Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review
Abstract
Simple Summary
Abstract
1. Introduction
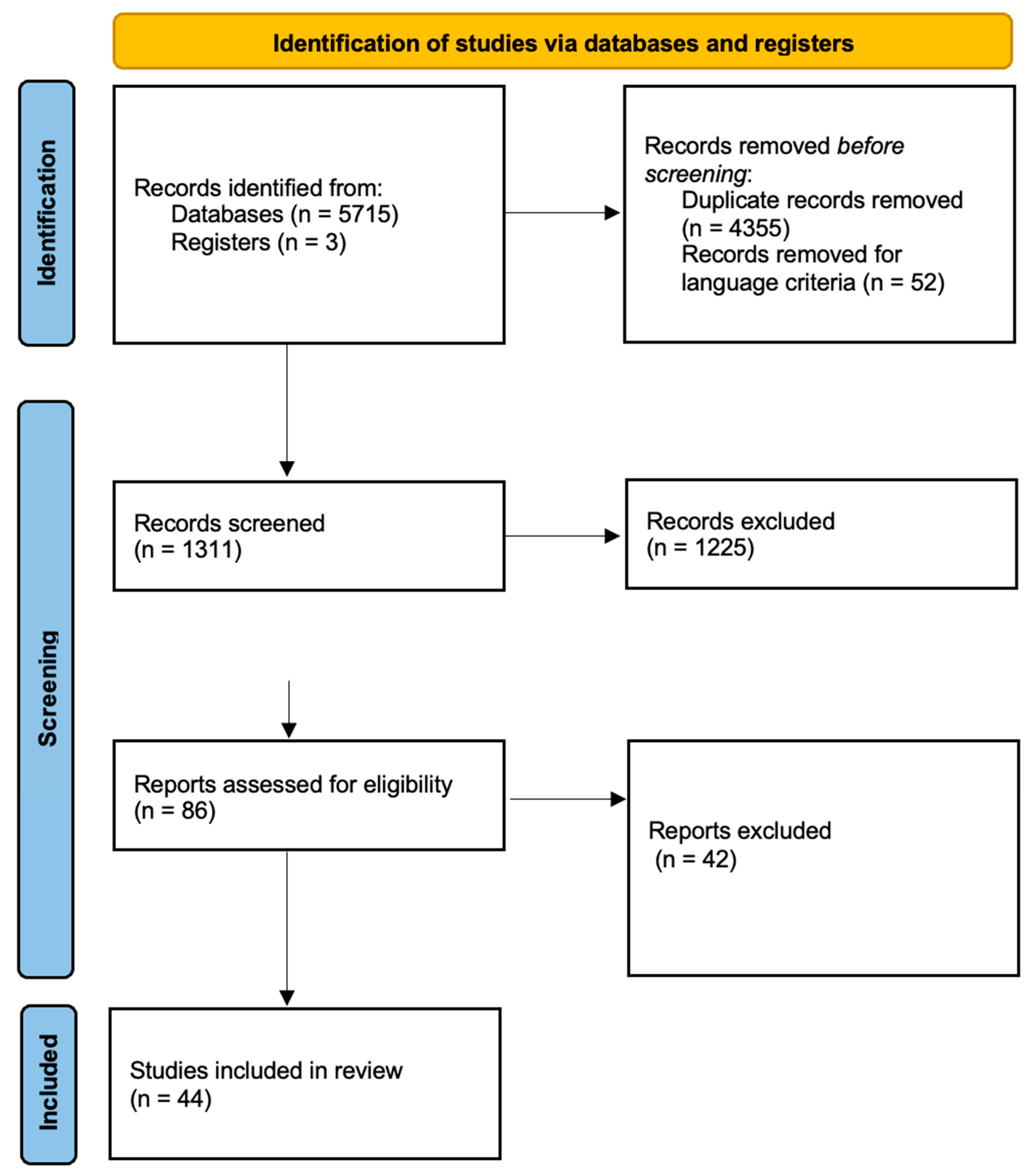
2. Search Methodology and Data Analysis
3. Biomarkers
3.1. Heat Shock Proteins (HSPs)
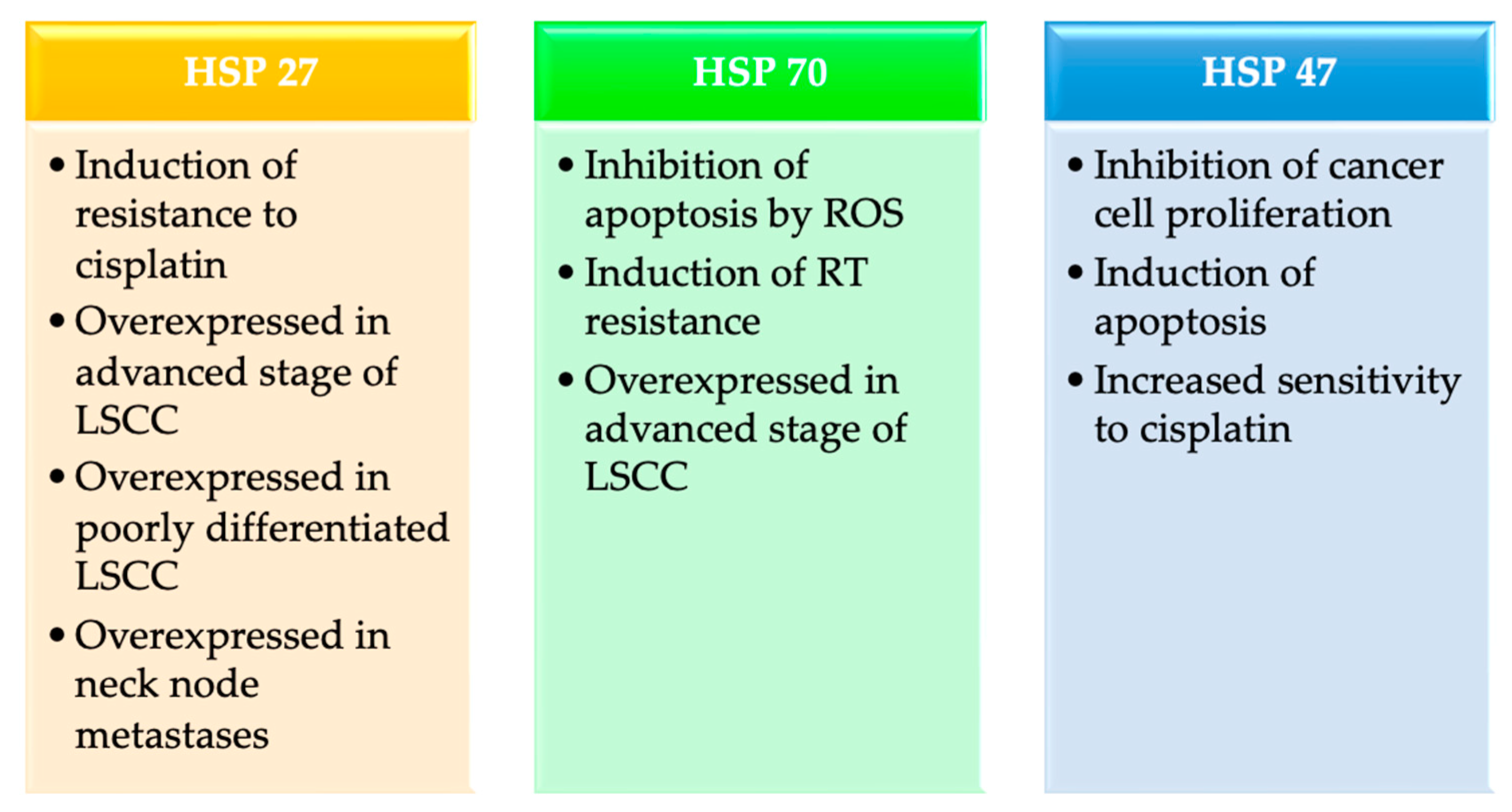
3.1.1. HSP27
| Authors | Lee [28] | Karam [29] | Kaigorodova [31] | Xu [32] | Song [26] |
|---|---|---|---|---|---|
| Year of publication | 2007 | 2017 | 2016 | 2010 | 2017 |
| Study design | Prospective | Prospective Case–control study | Retrospective | Prospective | Retrospective |
| Sample size | - | 44 | 50 | 50 | 62 |
| HSP | 27 | 27 | 27 | 70 | 47 |
| Expression level of HSP | Upregulation | Upregulation | Upregulation | Upregulation | Upregulation |
| Correlation between expression levels and LSCC | Cisplatin resistance | Advanced stage Poor differentiated | Neck node metastases | Advanced stage RT resistance | Inhibition of cancer cell proliferation Induction of apoptosis Increased sensitivity to cisplatin |
3.1.2. HSP70
3.1.3. HSP47
3.2. Metallothioneins (MTs)
3.3. Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
3.4. Heme Oxygenase (HO)
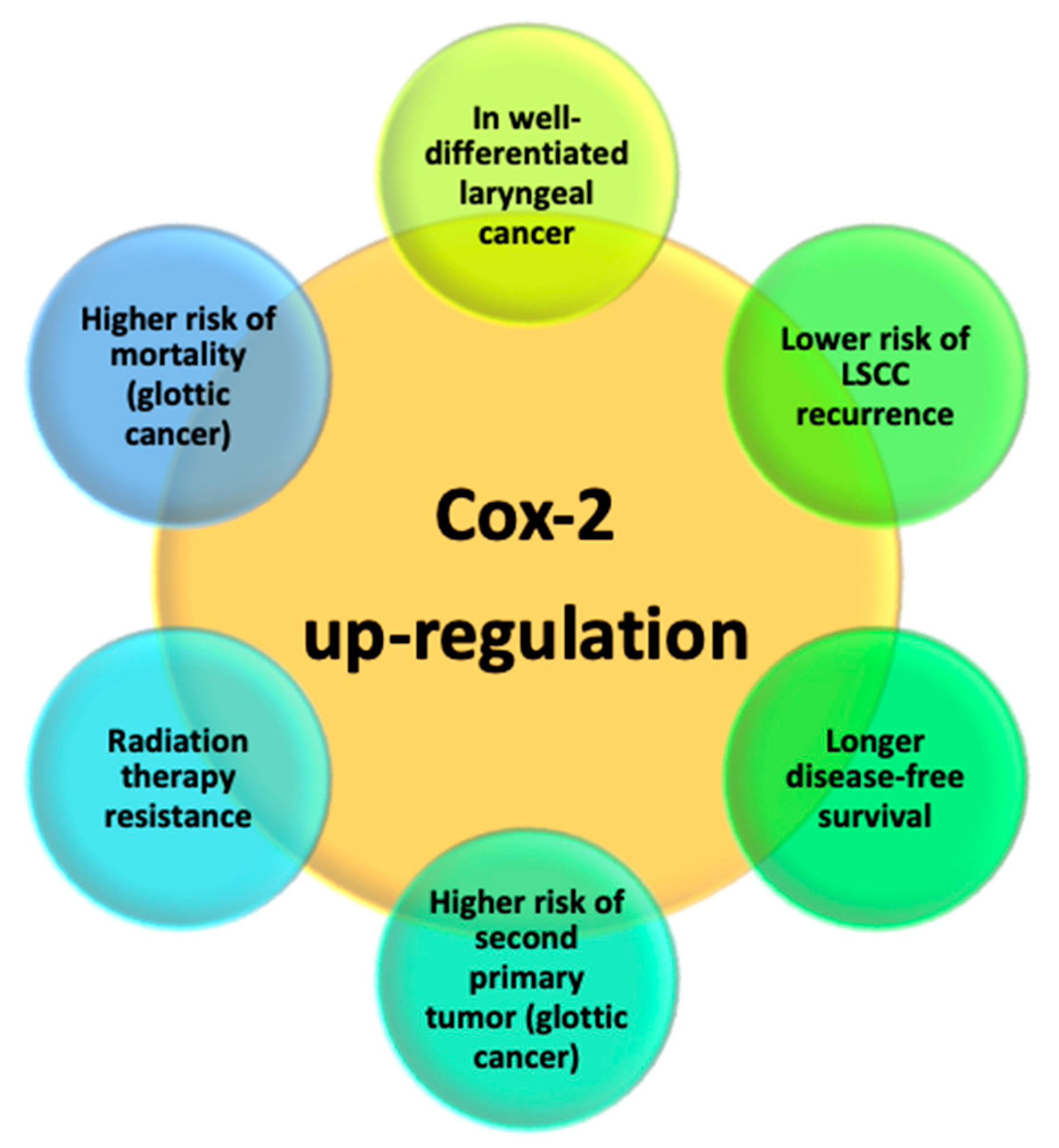
3.5. Cyclooxygenase-2 (COX-2)
3.6. Micro Ribonucleic Acids (miRNAs)
4. Conclusions
5. Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.J. Laryngeal cancer in nondrinker nonsmoker young patients: A distinct pathological entity? Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Bayer, O.; Cámara, R.; Zeissig, S.R.; Ressing, M.; Dietz, A.; Locati, L.D.; Ramroth, H.; Singer, S. Occupation and cancer of the larynx: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2016, 273, 9–20. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, B.; Lim, H.; Kim, M.; Kong, I.G.; Choi, H.G. Increased risk of larynx cancer in patients with gastroesophageal reflux disease from a national sample cohort. Clin. Otolaryngol. 2019, 44, 534–540. [Google Scholar] [CrossRef]
- Ciolofan, M.S.; Vlăescu, A.N.; Mogoantă, C.A.; Ioniță, E.; Ioniță, I.; Căpitănescu, A.N.; Mitroi, M.R.; Anghelina, F. Clinical, Histological and Immunohistochemical Evaluation of Larynx Cancer. Curr. Health Sci. J. 2017, 43, 367–375. [Google Scholar] [CrossRef]
- Markou, K.; Christoforidou, A.; Karasmanis, I.; Tsiropoulos, G.; Triaridis, S.; Constantinidis, I.; Vital, V.; Nikolaou, A. Laryngeal cancer: Epidemiological data from Νorthern Greece and review of the literature. Hippokratia 2013, 17, 313–318. [Google Scholar] [PubMed]
- Forastiere, A.A.; Ismaila, N.; Lewin, J.S.; Nathan, C.A.; Adelstein, D.J.; Eisbruch, A.; Fass, G.; Fisher, S.G.; Laurie, S.A.; Le, Q.T.; et al. Use of Larynx-Preservation Strategies in the Treatment of Laryngeal Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 1143–1169. [Google Scholar] [CrossRef]
- Saraniti, C.; Chianetta, E.; Greco, G.; Mat Lazim, N.; Verro, B. The Impact of Narrow-band Imaging on the Pre- and Intra- operative Assessments of Neoplastic and Preneoplastic Laryngeal Lesions. A Systematic Review. Int. Arch. Otorhinolaryngol. 2021, 25, e471–e478. [Google Scholar] [CrossRef]
- Steuer, C.E.; El-Deiry, M.; Parks, J.R.; Higgins, K.A.; Saba, N.F. An update on larynx cancer. CA Cancer J. Clin. 2017, 67, 31–50. [Google Scholar] [CrossRef]
- Cavaliere, M.; Bisogno, A.; Scarpa, A.; D’Urso, A.; Marra, P.; Colacurcio, V.; De Luca, P.; Ralli, M.; Cassandro, E.; Cassandro, C. Biomarkers of laryngeal squamous cell carcinoma: A review. Ann. Diagn. Pathol. 2021, 54, 151787. [Google Scholar] [CrossRef] [PubMed]
- Munck-Wikland, E.; Edström, S.; Jungmark, E.; Kuylenstierna, R.; Lindholm, J.; Auer, G. Nuclear DNA content, proliferating-cell nuclear antigen (PCNA) and p53 immunostaining in predicting progression of laryngeal cancer in situ lesions. Int. J. Cancer 1994, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Uhlman, D.L.; Adams, G.; Knapp, D.; Aeppli, D.M.; Niehans, G. Immunohistochemical staining for markers of future neoplastic progression in the larynx. Cancer Res. 1996, 56, 2199–2205. [Google Scholar] [PubMed]
