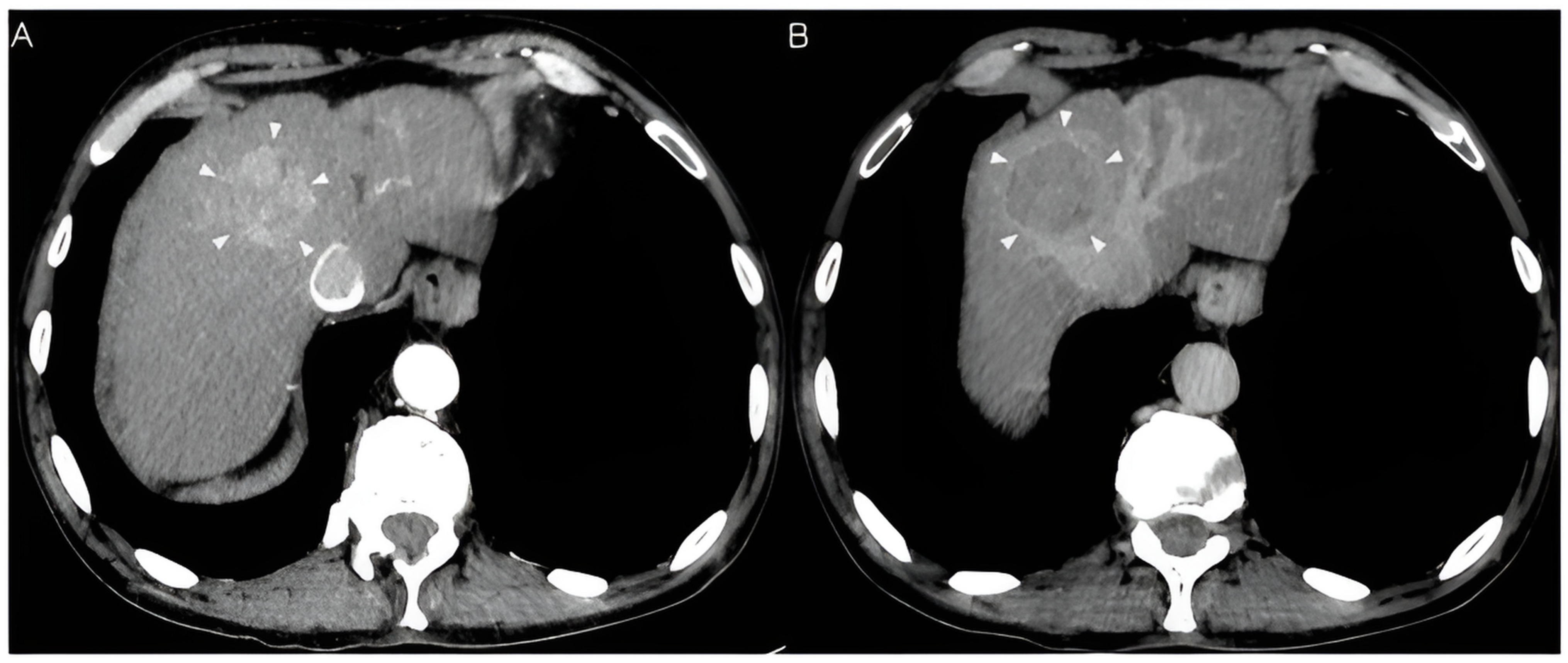
| Diagnostic Category | CT/MRI Criteria (Additional Major Features: Enhancing Capsule, Nonperipheral Washout, Threshold Growth) | Management Recommendation |
|---|---|---|
| LR-NC: noncategorizable | Cannot be categorized due to image degradation or omission | Repeat or alternative diagnostic imaging in ≤3 months |
| LR-1: definitely benign | Simple cyst, punctate perfusion alteration, focal fatty deposition/sparing, hemangioma, hypertrophic pseudomass, confluent fibrosis or focal scar No enhancement in both late arterial, portal venous and delayed phase | Return to surveillance in 6 months |
| LR-2: probably benign, 16% are HCC, 18% are malignant | Distinct nodules <20 mm with no additional major features CT images show lesions with enhancement that follows the blood pool (hemangioma) | Return to surveillance in 6 months OR consider repeat diagnostic imaging in ≤6 months |
| LR-3: intermediate probability 37% are HCC, 39% are malignant | Nonrim APHE AND ≤ 20 mm with no additional major features No APHE AND
| Repeat or alternative diagnostic imaging in 3–6 months |
| LR-4: probably HCC, 74% are HCC, 81% are malignant | Nonrim APHE AND:
| Multidisciplinary discussion for tailored workup
|
| LR-5: definitely HCC | Nonrim APHE AND:
| HCC confirmed Multidisciplinary discussion for consensus management |
| LR-M: probably or definitely malignant, not specific for HCC | Targetoid mass:
Nontargetoid mass not meeting LR-5 criteria AND no TIV, with ≥1 of the following
| Multidisciplinary discussion for tailored workup
|
| LR-TIV: malignancy with TIV | Unequivocal enhancing soft tissue in vein, regardless of visualization of parenchymal mass | Multidisciplinary discussion for tailored workup
|
| Primary Tumor (T) | Regional Lymph Nodes (N) | Metastases (M) | |
|---|---|---|---|
| AJCC TNM [57] | |||
| T1a | Solitary tumor ≤ 2 cm | Nx, regional lymph nodes cannot be assessed | M0, no distant metastasis |
| T1b | Solitary tumor ≥ 2 cm without vascular invasion | N0, no regional lymph node metastasis | M1, distant metastasis |
| T2 | Solitary tumor > 2 cm with vascular invasion, or multiple tumors, none > 5 cm | N1, regional lymph node metastasis | |
| T3 | Multiple tumors at least one of which is >5 cm | ||
| T4 | Single tumor or multiple tumors of any size involving a major branch of the portal vein or hepatic vein or tumor(s) with direction invasion of adjacent organs other than the gallbladder or with perforation of visceral peritoneum | ||
| Stage | |||
| Stage IA | T1a | N0 | M0 |
| Stage IB | T1b | N0 | M0 |
| Stage II | T2 | N0 | M0 |
| Stage IIIA | T3 | N0 | M0 |