- Nylander, K.; Dabelsteen, E.; Hall, P.A. The p53 molecule and its prognostic role in squamous cell carcinomas of the head and neck. J. Oral Pathol. Med. 2000, 29, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.R.; Kumar, B.; Bellile, E.; Lee, J.; Taylor, J.; D’Silva, N.; Cordell, K.; Kleer, C.; Kupfer, R.; Kumar, P.; et al. Biomarkers in advanced larynx cancer. Laryngoscope 2014, 124, 179–187. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
- Liang, Y.; Yeligar, S.M.; Brown, L.A. Chronic-alcohol-abuse-induced oxidative stress in the development of acute respiratory distress syndrome. Sci. World J. 2012, 2012, 740308. [Google Scholar] [CrossRef]
- Libalova, H.; Milcova, A.; Cervena, T.; Vrbova, K.; Rossnerova, A.; Novakova, Z.; Topinka, J.; Rossner, P. Kinetics of ROS generation induced by polycyclic aromatic hydrocarbons and organic extracts from ambient air particulate matter in model human lung cell lines. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 827, 50–58. [Google Scholar] [CrossRef]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The Double-Edged Sword Profile of Redox Signaling: Oxidative Events As Molecular Switches in the Balance between Cell Physiology and Cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Campanella, C.; Bucchieri, F.; Cappello, F. Extracellular heat shock proteins in cancer: From early diagnosis to new therapeutic approach. Semin. Cancer Biol. 2022, 86 Pt 1, 36–45. [Google Scholar] [CrossRef]
- D’anneo, A.; Bavisotto, C.C.; Gammazza, A.M.; Paladino, L.; Carlisi, D.; Cappello, F.; de Macario, E.C.; Macario, A.J.L.; Lauricella, M. Lipid chaperones and associated diseases: A group of chaperonopathies defining a new nosological entity with implications for medical research and practice. Cell Stress Chaperones 2020, 25, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Campanella, C.; Barone, R.; Bavisotto, C.C.; Gorska, M.; Wozniak, M.; Carini, F.; Cappello, F.; D’Anneo, A.; Lauricella, M.; et al. Doxorubicin anti-tumor mechanisms include Hsp60 post-translational modifications leading to the Hsp60/p53 complex dissociation and instauration of replicative senescence. Cancer Lett. 2017, 385, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.; D’Anneo, A.; Gammazza, A.M.; Bavisotto, C.C.; Barone, R.; Emanuele, S.; Cascio, F.L.; Mocciaro, E.; Fais, S.; De Macario, E.C.; et al. The histone deacetylase inhibitor SAHA induces HSP60 nitration and its extracellular release by exosomal vesicles in human lung-derived carcinoma cells. Oncotarget 2016, 7, 28849–28867. [Google Scholar] [CrossRef] [PubMed]
- Paladino, L.; Vitale, A.M.; Santonocito, R.; Pitruzzella, A.; Cipolla, C.; Graceffa, G.; Bucchieri, F.; Conway de Macario, E.; Macario, A.J.L.; Rappa, F. Molecular Chaperones and Thyroid Cancer. Int. J. Mol. Sci. 2021, 22, 4196. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liao, Z.; Zhou, C.; Lin, R.; Lu, J.; Cai, L.; Tan, X.; Zeng, W.; Lu, X.; Zheng, W.; et al. HSP47 is associated with the prognosis of laryngeal squamous cell carcinoma by inhibiting cell viability and invasion and promoting apoptosis. Oncol. Rep. 2017, 38, 2444–2452. [Google Scholar] [CrossRef][Green Version]
- Rappa, F.; Farina, F.; Zummo, G.; David, S.; Campanella, C.; Carini, F.; Tomasello, G.; Damiani, P.; Cappello, F.; DEMacario, E.C.; et al. HSP-molecular chaperones in cancer biogenesis and tumor therapy: An overview. Anticancer Res. 2012, 32, 5139–5150. [Google Scholar]
- Lee, J.-H.; Sun, D.; Cho, K.-J.; Kim, M.-S.; Hong, M.-H.; Kim, I.-K.; Lee, J.-S.; Lee, J.-H. Overexpression of human 27 kDa heat shock protein in laryngeal cancer cells confers chemoresistance associated with cell growth delay. J. Cancer Res. Clin. Oncol. 2007, 133, 37–46. [Google Scholar] [CrossRef]
- Karam, J.; Fadous-Khalifé, M.C.; Tannous, R.; Fakhreddine, S.; Massoud, M.; Hadchity, J.; Aftimos, G.; Hadchity, E. Role of Krüppel-like factor 4 and heat shock protein 27 in cancer of the larynx. Mol. Clin. Oncol. 2017, 7, 808–814. [Google Scholar] [CrossRef]
- Voll, E.A.; Ogden, I.M.; Pavese, J.M.; Huang, X.; Xu, L.; Jovanovic, B.D.; Bergan, R.C. Heat shock protein 27 regulates human prostate cancer cell motility and metastatic progression. Oncotarget 2014, 5, 2648–2663. [Google Scholar] [CrossRef]
- Kaigorodova, E.V.; Zavyalova, M.V.; Bychkov, V.A.; Perelmuter, V.M.; Choynzonov, E.L. Functional state of the Hsp27 chaperone as a molecular marker of an unfavorable course of larynx cancer. Cancer Biomark. 2016, 17, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, K.; Zhang, X.; Qiu, Y.; Huang, D.; Li, W.; Xiao, X.; Tian, Y. HSP70: A promising target for laryngeal carcinoma radiaotherapy by inhibiting cleavage and degradation of nucleolin. J. Exp. Clin. Cancer Res. 2010, 29, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Xiong, G.; Fu, H.; Evers, B.M.; Zhou, B.P.; Xu, R. Chaperone Hsp47 Drives Malignant Growth and Invasion by Modulating an ECM Gene Network. Cancer Res. 2015, 75, 1580–1591. [Google Scholar] [CrossRef] [PubMed]
- Si, M.; Lang, J. The roles of metallothioneins in carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Ioachim, E.; Assimakopoulos, D.; Peschos, D.; Zissi, A.; Skevas, A.; Agnantis, N.J. Immunohistochemical expression of metallothionein in benign premalignant and malignant epithelium of the larynx: Correlation with p53 and proliferative cell nuclear antigen. Pathol. Res. Pract. 1999, 195, 809–814. [Google Scholar] [CrossRef]
- Nowinska, K.; Chmielewska, M.; Piotrowska, A.; Pula, B.; Pastuszewski, W.; Krecicki, T.; Podhorska-Okołow, M.; Zabel, M.; Dziegiel, P. Correlation between levels of expression of minichromosome maintenance proteins, Ki-67 proliferation antigen and metallothionein I/II in laryngeal squamous cell cancer. Int. J. Oncol. 2016, 48, 635–645. [Google Scholar] [CrossRef]
- Pastuszewski, W.; Dziegiel, P.; Krecicki, T.; Podhorska-Okolow, M.; Ciesielska, U.; Gorzynska, E.; Zabel, M. Prognostic significance of metallothionein, p53 protein and Ki-67 antigen expression in laryngeal cancer. Anticancer Res. 2007, 27, 335–342. [Google Scholar]
- Starska, K.; Krześlak, A.; Forma, E.; Olszewski, J.; Lewy-Trenda, I.; Osuch-Wójcikiewicz, E.; Bryś, M. Genetic polymorphism of metallothionein 2A and risk of laryngeal cancer in a Polish population. Med. Oncol. 2014, 31, 75. [Google Scholar] [CrossRef]
- Emanuele, S.; Celesia, A.; D’anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Giuliano, M. The Good and Bad of Nrf2: An Update in Cancer and New Perspectives in COVID-19. Int. J. Mol. Sci. 2021, 22, 7963. [Google Scholar] [CrossRef]
- Namani, A.; Matiur Rahaman, M.; Chen, M.; Tang, X. Gene-expression signature regulated by the KEAP1-NRF2-CUL3 axis is associated with a poor prognosis in head and neck squamous cell cancer. BMC Cancer 2018, 18, 46. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Shitara, M.; Yokota, K.; Hikosaka, Y.; Moriyama, S.; Yano, M.; Fujii, Y. Increased NRF2 gene (NFE2L2) copy number correlates with mutations in lung squamous cell carcinomas. Mol. Med. Rep. 2012, 6, 391–394. [Google Scholar] [CrossRef]
- Xia, D.; Zhang, X.R.; Ma, Y.L.; Zhao, Z.J.; Zhao, R.; Wang, Y.Y. Nrf2 promotes esophageal squamous cell carcinoma (ESCC) resistance to radiotherapy through the CaMKIIα-associated activation of autophagy. Cell Biosci. 2020, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Sheth, S.; Farquhar, D.R.; Schrank, T.P.; Stepp, W.; Mazul, A.; Hayward, M.; Lenze, N.; Little, P.; Jo, H.; Ben Major, M.; et al. Correlation of alterations in the KEAP1/CUL3/NFE2L2 pathway with radiation failure in larynx squamous cell carcinoma. Laryngoscope Investig. Otolaryngol. 2021, 6, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, H.; Wang, S.; Zhu, J. Expression and correlation of NRF2, KEAP1, NQO-1 and HO-1 in advanced squamous cell carcinoma of the larynx and their association with clinicopathologic features. Mol. Med. Rep. 2016, 14, 5171–5179. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, J.; Bao, X.; Shi, J.; Liu, B.; Chen, Y.; Li, J. Nuclear Nrf2 Activity in Laryngeal Carcinoma is Regulated by SENP3 After Cisplatin-Induced Reactive Oxygen Species Stress. J. Cancer 2019, 10, 3427–3434. [Google Scholar] [CrossRef]