| Stage IIIB | T4 | N0 | M0 |
| Stage IVA | Any T | N1 | M0 |
| Stage IVB | Any T | Any N | M1 |
| Histologic Grade (G) | Fibrosis Score (F) | ||
| Gx | Grade cannot be accessed | F0 | Fibrosis score 0–4 (none to moderate fibrosis) |
| G1 | Well differentiated | F1 | Fibrosis score 5–6 (severe fibrosis or cirrhosis) |
| G2 | Moderately differentiated | ||
| G3 | Poorly differentiated | ||
| G4 | Undifferentiated | ||
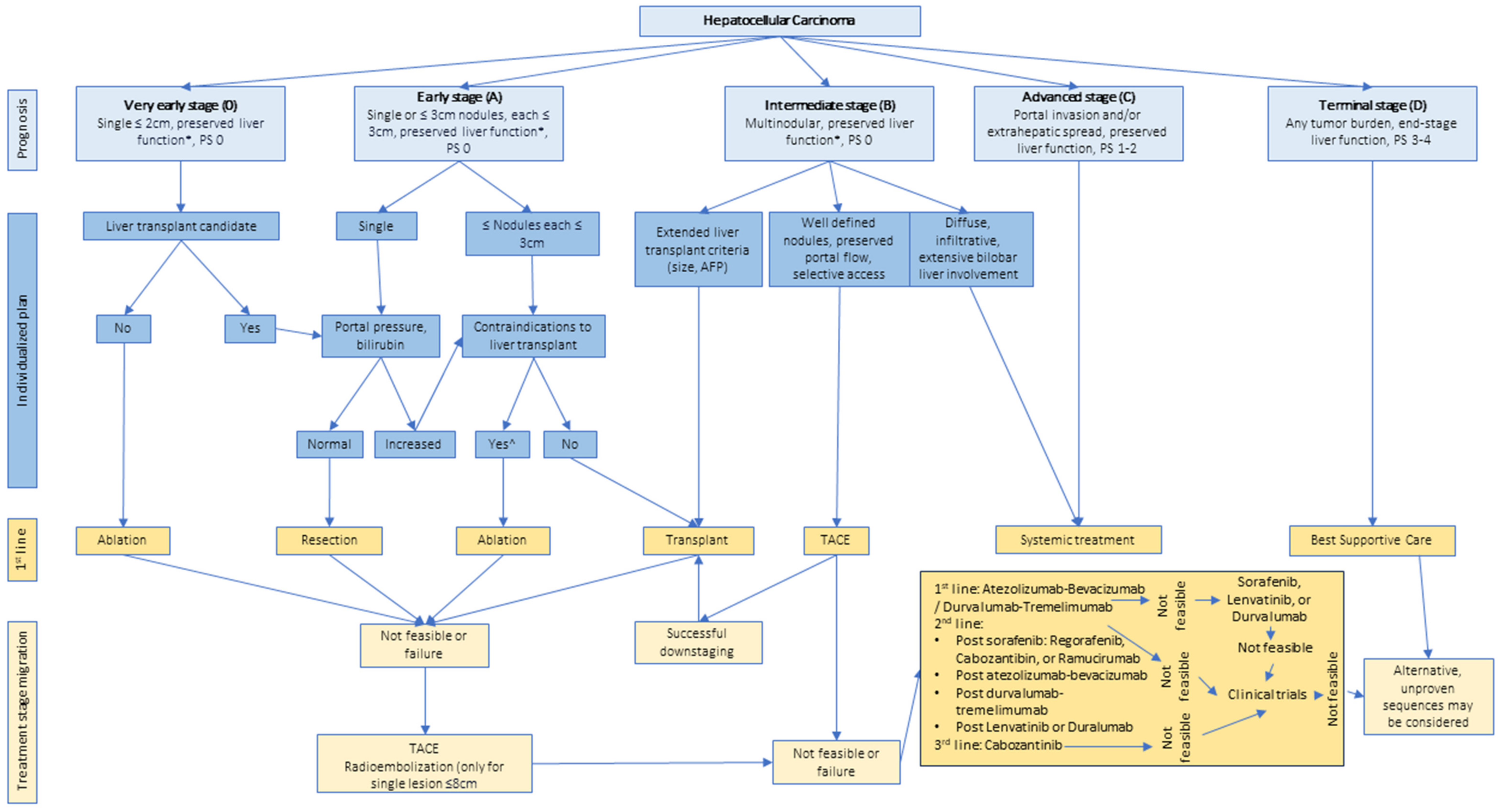
| BCLC (47) | |||
| BCLC-0 (very early stage) |
| ||
| BCLC-A (early stage) |
| ||
| BCLC-B (intermediate stage) |
| ||
| BCLC-C (advanced stage) |
| ||
| BCLC-D (terminal stage) |
| ||
| Study Name | Treatment | Inhibited Molecules | Dose |
|---|---|---|---|
| First-Line Therapies | |||
| IMbrave150 [122] | Atezolizumab plus bevacizumab | PD-L1 (immune checkpoint), VEGF (angiogenesis) | Atezolizumab: 1200 mg IV every 3 weeks Bevacizumab: 15 mg/kg IV every 3 weeks |
| HIMALAYA [124,126] | Durvalumab + tremelimumab | PD1 and CTLA4 (immune checkpoints) | Tremelimumab 300 mg IV infusion + durvalumab 1500 mg IV |
| SHARP [116] | Sorafenib | VEGFR, PDGFR (angiogenesis), MAPK (BRAF) | 400 mg oral twice daily |
| REFLECT [117] | Lenvatinib | VEGFR, PDGFR, FGFR (angiogenesis), KIT, RET | 12 mg oral once daily, if bodyweight ≥ 60 kg 8 mg oral once daily, if bodyweight < 60 kg |
| Second-line therapies | |||
| RESOURCE [127] | Regorafenib | VEGFR, PEDGR (angiogenesis), MAPK (BRAF) | 160 mg orally once daily on days 1–21 of each 28-day cycle |
| CELESTIAL [128] | Cabozantinib | MET (proliferation), VEGFR (angiogenesis), RET | 60 mg orally once daily |
| REACH-2 [129] | Ramucirumab | VEGFR2 (angiogenesis) | 8 mg/kg IV every 2 weeks |
| KEYNOTE-240 [130] | Pembrolizumab | PD1 (immune checkpoint) | 200 mg IV every 3 weeks |
| KEYNOTE-224 [131] | Pembrolizumab | PD1 (immune checkpoint) | 200 mg every 3 weeks for ≤35 cycles |
| CheckMate 040 [132] | Nivolumab + ipilimumab | PD1 and CTLA4 (immune checkpoints) | Nivolumab 1 mg/kg IV + ipilimumab 3 mg/kg IV every 3 weeks for four cycles, Nivolumab 240 mg IV every 2 weeks |
| RATIONALE-301 [133,134] | Tislelizumab | PD1 (immune checkpoint) | 200 mg IV once every three weeks |
| CARES-310 [135] | Camrelizumab + rivoceranib (apatinib) | PD1 (immune checkpoint) VEGFR2 (angiogenesis) | Camrelizumab 200 mg IV every two weeks and rivoceranib 250 mg tablet orally once daily |
| Study Name | Treatment | Inhibited Molecules | Primary Endpoint(s) | Dose |
|---|---|---|---|---|
| COSMIC-312 [136,137] NCT03755791 | Cabozantinib + atezolizumab versus sorafenib And Cabozantinib versus sorafenib | Cabozantinib is a multikinase inhibitor. Atezolizumab is an immune checkpoint inhibitor. | PFS, OS | Cabozantinib 40 mg oral, qd + atezolizumab 1200 mg infusion, q3w versus sorafenib 400 mg twice daily. |
| LEAP-002 [138] NCT03713593 | Lenvatinib + pembrolizumab versus lenvatinib + placebo | Lenvatinib targets VEGFR2-3, FGFR1-2, RET, PDGFR. Pembrolizumab is an anti-PD-1 antibody. | PFS, OS | Levatinib 12 mg (for participants with screening body weight ≥60 kg) or 8 mg (for participants with screening body weight <60 kg) orally once a day + pembrolizumab 200 mg by intravenous infusion on day 1 of each 21-day cycle (administered for up to 35 cycles). Lenvatinib 12 mg (for participants with screening body weight ≥60 kg) or 8 mg (for participants with screening body weight <60 kg) orally once a day plus saline placebo by IV infusion on day 1 of each 21-day cycle. |
| CheckMate 9DW [139] NCT04039607 | Nivolumab + ipilimumab versus sorafenib or lenvatinib | Nivolumab is an anti-PD1 receptor antibody. Ipilimumab is an anti-CTLA-4 antibody. Sorafenib is a multikinase (RAF1, BRAF, VEGFR, −1, −2, −3, PDGFR, KIT, FGFR1, RET) inhibitor inhibiting cell proliferation and angiogenesis. | OS | Nivolumab IV infusion + ipilimumab IV infusion versus sorafenib oral tablet or Lenvatinib oral tablet. |
| GOING [140] NCT04170556 | Regorafenib (monotherapy for the first 8 weeks) + nivolumab | Regorafenib potently blocks multiple protein kinases involved in tumor angiogenesis (VEGFR, −2, −3, TIE2), oncogenesis (KIT, RET, RAF-1, BRAF, BRAFV600E), metastasis (VEGFR3, PDGFR, FGFR) and tumor immunity (CDF1R). Nivolumab is a IgG4 monoclonal antibody to (PD)-1 receptor. | Safety | Regorafenib 160 mg/day for 3 weeks on and 1 week off + nivolumab 1.5 mg/kg, 3 mg/kg, or 240 mg/infusion every 2 weeks. |
| N/A [141] NCT04183088 | Part 1: regorafenib + tislelizumab Part 2: regorafenib + tislelizumab versus regorafenib | Regorafenib potently blocks multiple protein kinases involved in tumor angiogenesis (VEGFR, −2, −3, TIE2), oncogenesis (KIT, RET, RAF-1, BRAF, BRAFV600E), metastasis (VEGFR3, PDGFR, FGFR), and tumor immunity (CDF1R). Tislelizumab is an anti-PD-1 antibody. | Part 1: safety Part 2: PFS, ORR | Part 1: Tislelizumab 200 mg IV on day 1 every 3 weeks + regorafenib orally 80 mg per day. Part 2 (group 1): receives tislelizumab 200 mg IV on day 1 + regorafenib (dosage in randomized cohort will be determined according to results in the safety cohort). Part 2 (group 2): regorafenib 80 mg daily for 1 week, regorafenib 120 mg daily for week 2, regorafenib 160 mg daily for week 3, dosing-free interval for week 4. |