- Chen, J.S.; Huang, P.H.; Wang, C.H.; Lin, F.Y.; Tsai, H.Y.; Wu, T.C.; Lin, S.J.; Chen, J.W. Nrf-2 mediated heme oxygenase-1 expression, an antioxidant-independent mechanism, contributes to anti-atherogenesis and vascular protective effects of Ginkgo biloba extract. Atherosclerosis 2011, 214, 301–309. [Google Scholar] [CrossRef]
- Dunn, L.L.; Midwinter, R.G.; Ni, J.; Hamid, H.A.; Parish, C.R.; Stocker, R. New insights into intracellular locations and functions of heme oxygenase-1. Antioxid. Redox Signal. 2014, 20, 1723–1742. [Google Scholar] [CrossRef]
- Lv, X.; Song, D.M.; Niu, Y.H.; Wang, B.S. Inhibition of heme oxygenase-1 enhances the chemosensitivity of laryngeal squamous cell cancer Hep-2 cells to cisplatin. Apoptosis 2016, 21, 489–501. [Google Scholar] [CrossRef]
- Tang, D.; Tang, W.J.; Shi, X.L.; Li, W.P.; Zhou, H.; Lu, L.M.; Tao, L. Association of the microsatellite (GT)n repeat polymorphisms of the HO-1 gene promoter and corresponding serum levels with the risk of laryngeal squamous cell carcinoma. Acta Otolaryngol. 2016, 136, 806–811. [Google Scholar] [CrossRef]
- Cui, J.; Jia, J. Natural COX-2 Inhibitors as Promising Anti-inflammatory Agents: An Update. Curr. Med. Chem. 2021, 28, 3622–3646. [Google Scholar] [CrossRef] [PubMed]
- Gallo, O.; Masini, E.; Bianchi, B.; Bruschini, L.; Paglierani, M.; Franchi, A. Prognostic significance of cyclooxygenase-2 pathway and angiogenesis in head and neck squamous cell carcinoma. Hum. Pathol. 2002, 33, 708–714. [Google Scholar] [CrossRef]
- Hugo, H.J.; Saunders, C.; Ramsay, R.G.; Thompson, E.W. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. J. Mammary Gland. Biol. Neoplasia 2015, 20, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.F.; Baldissera, V.D.; Chiela, E.C.F.; Cerski, C.T.S.; Fontes, P.R.O.; Fernandes, M.D.C.; Porawski, M.; Giovenardi, M. Altered expression of COX-2 and TNF-α in patients with hepatocellular carcinoma. Rev. Esp. Enferm. Dig. 2019, 111, 364–370. [Google Scholar] [CrossRef]
- Li, M.; Li, M.; Wei, Y.; Xu, H. Prognostic and Clinical Significance of Cyclooxygenase-2 Overexpression in Endometrial Cancer: A Meta-Analysis. Front. Oncol. 2020, 10, 1202. [Google Scholar] [CrossRef]
- Sayar, C.; Sayar, H.; Özdemir, S.; Selçuk, T.; Görgülü, O.; Akbaş, Y.; Kemal Olgun, M. Cyclooxygenase-2 expression and clinical parameters in laryngeal squamous cell carcinoma, vocal fold nodule, and laryngeal atypical hyperplasia. Head Neck 2013, 35, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ranelletti, F.O.; Almadori, G.; Rocca, B.; Ferrandina, G.; Ciabattoni, G.; Habib, A.; Galli, J.; Maggiano, N.; Gessi, M.; Lauriola, L. Prognostic significance of cyclooxygenase-2 in laryngeal squamous cell carcinoma. Int. J. Cancer 2001, 95, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, K.; Vandoros, G.; Kourelis, T.; Papadas, T.; Goumas, P.; Sotiropoulou-Bonikou, G. Low COX2 in tumor and upregulation in stroma mark laryngeal squamous cell carcinoma progression. Laryngoscope 2009, 119, 1723–1729. [Google Scholar] [CrossRef]
- Kawata, R.; Hyo, S.; Maeda, T.; Urade, Y.; Takenaka, H. Simultaneous expression of cyclooxygenase-2 and microsomal prostaglandin E synthase in squamous cell carcinoma of the larynx. Acta Otolaryngol. 2006, 126, 627–632. [Google Scholar] [CrossRef]
- Chen, Y.F.; Luo, R.Z.; Li, Y.; Cui, B.K.; Song, M.; Yang, A.K.; Chen, W.K. High expression levels of COX-2 and P300 are associated with unfavorable survival in laryngeal squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2013, 270, 1009–1017. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Wang, J.; Luo, L.; Chen, P.; Ding, J.; Gong, S. Expression of vascular endothelial growth factor and cyclooxygenase-2 in laryngeal squamous cell carcinoma and its significance. J. Huazhong Univ. Sci. Technol. Med. Sci. 2006, 26, 105–107. [Google Scholar] [CrossRef]
- Kourelis, K.; Sotiropoulou-Bonikou, G.; Vandoros, G.; Repanti, M.; Varakis, I.; Goumas, P. Coordinated upregulation of COX-2 and NF-kappaB is a steady feature of laryngeal carcinogenesis. ORL J. Otorhinolaryngol. Relat. Spec. 2007, 69, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ruiz, E.; Cazorla, O.; Redondo, M.; Pérez, L.; Álvarez, M.; Gallego, E.; Trigo, J.M.; Medina, J.A.; Matilla, A.; Rueda, A. Immunohistochemical expression of cyclooxygenase-2 in patients with advanced cancer of the larynx who have undergone induction chemotherapy with the intention of preserving phonation. Clin. Transl. Oncol. 2012, 14, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.I.; Kowalski, D.P.; Sasaki, C.T.; Haffty, B.G. Tissue microarray analysis reveals prognostic significance of COX-2 expression for local relapse in T1-2N0 larynx cancer treated with primary radiation therapy. Laryngoscope 2004, 114, 2001–2008. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, J.; Feng, J.; Luo, D.; Peng, L. Prognostic and Clinical Significance of COX-2 Overexpression in Laryngeal Cancer: A Meta-Analysis. Front. Oncol. 2022, 12, 854946. [Google Scholar] [CrossRef]
- Sackett, M.K.; Bairati, I.; Meyer, F.; Jobin, E.; Lussier, S.; Fortin, A.; Gélinas, M.; Nabid, A.; Brochet, F.; Têtu, B. Prognostic significance of cyclooxygenase-2 overexpression in glottic cancer. Clin. Cancer Res. 2008, 14, 67–73. [Google Scholar] [CrossRef]
- Nix, P.; Lind, M.; Greenman, J.; Stafford, N.; Cawkwell, L. Expression of Cox-2 protein in radioresistant laryngeal cancer. Ann. Oncol. 2004, 15, 797–801. [Google Scholar] [CrossRef]
- Tsujii, M.; DuBois, R.N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell 1995, 83, 493–501. [Google Scholar] [CrossRef]
- Pyo, H.; Choy, H.; Amorino, G.P.; Kim, J.S.; Cao, Q.; Hercules, S.K.; DuBois, R.N. A selective cyclooxygenase-2 inhibitor, NS-398, enhances the effect of radiation in vitro and in vivo preferentially on the cells that express cyclooxygenase-2. Clin. Cancer Res. 2001, 7, 2998–3005. [Google Scholar]
- Klatka, J.; Grywalska, E.; Hymos, A.; Guz, M.; Polberg, K.; Roliński, J.; Stepulak, A. Cyclooxygenase-2 Inhibition Enhances Proliferation of NKT Cells Derived from Patients with Laryngeal Cancer. Anticancer Res. 2017, 37, 4059–4066. [Google Scholar] [CrossRef][Green Version]
- Wang, R.; Wang, X.; Lin, F.; Gao, P.; Dong, K.; Zhang, H.Z. shRNA-targeted cyclooxygenase (COX)-2 inhibits proliferation, reduces invasion and enhances chemosensitivity in laryngeal carcinoma cells. Mol. Cell. Biochem. 2008, 317, 179–188. [Google Scholar] [CrossRef]
- Fabbri, M. miRNAs as molecular biomarkers of cancer. Expert Rev. Mol. Diagn. 2010, 10, 435–444. [Google Scholar] [CrossRef]
- Li, J.; Fu, H.; Xu, C.; Tie, Y.; Xing, R.; Zhu, J.; Qin, Y.; Sun, Z.; Zheng, X. miR-183 inhibits TGF-beta1-induced apoptosis by downregulation of PDCD4 expression in human hepatocellular carcinoma cells. BMC Cancer 2010, 10, 354. [Google Scholar] [CrossRef]
- Lowery, A.J.; Miller, N.; Dwyer, R.M.; Kerin, M.J. Dysregulated miR-183 inhibits migration in breast cancer cells. BMC Cancer 2010, 10, 502. [Google Scholar] [CrossRef]
- Tuncturk, F.R.; Akalin, I.; Uzun, L.; Zenginkinet, T. Comparison of miRNA expressions among benign, premalignant and malignant lesions of the larynx: Could they be transformation biomarkers? J. Otolaryngol. Head Neck Surg. 2021, 50, 14. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.J.; Xu, W.H.; Jin, X.J.; Li, J.P.; Tang, Y.J.; Huang, X.F.; Cui, H.J.; Sun, G.B. miR-21 and miR-375 microRNAs as candidate diagnostic biomarkers in squamous cell carcinoma of the larynx: Association with patient survival. Am. J. Transl. Res. 2014, 6, 604–613. [Google Scholar] [PubMed]