| REVERT [142] NCT05440708 | TTI-101 And TTI-101 + pembrolizumab And TTI-101 + atezolizumab + bevacizumab | TTI-101 is a STAT3 inhibitor. Pembrolizumab is a PD-1 inhibitor. Atezolizumab is a PD-L1 inhibitor. Bevacizumab targets angiogenesis by inhibiting VEGF. | Phase 1: safety, MTD, RP2D Phase 2: ORR | Phase 1: participants will receive up to 3 dose levels of TTI-101 oral tablet as single agent to determine RP2D. Then treated in Phase 2 with TTI-101 RP2D as single agent. TTI-101 oral tablet up to 3 dose levels + Pembrolizumab IV infusion to determine RP2D. Then in Phase 2 treat with RP2D of TTI-101 + pembrolizumab. TTI-101 oral tablet up to 3 dose levels to determine RP2D + atezolizumab IV infusion + bevacizumab IV infusion. Then in Phase 2 treat with RP2D of TTI-101 in combination with atezolizumab + bevacizumab. |
| ARTEMIS [143] NCT04797884 | TheraBionic Device versus Placebo Device | TheraBionic Device emits emitting radiofrequencies programmed with hepatocellular carcinoma-specific modulation frequencies. | OS, quality of life | TheraBionic Device emits hepatocellular carcinoma-specific modulation frequencies >200 for three courses of 60-min treatments of modulated radiofrequencies >200 administered in morning, noon, and evening for 6 weeks (very first treatment administered at recruiting site, all others at home) versus Placebo that does not emit any hepatocellular carcinoma-specific modulation frequencies for three courses of 60-min treatments administered morning, noon, and evening for 6 weeks (very first treatment administered at recruiting site, all others at home). |
| N/A [144] NCT02715362 | Vascular interventional therapy mediated GPC3-targeted chimeric antigen receptor T (CART) cells | Patient’s autologous T cells are activated and engineered to express chimeric antigen receptors (CARs) specific for GPC3, expanded, and returned to patient. | Safety | Transcatheter arterial infusion of: (1–10) × 106 CAR positive T cells/kg. A single dose of 1.5 g/m2 of cyclophosphamide will be given two days before CART cell infusion. |
| N/A [145] NCT03993743 | CD147-targeted CART cells by hepatic artery infusion | Patient’s autologous T cells are activated and engineered to express chimeric antigen receptors (CARs) specific for CD147, expanded, and returned to patient. | Safety | Three CD147-CART doses infused by hepatic artery at 1-week intervals. |
| N/A [146] NCT05323201 | fhB7H3-targeted CART cells by transhepatic arterial infusion | Patient’s autologous T cells are activated and engineered to express chimeric antigen receptors (CARs) specific for B7H3, expanded, and returned to patient. | Safety, ORR | Before infusion, cyclophosphamide (750 mg/m2 IV) and fludarabine (30 mg/m2 IV) will be administered for three consecutive days. Two days after lymphodepletion, fhB7H3 CART cells will be infused by transhepatic arterial infusion at three dose levels (1 × 106/kg, 3 × 106/kg, and 5 × 106/kg) only one time. |