- Erkul, E.; Yilmaz, I.; Gungor, A.; Kurt, O.; Babayigit, M.A. MicroRNA-21 in laryngeal squamous cell carcinoma: Diagnostic and prognostic features. Laryngoscope 2017, 127, E62–E66. [Google Scholar] [CrossRef]
- Liu, M.; Wu, H.; Liu, T.; Li, Y.; Wang, F.; Wan, H.; Li, X.; Tang, H. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009, 19, 828–837. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hanazawa, T.; Nohata, N.; Kikkawa, N.; Enokida, H.; Yoshino, H.; Yamasaki, T.; Hidaka, H.; Nakagawa, M.; Okamoto, Y.; et al. Tumor suppressive microRNA-218 inhibits cancer cell migration and invasion through targeting laminin-332 in head and neck squamous cell carcinoma. Oncotarget 2012, 3, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Gao, W.; Lei, W.B.; Zhao, J.; Chan, J.Y.; Wei, W.I.; Ho, W.K.; Wong, T.S. MicroRNA 744-3p promotes MMP-9-mediated metastasis by simultaneously suppressing PDCD4 and PTEN in laryngeal squamous cell carcinoma. Oncotarget 2016, 7, 58218–58233. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, J.; Xie, J.; Zhang, T.; Dong, P. Role of miR-138 in the regulation of larynx carcinoma cell metastases. Tumour Biol. 2016, 37, 15601–15606. [Google Scholar] [CrossRef] [PubMed]
- Russ, R.; Slack, F.J. Cigarette-Smoke-Induced Dysregulation of MicroRNA Expression and Its Role in Lung Carcinogenesis. Pulm. Med. 2012, 2012, 791234. [Google Scholar] [CrossRef]
- Ahrendt, S.A.; Chow, J.T.; Yang, S.C.; Wu, L.; Zhang, M.J.; Jen, J.; Sidransky, D. Alcohol consumption and cigarette smoking increase the frequency of p53 mutations in non-small cell lung cancer. Cancer Res. 2000, 60, 3155–3159. [Google Scholar] [PubMed]
- Bruzgielewicz, A.; Osuch-Wojcikiewicz, E.; Niemczyk, K.; Sieniawska-Buccella, O.; Siwak, M.; Walczak, A.; Nowak, A.; Majsterek, I. Altered Expression of miRNAs Is Related to Larynx Cancer TNM Stage and Patients’ Smoking Status. DNA Cell Biol. 2017, 36, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Y.P.; Yang, D.; Zhang, G.; Zhou, H.F. Clinical Significance of miR-149 in the Survival of Patients with Laryngeal Squamous Cell Carcinoma. Biomed. Res. Int. 2016, 2016, 8561251. [Google Scholar] [CrossRef]
- Karatas, O.F. Antiproliferative potential of miR-33a in laryngeal cancer Hep-2 cells via targeting PIM1. Head Neck 2018, 40, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Li, D.J.; Wang, X.; Yin, W.H.; Niu, K.; Zhu, W.; Fang, N. MiR-199a-5p suppresses proliferation and invasion of human laryngeal cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12200–12207. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.F.; Yuceturk, B.; Suer, I.; Yilmaz, M.; Cansiz, H.; Solak, M.; Ittmann, M.; Ozen, M. Role of miR-145 in human laryngeal squamous cell carcinoma. Head Neck 2016, 38, 260–266. [Google Scholar] [CrossRef]
- Shen, Z.; Zhan, G.; Ye, D.; Ren, Y.; Cheng, L.; Wu, Z.; Guo, J. MicroRNA-34a affects the occurrence of laryngeal squamous cell carcinoma by targeting the antiapoptotic gene survivin. Med. Oncol. 2012, 29, 2473–2480. [Google Scholar] [CrossRef]
- Chen, H.; Cai, X.; Du, B.; Cai, J.; Luo, Z. MicroRNA-150-5p inhibits the proliferation and invasion of human larynx epidermiod cancer cells though regulating peptidyl-prolyl cis/trans isomerase. Braz. J. Otorhinolaryngol. 2023, 89, 383–392. [Google Scholar] [CrossRef]
- Luo, M.; Sun, G.; Sun, J.W. MiR-196b affects the progression and prognosis of human LSCC through targeting PCDH-17. Auris Nasus Larynx 2019, 46, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cao, H. MicroRNA-143-3p suppresses cell growth and invasion in laryngeal squamous cell carcinoma via targeting the k-Ras/Raf/MEK/ERK signaling pathway. Int. J. Oncol. 2019, 54, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Maia, D.; de Carvalho, A.C.; Horst, M.A.; Carvalho, A.L.; Scapulatempo-Neto, C.; Vettore, A.L. Expression of miR-296-5p as predictive marker for radiotherapy resistance in early-stage laryngeal carcinoma. J. Transl. Med. 2015, 13, 262. [Google Scholar] [CrossRef] [PubMed]