| Complete Response (CR) | Partial Response (PR) | Stable Disease (SD) | Progressive Disease (PD) | |
|---|---|---|---|---|
| WHO [165] | Disappearance, confirmed at 4 weeks | 50% decrease in measurable lesions; confirmed at 4 weeks | Neither PR nor PD criteria met | 25% increase in measurable lesions; no CR, PR, or SD documented before increased disease |
| RECIST [166] | Disappearance; confirmed at 4 weeks | 30% decrease in target lesions; confirmed at 4 weeks | Neither PR nor PD criteria met | 20% increase in sum of diameter of target lesions; no CR, PR, or SD documented before increased disease |
| EASL [168] | Disappearance; determined by two observations not less than 4 weeks apart | 50% decrease in maximum diameter of the enhanced tumor area | Neither PR nor PD criteria met | 25% increase in sum of diameter of the enhanced tumor area |
| qEASL [167,169] | Disappearance | 65% decrease in enhanced tumor volume | Neither PR nor PD criteria | 73% increase in the enhanced tumor volume |
| mRECIST [167,169,170] | Disappearance of any intratumoral arterial enhancement in all target lesions | 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions | Neither PR nor PD criteria met | 20% increase in the sum of viable target lesions |
| vRECIST [167,169] | Disappearance | 65% decrease in enhanced tumor volume | Neither PR nor PD criteria | 73% increase in the enhanced tumor volume |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elderkin, J.; Al Hallak, N.; Azmi, A.S.; Aoun, H.; Critchfield, J.; Tobon, M.; Beal, E.W. Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management. Cancers 2023, 15, 5118. https://doi.org/10.3390/cancers15215118
Elderkin J, Al Hallak N, Azmi AS, Aoun H, Critchfield J, Tobon M, Beal EW. Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management. Cancers. 2023; 15(21):5118. https://doi.org/10.3390/cancers15215118
Chicago/Turabian StyleElderkin, Jessica, Najeeb Al Hallak, Asfar S. Azmi, Hussein Aoun, Jeffrey Critchfield, Miguel Tobon, and Eliza W. Beal. 2023. "Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management" Cancers 15, no. 21: 5118. https://doi.org/10.3390/cancers15215118
APA StyleElderkin, J., Al Hallak, N., Azmi, A. S., Aoun, H., Critchfield, J., Tobon, M., & Beal, E. W. (2023). Hepatocellular Carcinoma: Surveillance, Diagnosis, Evaluation and Management. Cancers, 15(21), 5118. https://doi.org/10.3390/cancers15215118