| Authors | Ioachim [35] | Nowinska [36] | Pastuszewski [37] | Starska [38] |
|---|---|---|---|---|
| Year of publication | 1999 | 2016 | 2007 | 2014 |
| Study design | Prospective | Prospective | Retrospective | Prospective |
| Sample size | 73 | 83 | 65 | 323 |
| MTs | MT | MT-I/II | MT | Loci—5 A/G (rs28366003) of the MT2A gene |
| Expression level of MTs | Upregulation | Upregulation | Upregulation | Genetic polymorphism |
| Correlation between expression levels and LSCC | Predictive of malignant transformation of laryngeal lesion | Higher in LSCC than in benign laryngeal lesions | Predictive of malignant transformation of laryngeal lesion | High risk of developing LSCC |
| Authors | Sheth [44] | Li [45] | Zhou [46] |
|---|---|---|---|
| Year of publication | 2021 | 2016 | 2019 |
| Study design | Retrospective | Prospective | Prospective |
| Sample size | 20 | 33 | 32 |
| Marker | Nrf2 | Nrf2 | Nrf2 |
| Expression level of Nrf2 | Changes in Nfr2/KEAP1 oxidative stress pathway | Upregulation | Upregulation |
| Correlation between expression levels and LSCC | RT resistance | Advanced stage | Cisplatin resistance |
| Authors | Lv X [49] | Tang [50] | Li [45] |
|---|---|---|---|
| Year of publication | 2016 | 2016 | 2016 |
| Study design | Prospective | Prospective Case–control study | Prospective |
| Sample size | - | 142 | 33 |
| Marker | HO-1 | HO-1 | HO-1 |
| Expression level of HO | Inhibition | Downregulation | Upregulation |
| Correlation between expression levels and LSCC | Increased cisplatin sensitivity | Advanced stage Neck node metastases | Advanced stage |
| Authors | Year of Publication | Study Design | Sample Size | Expression Level of COX-2 | Correlation between Expression Levels and LSCC |
|---|---|---|---|---|---|
| Sayar [56] | 2013 | Prospective | 105 | Upregulation | LSCC and smokers |
| Ranelletti [57] | 2001 | Prospective | 61 | Downregulation | Poorly differentiated LSCC and normal laryngeal tissue |
| Kourelis [58] | 2009 | Retrospective | 52 | Downregulation | Higher risk of local recurrence Shorter DFS |
| Kawata [59] | 2006 | Prospective | 24 | Upregulation | Well-differentiated |
| Chen [60] | 2013 | Retrospective | 80 | Upregulation | Advanced stage Poorly differentiated |
| Chen [61] | 2006 | Prospective Case–control study | 62 | Upregulation | LSCC |
| Kourelis [62] | 2007 | Prospective | 129 | Upregulation | LSCC |
| Pérez-Ruiz [63] | 2012 | Retrospective | 59 | Upregulation | Better OS Longer DFS |
| Cho [64] | 2004 | Prospective | 123 | Upregulation | Higher risk of local relapse in T1-T2N0 |
| Sackett [66] | 2008 | Prospective | 301 | Upregulation | Higher risk of second primary tumor Higher overall mortality |
| Nix [67] | 2004 | 122 | Upregulation | Higher resistance to RT | |
| Klatka [70] | 2017 | Prospective Case–control study | 78 | Inhibition (through celecoxib) | Higher sensitivity to immunotherapy |
| Wang [71] | 2008 | Prospective | - | Inhibition (through shRNA) | Induction of cancer cell apoptosis Inhibition of cancer proliferation and invasion |
| Authors | Year of Publication | Study Design | Sample Size | miRNAs | Expression Level of miRNAs | Correlation between Expression Levels and LSCC |
|---|---|---|---|---|---|---|
| Tuncturk [75] | 2021 | Prospective | 30 | miR-183_5p | Upregulation | Neck node metastasis in supraglottic cancer |
| miR-155_5p | Upregulation Upregulation | Both in premalignant and malignant lesions | ||||
| miR-106b_3p | ||||||
| Bruzgielewicz [84] | 2017 | Prospective | 48 | miR-29a | Upregulation | Early stage |
| miR-202 | Upregulation | Advanced stage Long-time smokers | ||||
| miR-4768-3p | Downregulation | Advanced stage with nodal metastases | ||||
| miR-548 | Upregulation | Advanced stage with nodal metastases | ||||
| Karatas [86] | 2018 | Prospective | - | miR-33a | Upregulation | Cancer cell apoptosis |
| Li [87] | 2020 | Prospective | 25 | miR-199a-5p | Upregulation | Cancer cell apoptosis |
| Hu [76] | 2014 | Prospective Case–control study | 46 | miR-21 | Upregulation | Biomarker for laryngeal cancers Worse prognosis |
| miR-375 | Downregulation | Supraglottic cancer Alcohol abuse | ||||
| Gao [81] | 2015 | Prospective Case–control study | 30 | miR-138 | Downregulation | Distal metastases Worse prognosis |
| Erkul [77] | 2017 | Retrospective Case–control study | 72 | miR-21 | Upregulation | Biomarker for laryngeal cancers |
| Karatas [88] | 2016 | Prospective Case–control study | 80 | miR-145 | Downregulation | Tumor suppressor |
| Shen [89] | 2012 | Prospective Case–control study | 69 | miR-34a | Downregulation | Shorter DFS Tumor suppressor |
| Xu [85] | 2016 | Prospective | 97 | miR-149 | Downregulation | Shorter OS |
| Luo [91] | 2018 | Prospective | 79 | miR-196b | Upregulation | Worse prognosis |
| Chen [90] | 2023 | Prospective Case–control study | 16 | miR-150-5p | Downregulation | Tumor suppressor |
| Li [80] | 2016 | Prospective | - | miR-744-3p | Upregulation | Neck node metastasis |
| Zhang [92] | 2019 | Prospective | 52 | miR-143-3p | Downregulation | Advanced stage Poorly differentiated Shorter OS |
| Maia [93] | 2015 | Retrospective | 34 | miR-296-5p | Upregulation | Resistance to RT Recurrence in early stage |
| Liu [78] | 2009 | Prospective | 210 | miR-21 | Upregulation | Biomarker for laryngeal cancers |
| Micro Ribonucleic Acid (miRNAs) | Overexpression |
|---|---|
| Hs_miR-21_5p | Biomarker for laryngeal cancers: only in the malignant laryngeal lesions |
| Hs_miR-210_3p | |
| Hs_miR-183_5p | Positively related to neck node metastasis in supraglottic cancer |
| Hs_miR-155_5p | “Transformation biomarkers”: both in premalignant and malignant lesions |
| Hs_miR-106b_3p | |
| Hs_miR-4768-3p | Negatively correlated with neck node metastasis Downregulated in smokers for less than 20 years |
| Hs_miR-29a | Positively related to tumor stage |
| Hs_miR-202 | Overexpressed in smokers for more than 20 years |
| Hs_miR-548 | Overexpressed in advanced laryngeal cancer (T4N+) |
| Hs_miR-33a Hs_miR-199a-5p Hs_miR- 150-5p | Tumor suppressor Cancer cell apoptosis |
| Hs_miR-375 | Downregulated in supraglottic cancer and in alcohol abuse |
| Hs_miR-138 | Negative related to distal metastases |
| Hs_miR-145 | Tumor suppressor |
| Hs_miR-34a | Positively related to disease-free survival |
| Hs_miR-149 | Negative related to prognosis Negative related to prognosis |
| Hs_miR-196b | |
| Hs_miR-744-3p | Neck node metastasis |
| Hs_miR-143-3p | Downregulated advanced and poorly differentiated laryngeal cancer |
| Hs_miR-296-5p | Resistance to radiation therapy Risk of recurrence in early stage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verro, B.; Saraniti, C.; Carlisi, D.; Chiesa-Estomba, C.; Maniaci, A.; Lechien, J.R.; Mayo, M.; Fakhry, N.; Lauricella, M. Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review. Cancers 2023, 15, 5096. https://doi.org/10.3390/cancers15205096
Verro B, Saraniti C, Carlisi D, Chiesa-Estomba C, Maniaci A, Lechien JR, Mayo M, Fakhry N, Lauricella M. Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review. Cancers. 2023; 15(20):5096. https://doi.org/10.3390/cancers15205096
Chicago/Turabian StyleVerro, Barbara, Carmelo Saraniti, Daniela Carlisi, Carlos Chiesa-Estomba, Antonino Maniaci, Jerome R. Lechien, Miguel Mayo, Nicolas Fakhry, and Marianna Lauricella. 2023. "Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review" Cancers 15, no. 20: 5096. https://doi.org/10.3390/cancers15205096
APA StyleVerro, B., Saraniti, C., Carlisi, D., Chiesa-Estomba, C., Maniaci, A., Lechien, J. R., Mayo, M., Fakhry, N., & Lauricella, M. (2023). Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review. Cancers, 15(20), 5096. https://doi.org/10.3390/cancers15205096








